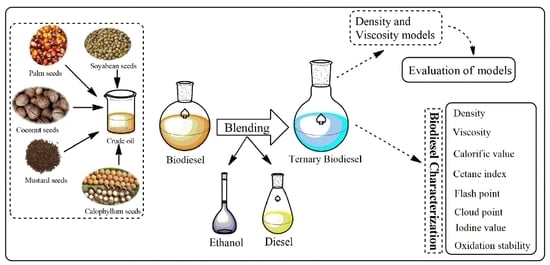
Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Biodiesel Blends and Characterization
2.2. Density and Viscosity Measurement
2.3. Equations for Density and Viscosity Modeling
2.4. Evaluation of Models
3. Results and Discussion
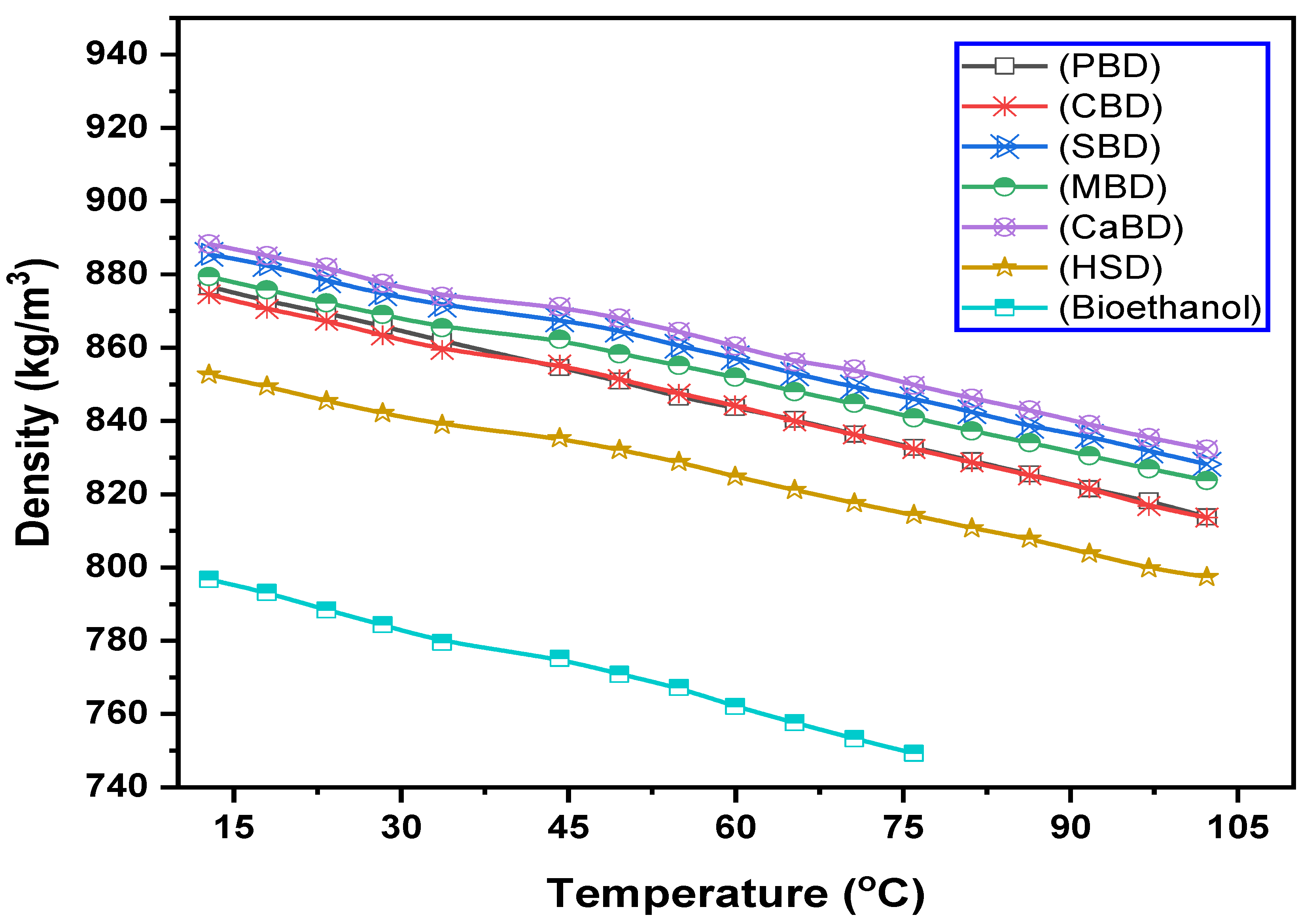
3.1. Effect of Temperature on the Density of Diesel, Ethanol, and Biodiesels
