Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fruit Material and Preparation of Extracts
2.2.1. Fruit Material
2.2.2. Preparation of Saco Cherry Extracts
2.3. Total Phenolic Content
2.4. Total Anthocyanins
2.5. Phenolic Compounds Identification by LC-ESI-QqTOF-HRMS
2.6. Phenolic Compounds Quantification by HPLC
2.7. Antioxidant Activity
2.7.1. The ABTS Method
2.7.2. The DPPH Assay
2.7.3. The Oxygen Radical Absorbance Capacity Assay (ORAC)
2.8. Statistical Analysis
3. Results and Discussion
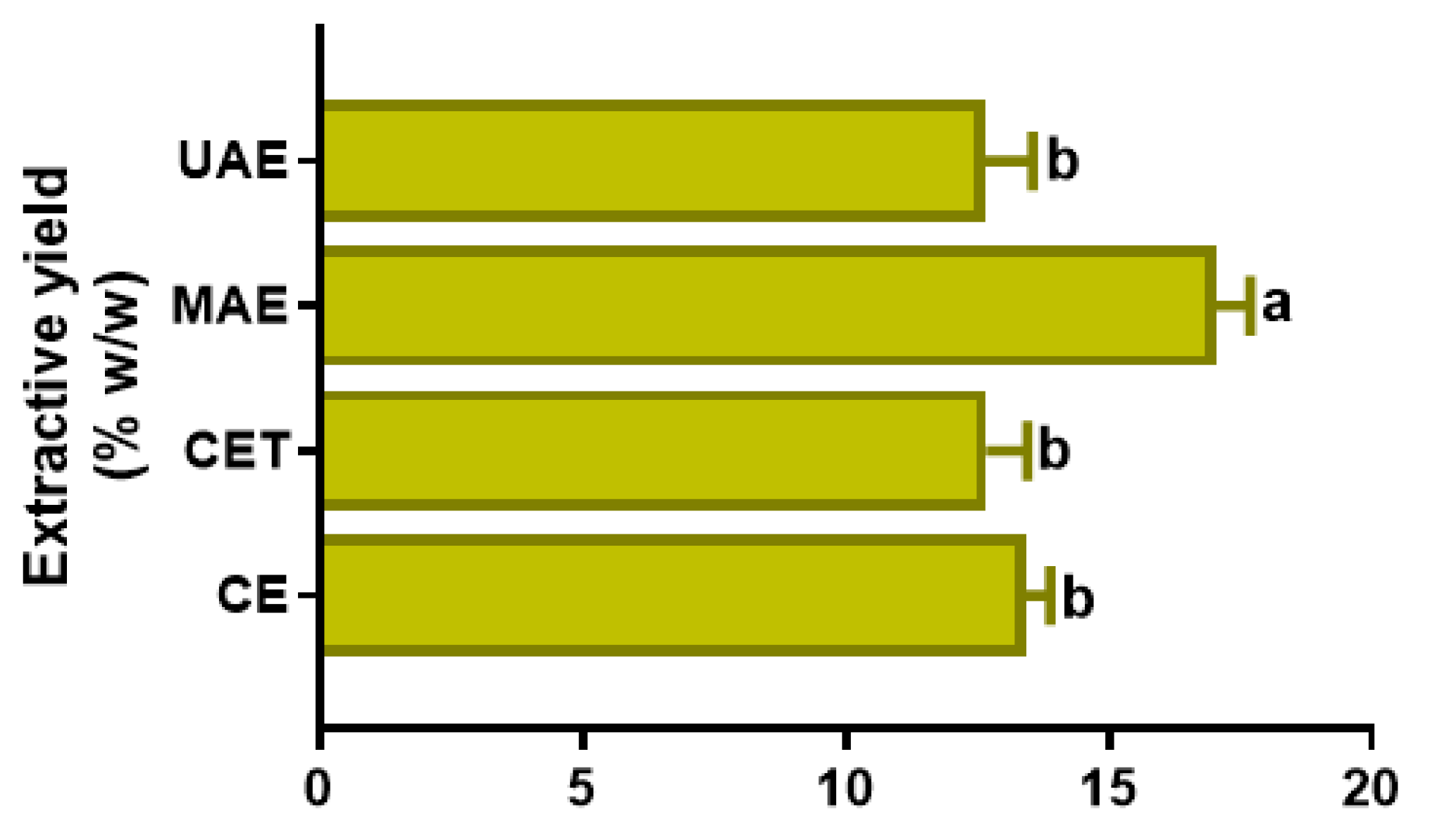
3.1. Extractive Yield
3.2. Total Phenolic Content and Total Anthocyanins
3.3. Phenolic Compounds Profile of Antioxidant Extracts
3.3.1. Identification of Major Phenolic Compounds by LC-ESI-QqTOF-HRMS
3.3.2. Quantification of Phenolic Compounds by HPLC-DAD
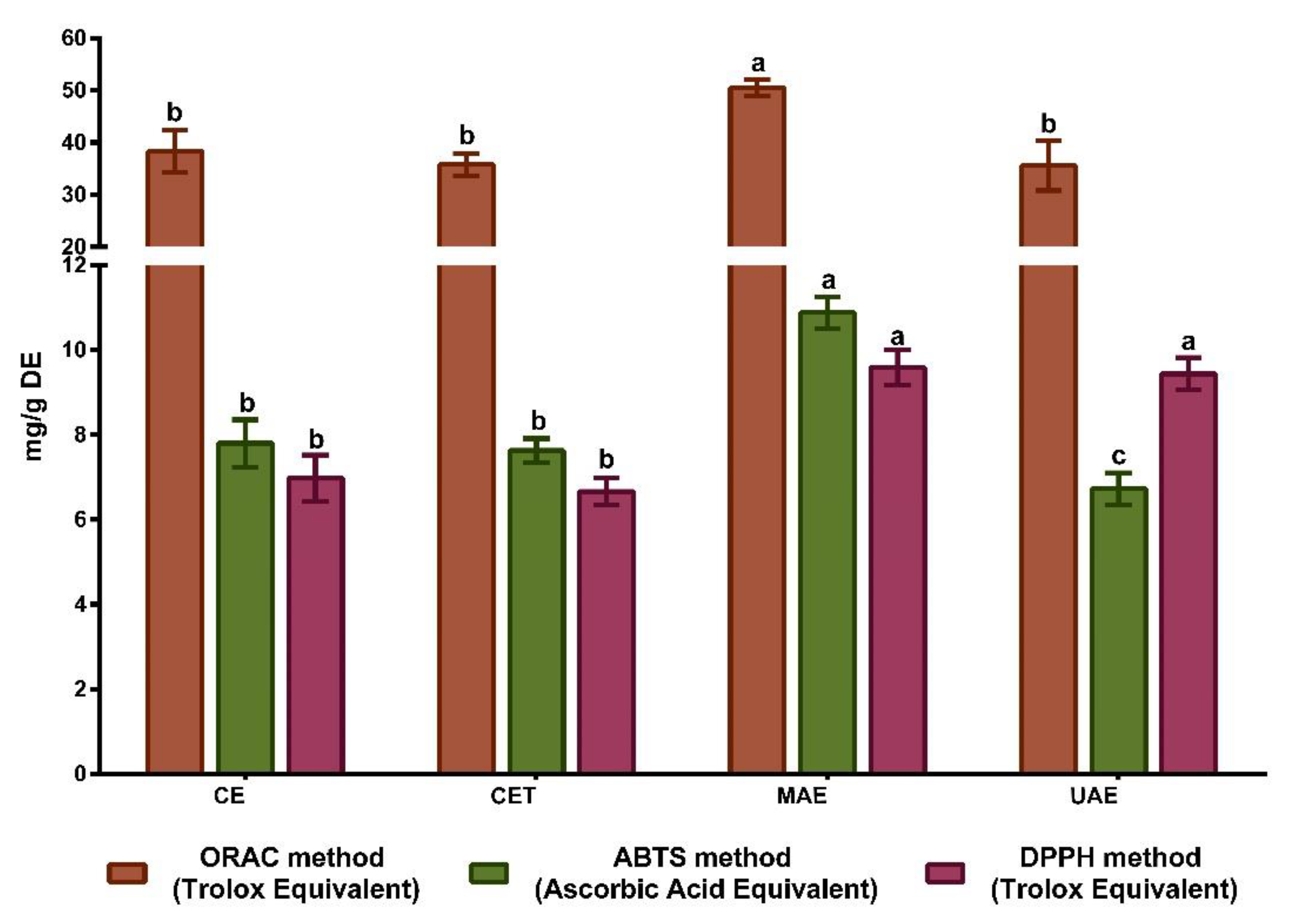
3.4. Antioxidant Activity
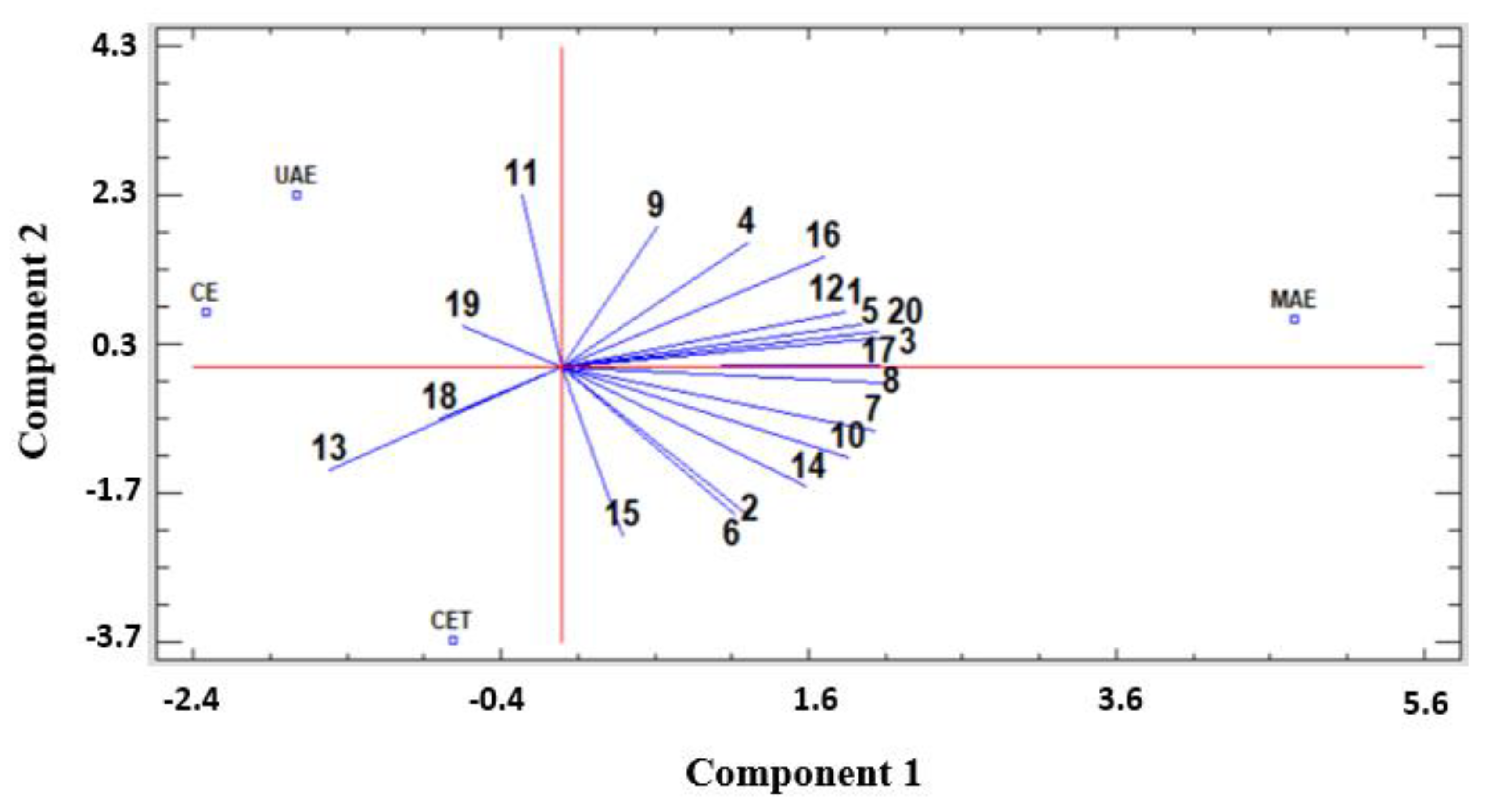
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Mingarro, D.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Hoque, M.Z.; Legay, S.; Cai, G.; Siddiqui, K.S.; Hausman, J.-F.; Andre, C.M.; Guerriero, G. Tuscan varieties of sweet cherry are rich sources of ursolic and oleanolic acid: Protein modeling coupled to targeted gene expression and metabolite analyses. Molecules 2019, 24, 1590. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, L.R. Nutrients, bioactive compounds and bioactivity: The health benefits of sweet cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2019, 15, 208–227. [Google Scholar]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mirto, A.; Iannuzzi, F.; Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Fuggi, A. Metabolic characterization and antioxidant activity in sweet cherry (Prunus avium L.) Campania accessions: Metabolic characterization of sweet cherry accessions. Food Chem. 2018, 240, 559–566. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.-F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef]
