Assessment of Nitrogen Uptake and Biological Nitrogen Fixation Responses of Soybean to Nitrogen Fertiliser with SPACSYS
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
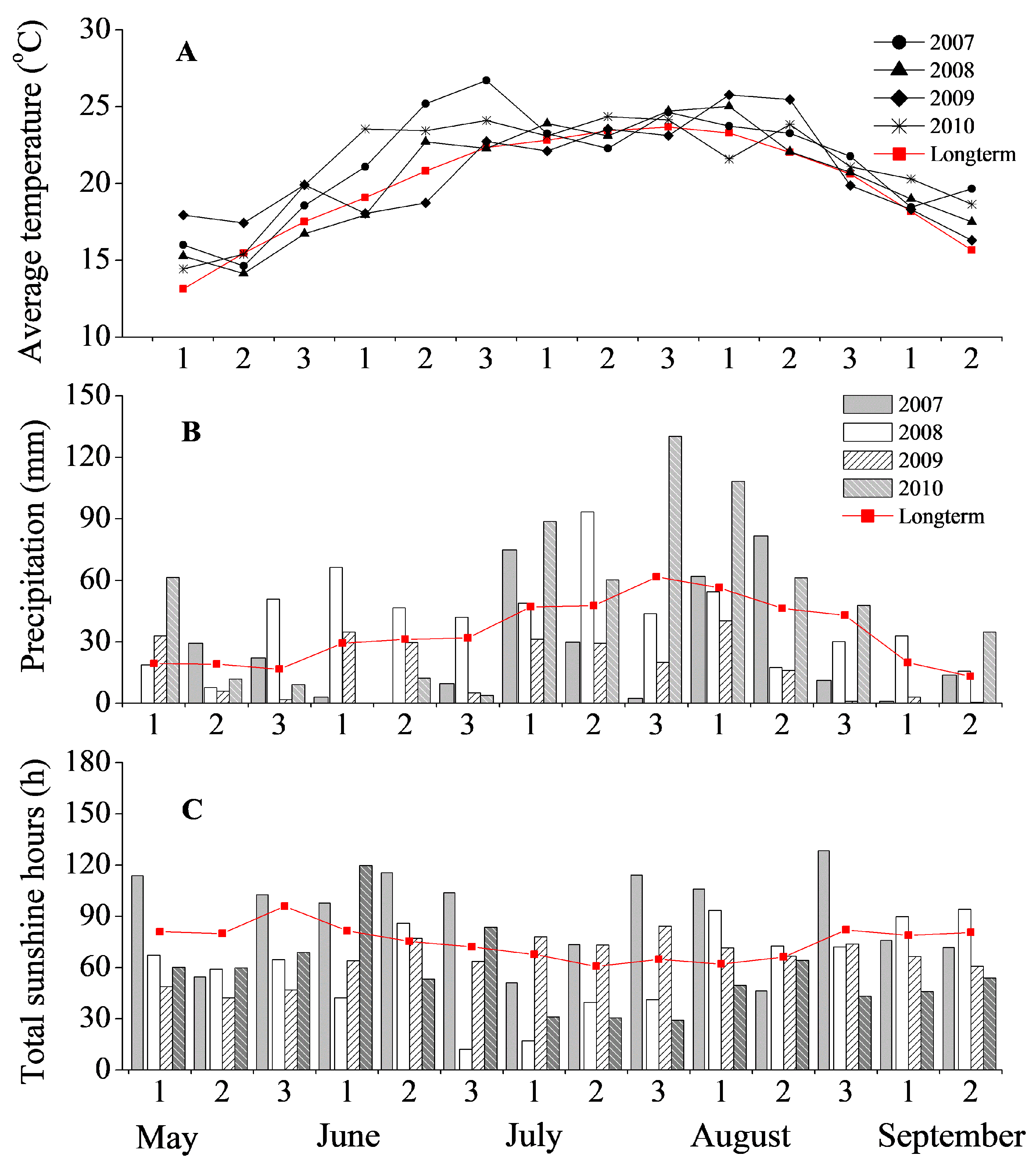
2.2. Experimental Design and Data Collection
2.3. The SPACSYS Model
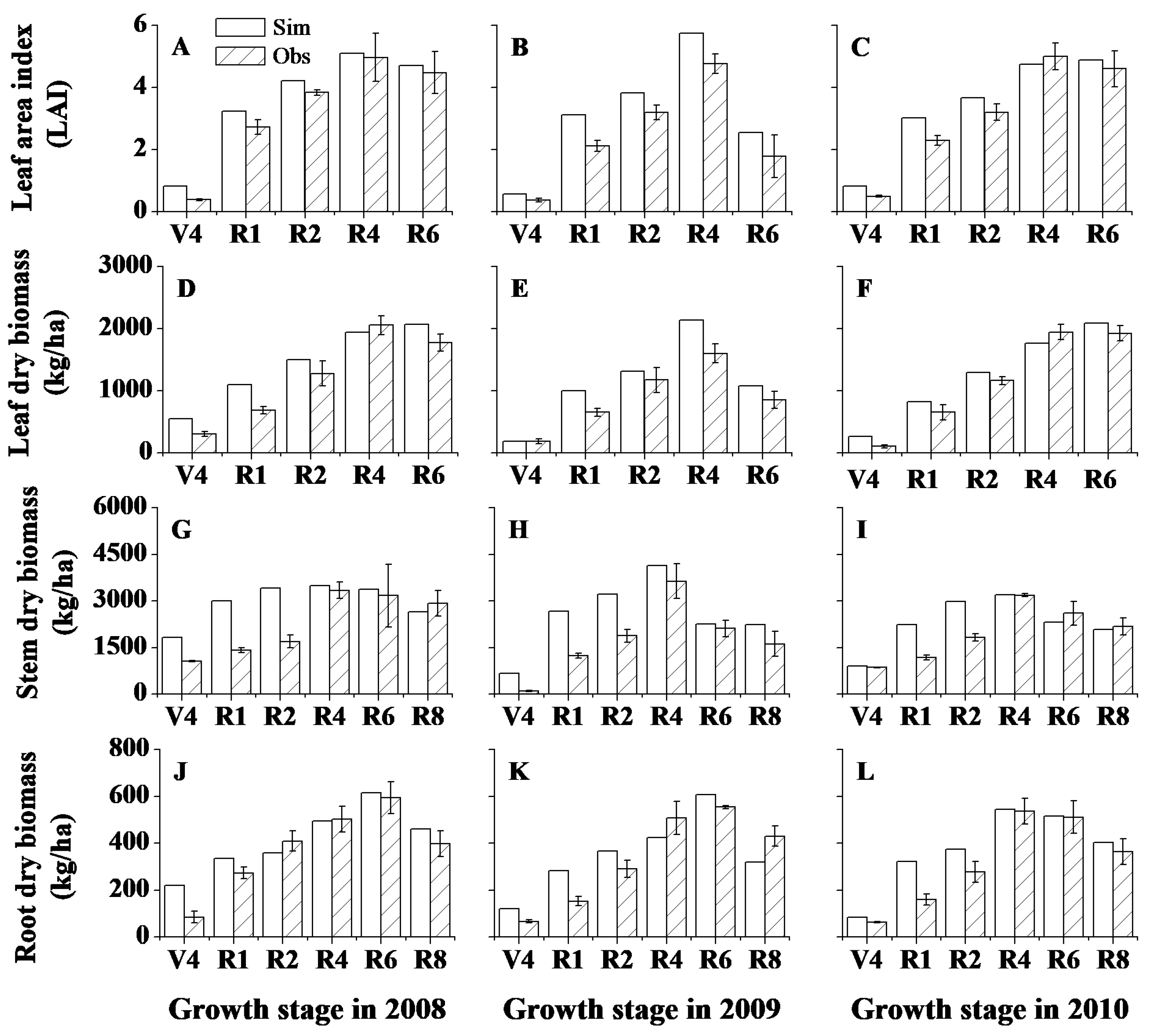
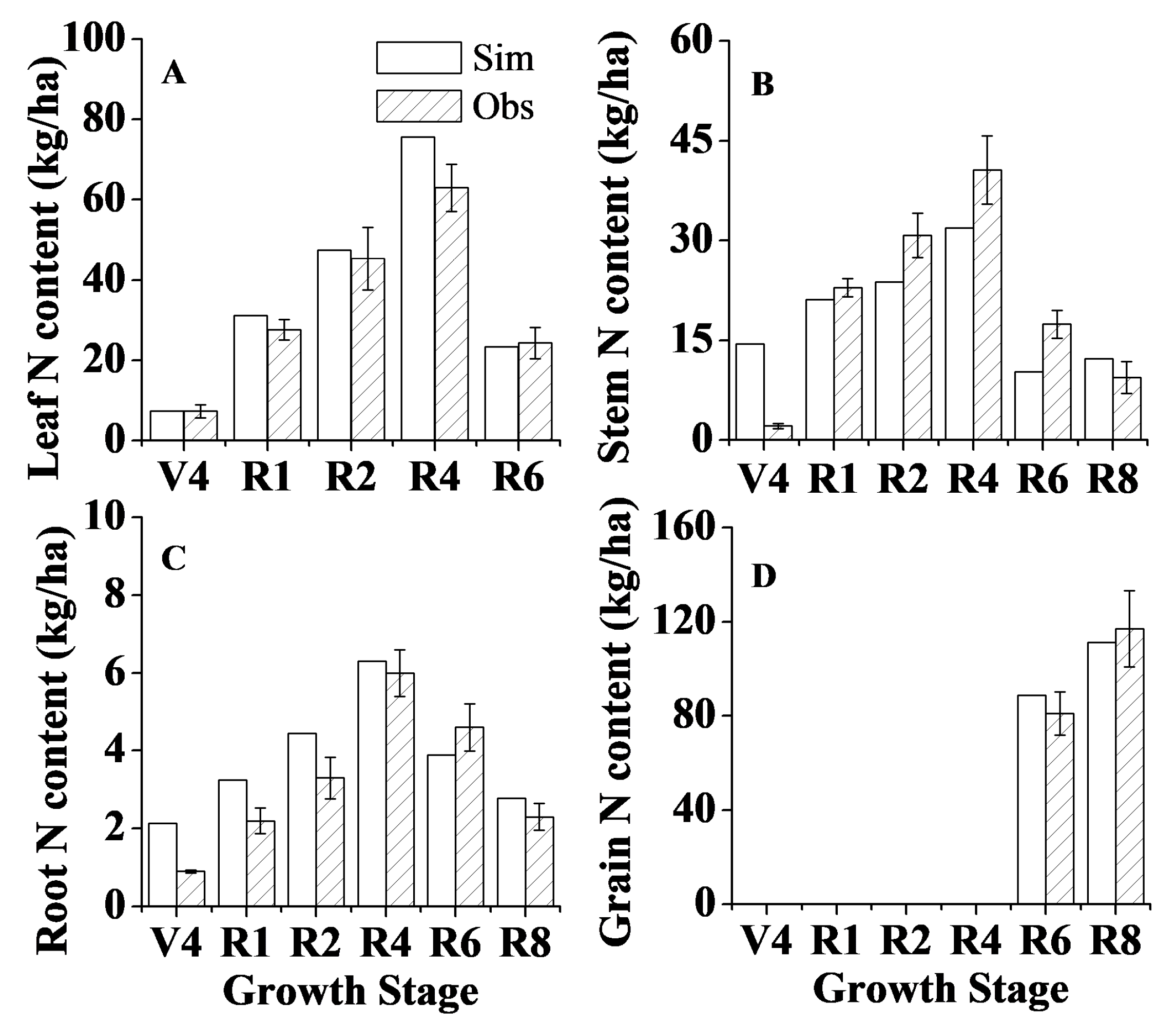
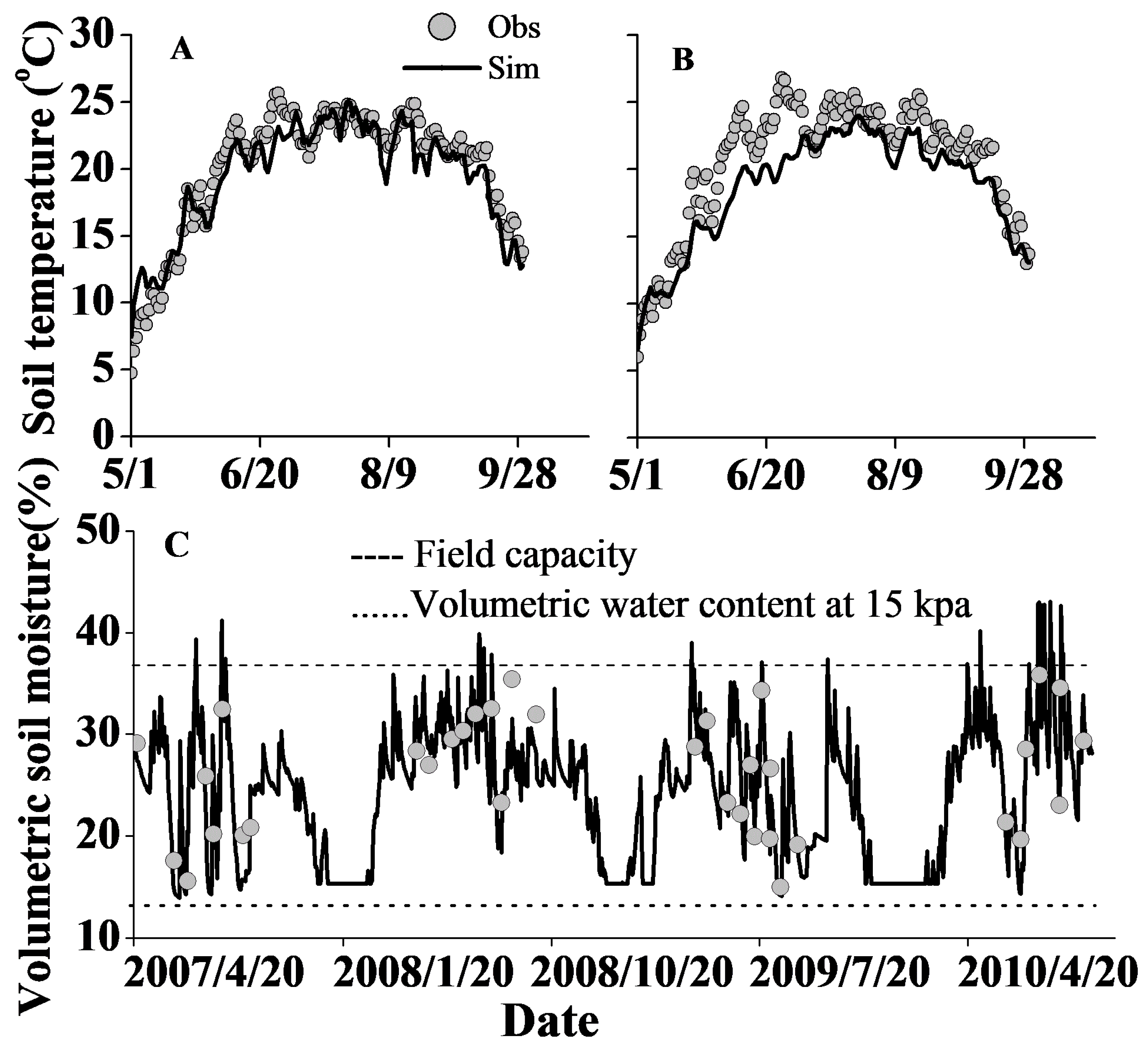
2.4. Model Calibration and Validation
2.5. Simulation Scenarios Design
2.6. Nitrogen Budget Calculation
3. Results
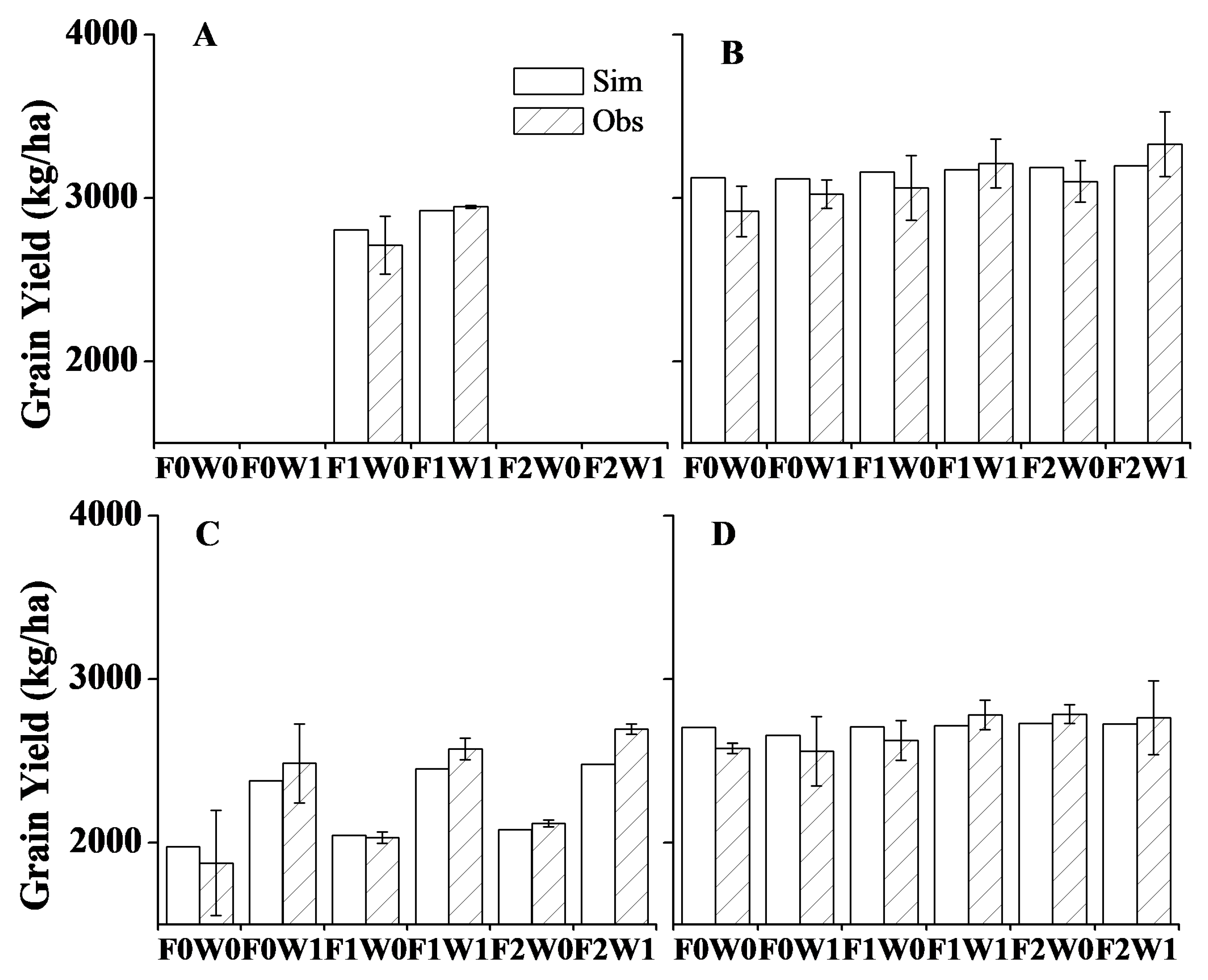
3.1. Model Calibration and Validation for Plant Growth, Soil Water Content and Soil Temperature
3.2. Response of Seed Yield to Simulated Field Management Strategies
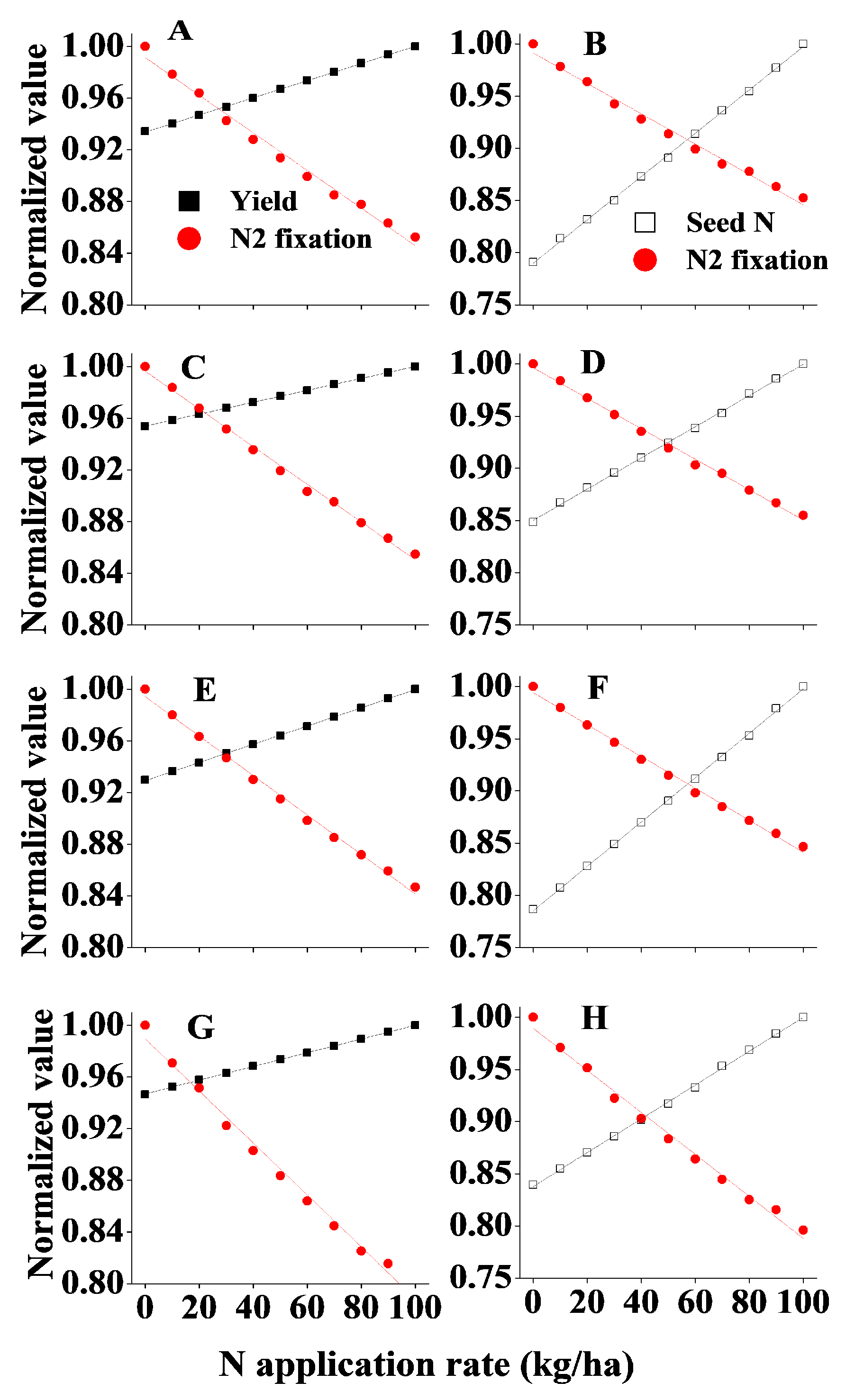
3.3. Response of BNF to Field Management Strategies
3.4. Response of N Content in Seed to Field Management Strategies
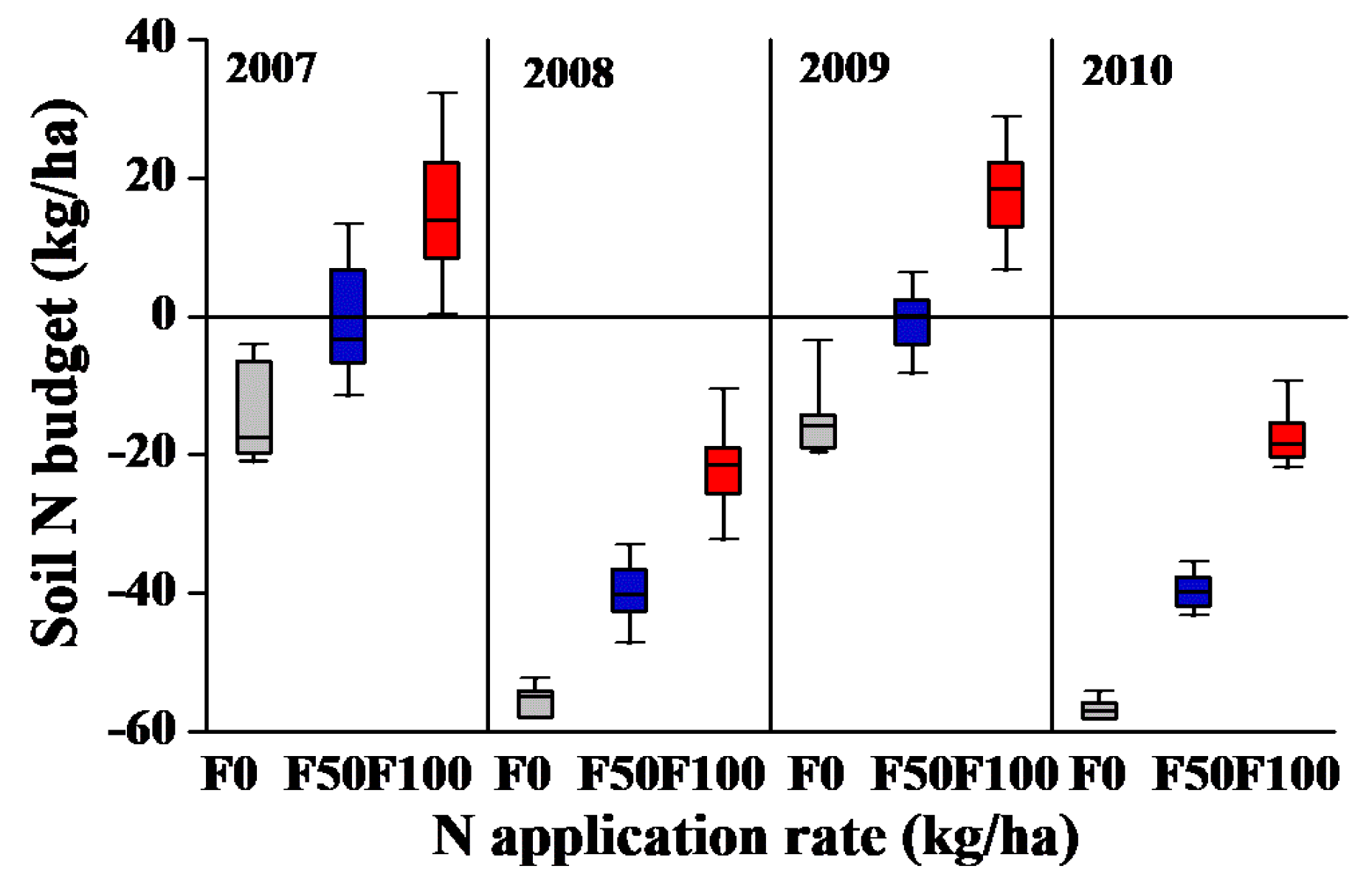
3.5. Soil Mineral N Budget under Various Field Management Strategies
3.6. Recommendations for Fertiliser N Application Rate in Northeast China
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.B.; Herbert, S.J. Fifteen years of research examining cultivation of continuous soybean in northeast China: A review. Field Crops Res. 2002, 79, 1–7. [Google Scholar] [CrossRef]
- Sheng, Y. National Bureau of Statistics of China 2016; China Statistics Press: Beijing, China, 2016.
- Liu, X.; Jin, J.; Wang, G.; Herbert, S.J. Soybean yield physiology and development of high-yielding practices in Northeast China. Field Crops Res. 2008, 105, 157–171. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, S.J.; Jin, J.; Zhang, Q.; Wang, G. Responses of photosynthetic rates and yield/quality of main crops to irrigation and manure application in the black soil area of Northeast China. Plant Soil 2004, 261, 55–60. [Google Scholar] [CrossRef]
- Córdova, S.C.; Castellano, M.J.; Dietzel, R.; Licht, M.A.; Togliatti, K.; Martinez-Feria, R.; Archontoulis, S.V. Soybean nitrogen fixation dynamics in Iowa, USA. Field Crops Res. 2019, 236, 165–176. [Google Scholar] [CrossRef]