3.2. Effect of Bioethanol on Density of the Binary Biodiesel Blends
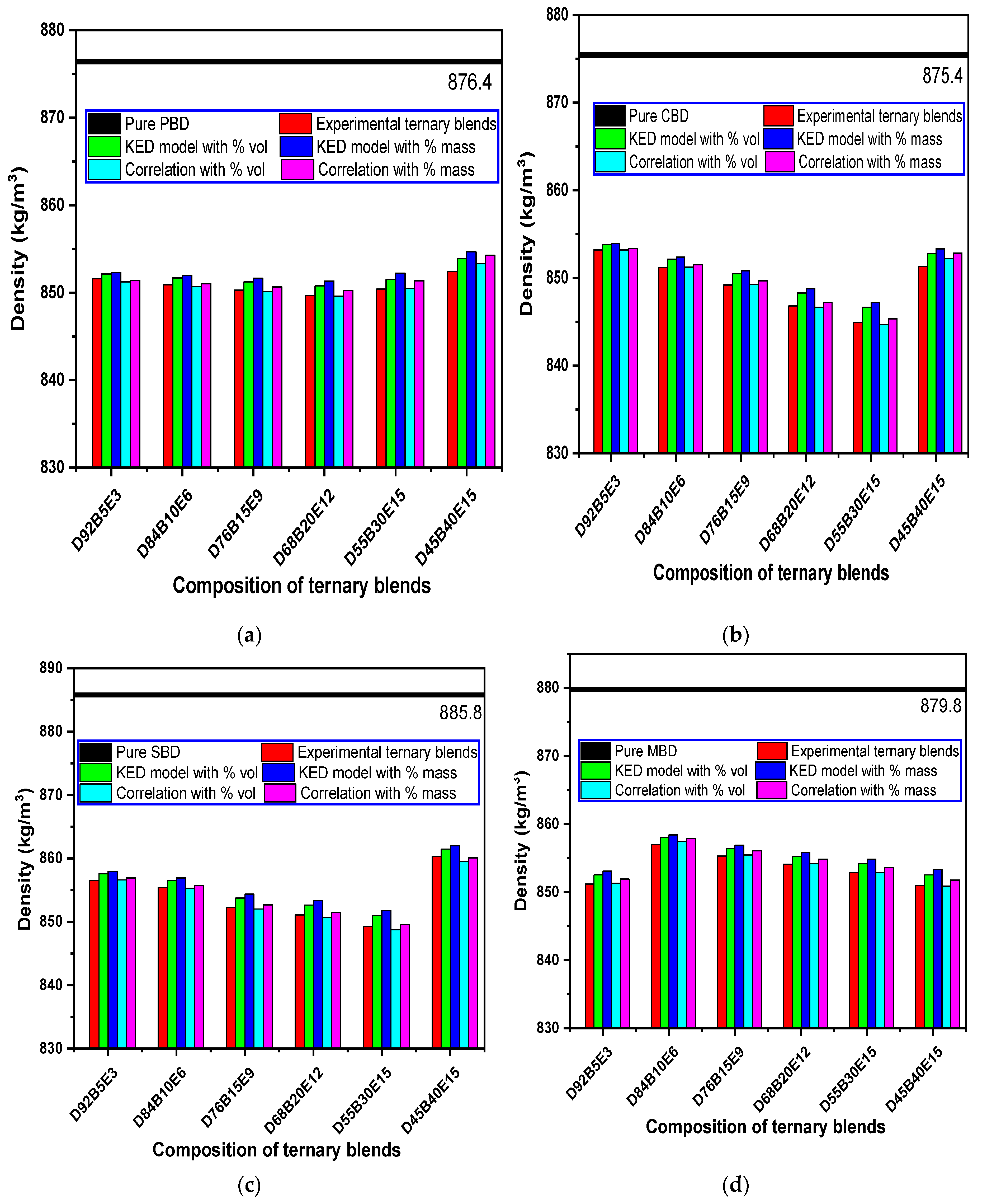
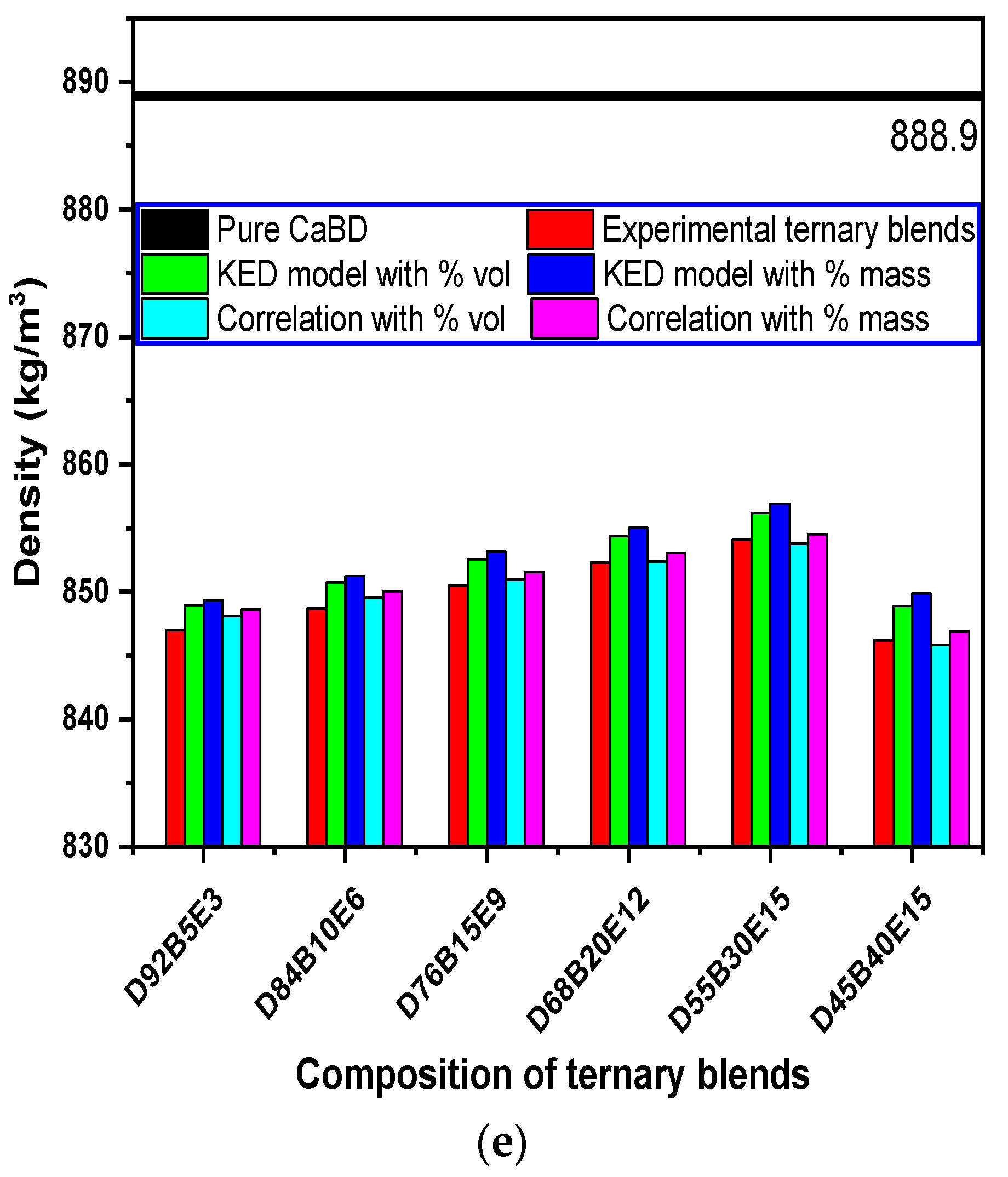
3.3. Evaluation of Density Models