- Matias, A.; Rosado-Ramos, R.; Nunes, S.; Figueira, I.; Serra, A.; Bronze, M.; Santos, C.; Duarte, C. Protective effect of a (poly) phenol-rich extract derived from sweet cherries culls against oxidative cell damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. C. R. Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Okur, İ.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis 2018, 39, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Hatti-Kaul, R.; Törnvall, U.; Gustafsson, L.; Börjesson, P. Industrial biotechnology for the production of bio-based chemicals–a cradle-to-grave perspective. Trends Biotechnol. 2007, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current extraction techniques towards bioactive compounds from brewer’s spent grain—A review. Crit. Rev. Food Sci. Nutr. 2019, 1–12. [Google Scholar] [CrossRef]
- Gonçalves, A.; Rodrigues, M.; Santos, A.; Alves, G.; Silva, L. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.; Bronze, M.; Alves, P.M.; de Sousa, H.C.; Duarte, C.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Alexandre, E.M.; Silva, S.; Santos, S.A.; Silvestre, A.J.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Monforte, A.R.; Martins, S.I.; Silva Ferreira, A.C. Strecker aldehyde formation in wine: New insights into the role of gallic acid, glucose, and metals in phenylacetaldehyde formation. J. Agric. Food Chem. 2017, 66, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the temperature and oxygen exposure in red Port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC− fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.-F.; Guerriero, G.; Cai, G. Functional molecules in locally-adapted crops: The case study of tomatoes, onions, and sweet cherry fruits from Tuscany in Italy. Front. Plant Sci. 2019, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Karabegović, I.T.; Stojičević, S.S.; Veličković, D.T.; Todorović, Z.B.; Nikolić, N.Č.; Lazić, M.L. The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind. Crop. Prod. 2014, 54, 142–148. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Dragović-Uzelac, V.; Jambrak, A.R.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar]