- Gan, Y.; Peoples, M.B.; Rerkasem, B. The effect of N fertilizer strategy on N2 fixation, growth and yield of vegetable soybean. Field Crops Res. 1997, 51, 221–229. [Google Scholar] [CrossRef]
- Zapata, F.; Danso, S.K.A.; Hardarson, G.; Fried, M. Time course of nitrogen fixation in field-grown soybean using nitrogen-15 methodology. Agron. J. 1987, 79, 172–176. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Crispino, C.C.; Moraes, J.Z.; Rnr, S.; Mendes, I.C.; Arihara, J. Nitrogen nutrition of soybean in Brazil: Contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 2006, 86, 927–939. [Google Scholar] [CrossRef]
- Mastrodomenico, A.T.; Purcell, L.C.; Andy King, C. The response and recovery of nitrogen fixation activity in soybean to water deficit at different reproductive developmental stages. Environ. Exp. Bot. 2013, 85, 16–21. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Lamb, J.A.; Randall, G.W.; Orf, J.H.; Rehm, G.W. In-season fertilizer nitrogen applications for soybean in minnesota. Agron. J. 2001, 93, 983–988. [Google Scholar] [CrossRef]
- Miransari, M.; Riahi, H.; Eftekhar, F.; Minaie, A.; Smith, D.L. Improving soybean (Glycine max L.) N2 fixation under stress. J. Plant Growth Regul. 2013, 32, 909–921. [Google Scholar] [CrossRef]
- van Kessel, C.; Hartley, C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation? Field Crops Res. 2000, 65, 165–181. [Google Scholar] [CrossRef]
- Barker, D.W.; Sawyer, J.E. Nitrogen application to soybean at early reproductive development. Agron. J. 2005, 97, 615–619. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Salvagiotti, F. New insights into soybean biological nitrogen fixation. Agron. J. 2018, 110, 1185–1196. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Kaschuk, G.; Nogueira, M.A.; De Luca, M.J.; Hungria, M. Response of determinate and indeterminate soybean cultivars to basal and topdressing N fertilization compared to sole inoculation with Bradyrhizobium. Field Crops Res. 2016, 195, 21–27. [Google Scholar] [CrossRef]
- Eaglesham, A.R.J.; Hassouna, S.; Seegers, R. Fertilizer-n effects on N2 fixation by cowpea and soybean. Agron. J. 1983, 75, 61–66. [Google Scholar] [CrossRef]