- Cacace, J.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Alexandre, E.M.; Moreira, S.A.; Castro, L.M.; Pintado, M.; Saraiva, J.A. Emerging technologies to extract high added value compounds from fruit residues: Sub/supercritical, ultrasound-, and enzyme-assisted extractions. Food Rev. Int. 2018, 34, 581–612. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.M. Thermosonication process for optimal functional properties in carrot juice containing orange peel and pulp extracts. Food Chem. 2018, 245, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. Int. J. Plant. Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Ferrentino, G.; Asaduzzaman, M.; Scampicchio, M.M. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Crit. Rev. Food Sci. Nutr. 2018, 58, 386–404. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78. [Google Scholar]
- Mateus, N.; de Freitas, V. Anthocyanins as food colorants. In Anthocyanins; Springer: Berlin, Germany, 2008; pp. 284–304. [Google Scholar]
- Oliveira, A.; Pintado, M. Stability of polyphenols and carotenoids in strawberry and peach yoghurt throughout In Vitro gastrointestinal digestion. Food Funct. 2015, 6, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Lopes, G.R.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Nunes, F.M.; Coimbra, M.A. Carbohydrate content, dietary fibre and melanoidins: Composition of espresso from single-dose coffee capsules. Food Res. Int. 2016, 89, 989–996. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388. [Google Scholar]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Bleve, G.; Tufariello, M.; Corbo, F.; Clodoveo, M.L.; Tarricone, L. Comprehensive identification and quantification of chlorogenic acids in sweet cherry by tandem mass spectrometry techniques. J. Food Compos. Anal. 2018, 73, 103–111. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jauregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; dos Santos, S.M.D.; Pintado, M.E.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chemical composition and in vitro antimicrobial, antifungal and antioxidant properties of essential oils obtained from some herbs widely used in Portugal. Food Control 2013, 32, 371–378. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of Extraction Conditions for the Antioxidant Potential of Different Pumpkin Varieties (Cucurbita maxima). Sustainability 2020, 12, 1305. [Google Scholar] [CrossRef]
- Ribeiro, T.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated digestion of olive pomace water-soluble ingredient: Relationship between the compounds bioaccessibility and their potential health benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef]
- Milea, A.Ș.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products. Foods 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Seabra, I.J.; Braga, M.E.; Bronze, M.; de Sousa, H.C.; Duarte, C.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds. J. Supercrit. Fluids 2010, 55, 184–191. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
| Phenolic Compounds | Validation Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Slope | Intercept | R2 | LoD (mg/mL) | LoQ (mg/mL) | Repeatability (% RSD) | % Recovery | |
| Hydroxycinnamic acids | |||||||
| 3-Caffeoylquinic acid | 54,993,453 | 195,577 | 0.998 | 0.02 | 0.06 | 0.382 | 92.684 |
| 5-Caffeoylquinic acid | 57,646,292 | 302,485 | 0.999 | 0.01 | 0.04 | 0.838 | 101.219 |
| 4-Caffeoylquinic acid | 55,692,713 | 116,655 | 0.999 | 0.01 | 0.05 | 0.430 | 98.995 |
| 4,5-dicaffeoylquinic acid | 31,929,586 | 91,582 | 0.999 | 0.01 | 0.03 | 0.566 | 97.548 |
| Caffeic acid | 109,109,858 | 485,096 | 0.998 | 0.01 | 0.06 | 0.139 | 99.510 |
| p-coumaric acid | 110,590,209 | 213,534 | 0.999 | 0.01 | 0.04 | 0.331 | 99.801 |
| Flavan-3-ols | |||||||