- Gai, Z.; Zhang, J.; Li, C. Effects of starter nitrogen fertilizer on soybean root activity, leaf photosynthesis and grain yield. PLoS ONE 2017, 12, e0174841. [Google Scholar] [CrossRef]
- Martinez-Feria, R.A.; Castellano, M.J.; Dietzel, R.N.; Helmers, M.J.; Liebman, M.; Huber, I.; Archontoulis, S.V. Linking crop- and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 2018, 256, 131–143. [Google Scholar] [CrossRef]
- Carranca, C.; Torres, M.O.; Madeira, M. Underestimated role of legume roots for soil N fertility. Agron. Sustain. Dev. 2015, 35, 1095–1102. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Yang, J.Y.; Zhang, X.Y.; Drury, C.F.; Reynolds, W.D.; Hoogenboom, G. Modelling crop yield, soil water content and soil temperature for a soybean–maize rotation under conventional and conservation tillage systems in Northeast China. Agric. Water Manag. 2013, 123, 32–44. [Google Scholar] [CrossRef]
- Wu, L.; McGechan, M.B.; McRoberts, N.; Baddeley, J.A.; Watson, C.A. SPACSYS: Integration of a 3D root architecture component to carbon, nitrogen and water cycling—Model description. Ecol. Model. 2007, 200, 343–359. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Pierozan Junior, C.; Kawakami, J. Efficiency of the leaf disc method for estimating the leaf area index of soybean plants. Acta Sci. Agron. 2013, 35, 487–493. [Google Scholar] [CrossRef]
- Böhm, W. Auger Methods. In Methods of Studying Root Systems. Ecological Studies (Analysis and Synthesis); Springer: Berlin, Germany, 1979; Volume 33, pp. 39–47. [Google Scholar]
- China Meteorological Data Network. Available online: http://data.cma.cn/ (accessed on 22 July 2020).
- Ångström, A. Solar and terrestrial radiation. Q. J. R. Meteorol. Soc. 1924, 50, 121–126. [Google Scholar]
- Wu, L.; McGechan, M.B. Simulation of nitrogen uptake, fixation and leaching in a grass/white clover mixture. Grass Forage Sci. 1999, 54, 30–41. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Watson, C.A.; Baddeley, J.A.; Pan, X.; Zhang, L. Modeling biological dinitrogen fixation of field pea with a process-based simulation model. Agron. J. 2013, 105, 670. [Google Scholar] [CrossRef]