| (−)-Epicatechin-3-gallate | 35,497,526 | 38,356 | 0.999 | 0.01 | 0.02 | 0.417 | 98.887 |
| Flavonols | |||||||
| Quercetin-3-rutinoside | 31,584,226 | 132,541 | 0.998 | 0.02 | 0.05 | 0.162 | 98.591 |
| Kaempferol-3-glucoside | 40,663,899 | 30,580 | 0.999 | 0.01 | 0.04 | 1.087 | 95.975 |
| Quercetin | 86,817,569 | 360,940 | 0.998 | 0.02 | 0.06 | 0.210 | 96.823 |
| Anthocyanins | |||||||
| Cyanidin-3-rutinoside | 53,088,951 | 91,623 | 0.999 | 0.01 | 0.02 | 0.855 | 100.749 |
| Cyanidin-3-glucoside | 13,535,732 | −39,433 | 0.999 | 0.01 | 0.03 | 0.346 | 97.235 |
| Peonidin-3-rutinoside | 51,163,043 | 256,007 | 0.998 | 0.02 | 0.06 | 0.411 | 98.820 |
| Pelargonidin-3-rutinoside | 46,960,315 | −54,554 | 0.999 | 0.01 | 0.04 | 0.471 | 100.005 |
| Saco Cherry Extracts | TPC (mg GAE/g DE) | TAC (mg Cy-3-glu/g DE) |
|---|---|---|
| CE | 8.75 ± 0.81 b | 1.93 ± 0.21 b |
| CET | 7.11 ± 0.33 c | 2.78 ± 0.18 a |
| MAE | 12.65 ± 0.81 a | 2.53 ± 0.13 a |
| UAE | 7.16 ± 0.81 c | 1.76 ± 0.06 b |
| Proposed Compound | Molecular Formula | RT (min) | m/z calcd ([M-H]-) | Error (mDa) | Major Fragments Negative MS/MS Ions (m/z) | Saco Cherry Extracts | |||
|---|---|---|---|---|---|---|---|---|---|
| CE | CET | MAE | UAE | ||||||
| Hydroxycinnamic acids | |||||||||
| 3-Caffeoylquinic acid cis | C16H18O9 | 7.7 | 353.1 | 1.0 | 191(100), 179(80), 135(16) | D. | D. | D. | D. |
| 3-Caffeoylquinic acid trans | C16H18O9 | 7.8 | 353.1 | 1.3 | 191(100), 179(54), 135(28) | D. | D. | D. | D. |
| 5-Caffeoylquinic acid trans | C16H18O9 | 8.8 | 353.1 | 1.1 | 191.05(100) | D. | D. | D. | D. |
| 4-Caffeoylquinic acid trans | C16H18O9 | 9 | 353.1 | 1.2 | 173(100), 179(70), 191(40), 135(20) | D. | D. | D. | D. |
| 3-Coumaroylquinic acid | C16H18O8 | 8.6 | 337.1 | 1.3 | 163.(100), 119(18), 191(10) | D. | D. | D. | D. |
| 4-Coumaroylquinic acid cis | C16H18O8 | 9.8 | 337.1 | 0.6 | 173(100), 163(16), 191(15) | D. | D. | D. | D. |
| 4-Coumaroylquinic acid trans | C16H18O8 | 10.2 | 337.1 | 1.1 | 173(86), 163.03(24) | D. | D. | D. | D. |