- Wu, L.; Rees, R.M.; Tarsitano, D.; Zhang, X.; Jones, S.K.; Whitmore, A.P. Simulation of nitrous oxide emissions at field scale using the SPACSYS model. Sci. Total Environ. 2015, 530–531, 76–86. [Google Scholar] [CrossRef]
- Wu, L.; Blackwell, M.; Dunham, S.; Hernández-Allica, J.; McGrath, S.P. Simulation of phosphorus chemistry, uptake and utilisation by winter wheat. Plants 2019, 8, 404. [Google Scholar] [CrossRef]
- Macduff, J.H.; Jarvis, S.C.; Davidson, I.A. Inhibition of N2 fixation by white clover (Trifolium repens L.) at low concentrations of NO3- in flowing solution culture. Plant Soil 1996, 180, 287–295. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Specht, J.E.; Cassman, K.G.; Walters, D.T.; Weiss, A.; Dobermann, A. Growth and nitrogen fixation in high-yielding soybean: Impact of nitrogen fertilization. Agron. J. 2009, 101, 958–970. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; Mcgill, W.B.; Arah, J.R.M.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Li, Q.S.; Willardson, L.S.; Deng, W.; Li, X.J.; Liu, C.J. Crop water deficit estimation and irrigation scheduling in western Jilin province, Northeast China. Agric. Water Manag. 2005, 71, 47–60. [Google Scholar] [CrossRef]
- Timsina, J.; Boote, K.J.; Duffield, S. Evaluating the CROPGRO soybean model for predicting impacts of insect defoliation and depodding. Agron. J. 2007, 99, 148–157. [Google Scholar] [CrossRef]
- Battisti, R.; Sentelhas, P.C.; Boote, K.J. Inter-comparison of performance of soybean crop simulation models and their ensemble in southern Brazil. Field Crops Res. 2017, 200, 28–37. [Google Scholar] [CrossRef]
- Wei, Z.; Paredes, P.; Liu, Y.; Chi, W.W.; Pereira, L.S. Modelling transpiration, soil evaporation and yield prediction of soybean in North China Plain. Agric. Water Manag. 2015, 147, 43–53. [Google Scholar] [CrossRef]
- Gelfand, I.; Robertson, G.P. A reassessment of the contribution of soybean biological nitrogen fixation to reactive N in the environment. Biogeochemistry 2015, 123, 175–184. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; dos Santos, M.B.; Terassi, F.; Hungria, M.; Nogueira, M.A. Strategies to promote early nodulation in soybean under drought. Field Crops Res. 2016, 196, 160–167. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Kaur, G.; Orlowski, J.M.; Shapiro, C.A.; Lee, C.D.; Wortmann, C.; Holshouser, D.; Nafziger, E.D.; Kandel, H.; Niekamp, J.; et al. Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crops Res. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- 43 Osborne, S.L.; Riedell, W.E. Starter nitrogen fertilizer impact on soybean yield and quality in the Northern Great Plains. Agron. J. 2006, 98, 1569–1574. [Google Scholar] [CrossRef]
| Depth | Bulk Density | Volumetric Water Content at 15 kPa | Field Capacity | Soil Organic Matter | Total N | NH4-N | NO3-N | pH |
|---|---|---|---|---|---|---|---|---|
| (cm) | (g/cm3) | (%) | (%) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (―) |
| 0–10 | 1.30 | 14.3 | 35.9 | 25.1 | 1.59 | 6.65 | 16.77 | 6.34 |
| 10–20 | 1.32 | 14.2 | 34.9 | 24.8 | 1.55 | 6.82 | 10.48 | 6.27 |
| 20–40 | 1.34 | 14.0 | 33.8 | 23.6 | 1.02 | 4.46 | 9.28 | 6.56 |
| 40–60 | 1.52 | 19.0 | 43.8 | 6.7 | 0.33 | ― | ― | 6.52 |
| 60–80 | 1.63 | 18.9 | 37.5 | 5.8 | 0.26 | ― | ― | 6.56 |
| Parameter | Unit | Value |
|---|---|---|
| Crop growth and development | ||
| Accumulated temperature required from sowing to emergence | °C·d | 160 |
| Accumulated temperature required from sowing to pod setting | °C·d | 1550 |
| Accumulated temperature required from pod setting to maturity | °C·d | 1120 |
| Critical photoperiod for vegetative stage over which plant development will stop | h | 14.0 |
| Threshold temperature for vegetative stage | °C | 9.0 |
| Threshold temperature for reproductive stage | °C | 10.0 |
| Optimum temperature for photosynthesis | °C | 20.0 |
| Extinct coefficient | ― | 1.0 |
| Leaf transmission coefficient | ― | 0.15 |
| Minimum leaf nitrogen concentration below which photosynthesis ceases | gN·g−1·DM | 0.01 |
| Optimum leaf nitrogen concentration above which photosynthesis is in unify | gN·g−1·DM | 0.06 |
| Q10 value for plant maintenance respiration | ― | 3.0 |
| Specific leaf area | m2·g−1·DM | 0.0325 |
| Root death rate as litter | d−1 | 0.04 |
| Rate of stem-carbon lost as litter | d−1 | 0.02 |
| N2 fixation | ||
| Fraction of nodule in root biomass | ― | 0.20 |
| The lower threshold of optimum temperature for N fixation | °C | 13.0 |
| The upper threshold of optimum temperature for N fixation | °C | 26.0 |
| The minimum temperature below which N fixation ceases | °C | 9.0 |
| The maximum temperature over which N fixation stops | °C | 30.0 |
| Calibration | Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | R2 | nRMSE% | EF | D | No | R2 | nRMSE% | EF | D | |
| Soil Temperature (°C) | 306 | 0.88 * | 10.1 | 0.81 | 0.97 | |||||
| Soil water content (%) | 110 | 0.72 * | 14.4 | 0.57 | 0.90 | 171 | 0.65 * | 15.5 | 0.48 | 0.90 |
| Leaf area index (LAI) | 15 | 0.96 * | 18.8 | 0.88 | 0.97 | 83 | 0.86 * | 22.1 | 0.83 | 0.90 |
| Leaf dry matter (kg/ha) | 15 | 0.92 * | 23.4 | 0.84 | 0.96 | 83 | 0.90 * | 17.6 | 0.90 | 0.97 |
| Stem dry matter (kg/ha) | 18 | 0.52 * | 44.9 | 0.22 | 0.72 | 100 | 0.52 * | 38.8 | 0.25 | 0.77 |
| Root dry matter (kg/ha) | 18 | 0.84 * | 23.3 | 0.79 | 0.94 | 100 | 0.83 * | 20.1 | 0.82 | 0.94 |
| Seed Yield (kg/ha) | 6 | 0.99 * | 6.1 | 0.96 | 0.99 | 38 | 0.86 * | 12.1 | 0.85 | 0.95 |
| Leaf N (kg/ha) | 5 | 0.99 * | 17.8 | 0.90 | 0.98 | 40 | 0.92 * | 15.7 | 0.91 | 0.98 |
| Stem N (kg/ha) | 6 | 0.75 * | 36.6 | 0.66 | 0.85 | 48 | 0.61 * | 29.0 | 0.61 | 0.88 |
| Root N (kg/ha) | 6 | 0.85 * | 27.7 | 0.72 | 0.91 | 48 | 0.71 * | 29.5 | 0.61 | 0.89 |
| Grain N (kg/ha) | 16 | 0.94 * | 7.7 | 0.92 | 0.97 | |||||
| Time (Stage) | BS 1 | BR1 2 | BR3 3 | R1 4 | R3 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate (kg/ha) | 0 | 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 | |
| Yield (kg/ha) | ||||||||||||
| 2007 | W0 6 | 2716 | 2839 | 2953 | 2823 | 2934 | 2825 | 2937 | 2793 | 2880 | 2801 | 2899 |
| Wr 7 | 2833 | 2957 | 3074 | 2935 | 3050 | 2941 | 3055 | 2902 | 2988 | 2914 | 3011 | |
| W1 8 | 3084 | 3192 | 3301 | 3182 | 3286 | 3185 | 3292 | 3162 | 3247 | 3174 | 3277 | |
| W2 9 | 3129 | 3237 | 3346 | 3227 | 3331 | 3230 | 3335 | 3207 | 3291 | 3218 | 3321 | |
| 2008 | W0 | 3125 | 3126 | 3202 | 3121 | 3193 | 3122 | 3196 | 3108 | 3172 | 3114 | 3187 |
| Wr | 3052 | 3127 | 3200 | 3122 | 3191 | 3124 | 3195 | 3109 | 3168 | 3118 | 3189 | |