| Feruloylquinic acid isomer | C16H16O10 | 6,9 | 367.1 | 1.1 | 163(18), 205(16) | D. | D. | D. | D. |
| 3-Feruloylquinic acid cis | C17H20O9 | 8.4 | 367.1 | 0.9 | 193(100), 134(10), 149(3) | D. | D. | D. | D. |
| 5-Feruloylquinic acid cis | C17H20O9 | 10.1 | 367.1 | 0.8 | 173(100) | D. | D. | N.D. | D. |
| Caffeoylquinic acid-glycoside | C22H27O14 | 6.4 | 515.1 | 1.6 | 341(35), 179(27), 335(11), 191(8), 353(6), 323(3) | D. | D. | D. | D. |
| 4,5-diCaffeoylquinic acid | C25H24O12 | 12.9 | 515.1 | 0.7 | 335(91), 191(32), 179(27), | D. | D. | N.D. | D. |
| 4-Caffeoylquinic acid lactone | C16H16O8 | 10.5 | 335.1 | 1.0 | 161(77), 191(15), 135(15) | D. | D. | D. | D. |
| 4-Coumaroylquinic acid lactone | C16H16O7 | 12.2 | 319.0 | 1.1 | 145(100), 119(14), 163(9), 173(4) | D. | D. | D. | D. |
| p-Coumaric acid derivative | C9H8O3 | 6.9 | 163.0 | 0.8 | 119(100) | D. | D. | D. | D. |
| Coumaroyl hexose | C15H18O8 | 8.2 | 325.1 | 0.8 | 145(100), 163(18), 265(4.5), 187(3), 205(3) | D. | D. | D. | D. |
| Caffeic acid-glycoside | C15H17O9 | 8.7 | 341.1 | 0.9 | 179(100), 135(13) | D. | D. | D. | D. |
| Caffeoyl alcohol 3/4-o-hexoside | C15H19O8 | 8.5 | 327.1 | 0.7 | 165(100), 121(2) | D. | D. | D. | D. |
| Feruloyl hexose | C16H19O9 | 9.6 | 355.1 | 1.5 | 175(100), 193(46), 295(8) | D. | D. | D. | D. |
| Sinapoyl hexose | C17H21O10 | 8.1 | 385.1 | 1.1 | 223(100), 208(12) | D. | D. | D. | D. |
| Flavanols | |||||||||
| Epicatechin-3-gallate | C22H18O10 | 12.8 | 441.1 | 395(100), 263(81), 441(6) | D. | D. | D. | D. | |
| Flavonols | |||||||||
| Quercetin-7-O-glucoside-3-O-rutinoside | C33H40O21 | 10.5 | 771.2 | 1.4 | 609(100) | D. | D. | D. | D. |
| Kaempferol-3-glucoside | C21H20O11 | 17.6 | 447.1 | 1.2 | 285(92) | D. | N.D. | D. | N.D. |
| Kaempferol-3-rutinoside | C27H30O16 | 12.5 | 593.1 | 1.7 | 285(29) | D. | D. | D. | D. |
| Other flavonoids | |||||||||
| Taxifolyn-rutinoside | C27H31O16 | 9 | 611.1 | 2.2 | 285(93), 475(76), 501(17), 241(13), 485(10), 303(4) | D. | D. | D. | D. |
| Hydroxybenzoic acids | |||||||||
| Protocatechuic acid-glycoside | C13H15O9 | 6.3 | 315.1 | 0.6 | 109(5), 153(100) | D. | D. | D. | D. |
| Protocatechuic acid | C7H6O4 | 7,2 | 153.0 | 0.1 | 109(100) | D. | D. | D. | D. |
| Hydroxybenzoic acid-glycoside | C13H16O8 | 6,3 | 299.1 | 0.9 | 137(100) | D. | D. | D. | D. |
| Hydroxybenzoyl hexose | C13H16O8 | 7.4 | 299.1 | 1.0 | 137(29), 179(20), 239(14) | D. | D. | D. | D. |
| Vanillic acid-glycoside | C14H17O9 | 7.2 | 329.1 | 0.6 | 167(100) | D. | D. | D. | D. |
| Anthocyanins | |||||||||
| Cyanidin-3-glucoside | C21H21O11 | 12.3 | 449.2 | 0.1 | 287(100) | D. | D. | D. | D. |
| Cyanidin-3-rutinoside | C27H31O15 | 595.1 | 0.6 | 287(100), 449(14) | D. | D. | D. | D. | |
| Peonidin-3-glucoside | C22H23O11 | 12.4 | 463.1 | 0.6 | 301(100) | D. | D. | D. | D. |
| Peonidin-3-rutinoside | C28H33O15 | 11.4 | 609.1 | 1.0 | 301(100), 463 (15) | D. | D. | D. | D. |
| Pelargonidin-3-rutinoside | C27H31O14 | 14 | 579.1 | 1.3 | 271(100), 433 (20) | D. | N.D. | D. | D. |
| Phenolic Compounds | Saco Cherry Extracts | |||
|---|---|---|---|---|
| CE | CET | MAE | UAE | |
| Hydroxycinnamic acids | ||||
| 3-Caffeoylquinic acid | 0.54 ± 0.05 c | 0.87 ± 0.02 b | 0.99 ± 0.07 a | 0.54 ± 0.00 c |
| 5-Caffeoylquinic acid | 0.40 ± 0.08 ab | 0.26 ± 0.05 c | 0.38 ± 0.01 b | 0.46 ± 0.01 a |
| 4-Caffeoylquinic acid | B.Q.L | 0.06 ± 0.03 b | 0.15 ± 0.04 a | 0.09 ± 0.02 a |
| 4,5-dicaffeoylquinic acid | 0.08 ± 0.02 a | 0.08 ± 0.00 a | N.D | 0.02 ± 0.00 b |
| Caffeic acid | B.D.L | 0.09 ± 0.03 a | 0.08 ± 0.02 a | B.Q.L |
| p-coumaroylquinic acid | 0.47 ± 0.20 c | 1.03 ± 0.05 a | 0.63 ± 0.00 b | 0.39 ± 0.00 d |
| p-coumaric acid | 0.20 ± 0.03 c | B.Q.L | 0.62 ± 0.05 a | 0.31 ± 0.00 b |
| Flavan-3-ols | ||||
| (−)-Epicatechin-3-gallate | B.Q.L | B.Q.L | 0.03 ± 0.08 a | B.Q.L |
| Flavonols | ||||
| Quercetin-3-rutinoside | 0.42 ± 0.63 a | 0.24 ± 0.02 b | 0.14 ± 0.00 c | 0.05 ± 0.01 d |