| W1 | 3059 | 3133 | 3207 | 3127 | 3195 | 3130 | 3201 | 3112 | 3171 | 3123 | 3195 | |
| W2 | 3083 | 3154 | 3210 | 3147 | 3214 | 3149 | 3219 | 3108 | 3188 | 3141 | 3209 | |
| 2009 | W0 | 1977 | 2068 | 2161 | 2065 | 2155 | 2061 | 2146 | 2052 | 2139 | 2043 | 2118 |
| Wr | 2551 | 2611 | 2746 | 2640 | 2730 | 2633 | 2716 | 2629 | 2725 | 2619 | 2702 | |
| W1 | 2432 | 2522 | 2616 | 2520 | 2608 | 2514 | 2596 | 2504 | 2587 | 2493 | 2573 | |
| W2 | 2635 | 2727 | 2823 | 2725 | 2816 | 2719 | 2808 | 2711 | 2799 | 2703 | 2793 | |
| 2010 | W0 | 2635 | 2710 | 2786 | 2699 | 2770 | 2693 | 2758 | 2675 | 2729 | 2658 | 2693 |
| Wr | 2694 | 2771 | 2849 | 2758 | 2828 | 2754 | 2819 | 2727 | 2778 | 2717 | 2752 | |
| W1 | 2684 | 2744 | 2818 | 2731 | 2799 | 2725 | 2789 | 2704 | 2754 | 2688 | 2724 | |
| W2 | 2680 | 2759 | 2831 | 2744 | 2812 | 2738 | 2801 | 2717 | 2734 | 2701 | 2734 | |
| N2 fixation (kg N/ha) | ||||||||||||
| 2007 | W0 | 130 | 119 | 109 | 116 | 105 | 116 | 103 | 110 | 98 | 109 | 97 |
| Wr | 144 | 135 | 128 | 129 | 120 | 130 | 120 | 121 | 107 | 122 | 107 | |
| W1 | 139 | 127 | 119 | 125 | 114 | 124 | 112 | 120 | 106 | 119 | 104 | |
| W2 | 142 | 130 | 121 | 127 | 116 | 127 | 114 | 122 | 108 | 121 | 106 | |
| 2008 | W0 | 124 | 111 | 103 | 109 | 99 | 108 | 96 | 105 | 92 | 101 | 88 |
| Wr | 125 | 115 | 106 | 112 | 102 | 111 | 99 | 108 | 96 | 104 | 89 | |
| W1 | 125 | 114 | 106 | 112 | 102 | 111 | 99 | 108 | 96 | 104 | 89 | |
| W2 | 124 | 113 | 104 | 110 | 101 | 109 | 97 | 105 | 95 | 102 | 89 | |
| 2009 | W0 | 96 | 87 | 79 | 85 | 76 | 83 | 72 | 80 | 70 | 76 | 68 |
| Wr | 121 | 110 | 102 | 109 | 99 | 105 | 92 | 101 | 90 | 96 | 81 | |
| W1 | 120 | 110 | 102 | 108 | 99 | 105 | 93 | 101 | 88 | 95 | 81 | |
| W2 | 134 | 124 | 116 | 123 | 114 | 120 | 109 | 115 | 104 | 110 | 97 | |
| 2010 | W0 | 99 | 87 | 79 | 84 | 75 | 83 | 74 | 80 | 70 | 79 | 25 |
| Wr | 105 | 94 | 86 | 89 | 80 | 88 | 79 | 84 | 73 | 83 | 73 | |
| W1 | 103 | 91 | 82 | 87 | 78 | 86 | 76 | 83 | 72 | 81 | 71 | |
| W2 | 105 | 93 | 84 | 89 | 80 | 88 | 78 | 82 | 72 | 82 | 72 | |
| Seed N content (kg N/ha) | ||||||||||||
| 2007 | W0 | 155 | 178 | 201 | 176 | 198 | 177 | 199 | 173 | 192 | 175 | 197 |