| Kaempferol-3-glucoside | 0.07 ± 0.00 a | N.D. | 0.06 ± 0.02 b | N.D. |
| Quercetin | B.D.L. | B.D.L. | 0.12 ± 0.00 a | B.D.L. |
| Anthocyanins | ||||
| Cyanidin-3-rutinoside | 2.13 ± 0.25 b | 2.54 ± 0.16 a | 2.46 ± 0.13 a | 2.21 ± 0.19 b |
| Cyanidin-3-glucoside | 0.58 ± 0.04 c | 0.87 ± 0.11 b | 1.14 ± 0.01 a | 0.54 ± 0.06 c |
| Peonidin-3-rutinoside | 0.08 ± 0.00 b | 0.07 ± 0.05 b | 0.16 ± 0.03 a | 0.06 ± 0.01 b |
| Pelargonidin-3-rutinoside | 0.06 ± 0.01 a | N.D. | 0.06 ± 0.05 a | 0.04 ± 0.00 a |
| Saco Cherry Extracts | |||
|---|---|---|---|
| Variable Number | Variable Designation | Variable Number | Variable Designation |
| 1 | Total Phenolic Content | 11 | 5-Caffeoylquinic acid |
| 2 | Total Anthocyanins Content | 12 | 4-Caffeoylquinic acid |
| 3 | ABTS assay | 13 | 4,5-Di-O-Caffeoylquinic acid |
| 4 | DPPH assay | 14 | Caffeic acid |
| 5 | ORAC assay | 15 | p-coumaroylquinic acid |
| 6 | Cyanidin-3-rutinoside | 16 | p-coumaric acid |
| 7 | Cyanidin-3-glucoside | 17 | Epicatechin-3-gallate |
| 8 | Peonidin-3-rutinoside | 18 | Quercetin-3-rutinoside |
| 9 | Pelargonidin-3-rutinoside | 19 | Kaempferol |
| 10 | 3-Caffeoylquinic acid | 20 | Quercetin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability 2020, 12, 5556. https://doi.org/10.3390/su12145556
Vilas-Boas AA, Campos DA, Nunes C, Ribeiro S, Nunes J, Oliveira A, Pintado M. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability. 2020; 12(14):5556. https://doi.org/10.3390/su12145556
Chicago/Turabian StyleVilas-Boas, Ana A., Débora A. Campos, Catarina Nunes, Sónia Ribeiro, João Nunes, Ana Oliveira, and Manuela Pintado. 2020. "Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract" Sustainability 12, no. 14: 5556. https://doi.org/10.3390/su12145556
APA StyleVilas-Boas, A. A., Campos, D. A., Nunes, C., Ribeiro, S., Nunes, J., Oliveira, A., & Pintado, M. (2020). Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability, 12(14), 5556. https://doi.org/10.3390/su12145556