| Wr | 167 | 190 | 214 | 187 | 210 | 188 | 211 | 182 | 202 | 185 | 207 | |
| W1 | 174 | 197 | 220 | 195 | 217 | 196 | 218 | 192 | 212 | 195 | 219 | |
| W2 | 175 | 197 | 221 | 196 | 218 | 197 | 219 | 193 | 213 | 196 | 220 | |
| 2008 | W0 | 183 | 196 | 212 | 195 | 210 | 195 | 211 | 193 | 208 | 194 | 211 |
| Wr | 179 | 194 | 210 | 193 | 208 | 194 | 209 | 191 | 205 | 193 | 210 | |
| W1 | 179 | 195 | 211 | 194 | 209 | 195 | 210 | 192 | 205 | 194 | 211 | |
| W2 | 181 | 196 | 208 | 195 | 209 | 195 | 210 | 193 | 205 | 194 | 210 | |
| 2009 | W0 | 115 | 130 | 150 | 129 | 149 | 129 | 147 | 128 | 147 | 126 | 143 |
| Wr | 156 | 172 | 196 | 176 | 196 | 174 | 193 | 172 | 193 | 169 | 188 | |
| W1 | 151 | 171 | 192 | 170 | 190 | 169 | 187 | 167 | 186 | 165 | 183 | |
| W2 | 164 | 188 | 209 | 187 | 208 | 186 | 206 | 184 | 204 | 183 | 203 | |
| 2010 | W0 | 160 | 176 | 192 | 174 | 189 | 172 | 186 | 169 | 182 | 166 | 174 |
| Wr | 167 | 183 | 200 | 180 | 196 | 179 | 194 | 175 | 186 | 172 | 180 | |
| W1 | 163 | 177 | 193 | 175 | 190 | 174 | 187 | 173 | 182 | 167 | 175 | |
| W2 | 162 | 178 | 193 | 175 | 190 | 174 | 187 | 170 | 181 | 167 | 174 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Misselbrook, T.H.; Feng, L.; Wu, L. Assessment of Nitrogen Uptake and Biological Nitrogen Fixation Responses of Soybean to Nitrogen Fertiliser with SPACSYS. Sustainability 2020, 12, 5921. https://doi.org/10.3390/su12155921
Wu L, Misselbrook TH, Feng L, Wu L. Assessment of Nitrogen Uptake and Biological Nitrogen Fixation Responses of Soybean to Nitrogen Fertiliser with SPACSYS. Sustainability. 2020; 12(15):5921. https://doi.org/10.3390/su12155921
Chicago/Turabian StyleWu, Lu, Thomas H. Misselbrook, Liping Feng, and Lianhai Wu. 2020. "Assessment of Nitrogen Uptake and Biological Nitrogen Fixation Responses of Soybean to Nitrogen Fertiliser with SPACSYS" Sustainability 12, no. 15: 5921. https://doi.org/10.3390/su12155921
APA StyleWu, L., Misselbrook, T. H., Feng, L., & Wu, L. (2020). Assessment of Nitrogen Uptake and Biological Nitrogen Fixation Responses of Soybean to Nitrogen Fertiliser with SPACSYS. Sustainability, 12(15), 5921. https://doi.org/10.3390/su12155921