3.4. Effect of Temperature on the Viscosity of Diesel, Ethanol, and Biodiesels
3.5. Effect of Bioethanol on Viscosity of the Binary Biodiesel Blends
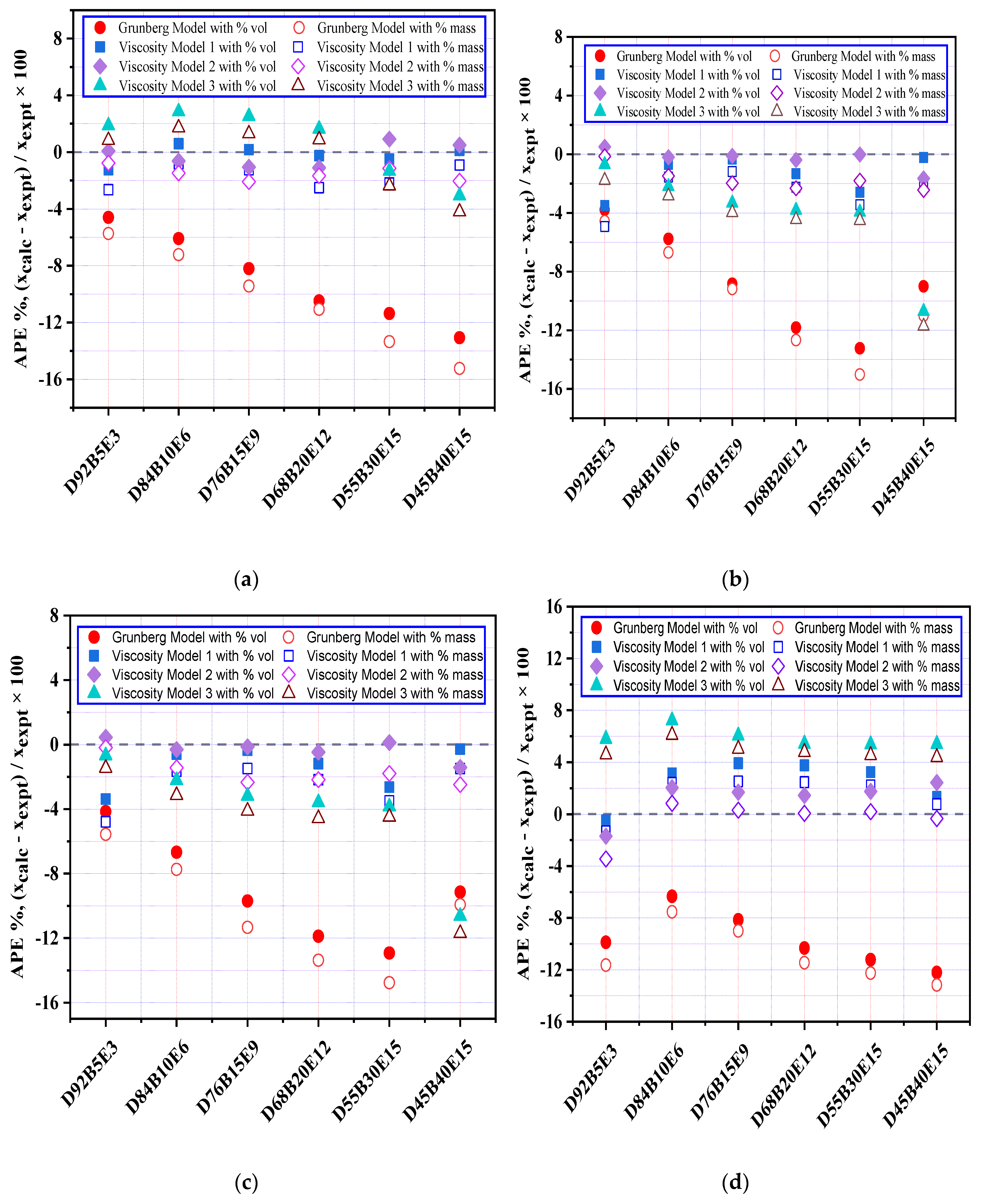
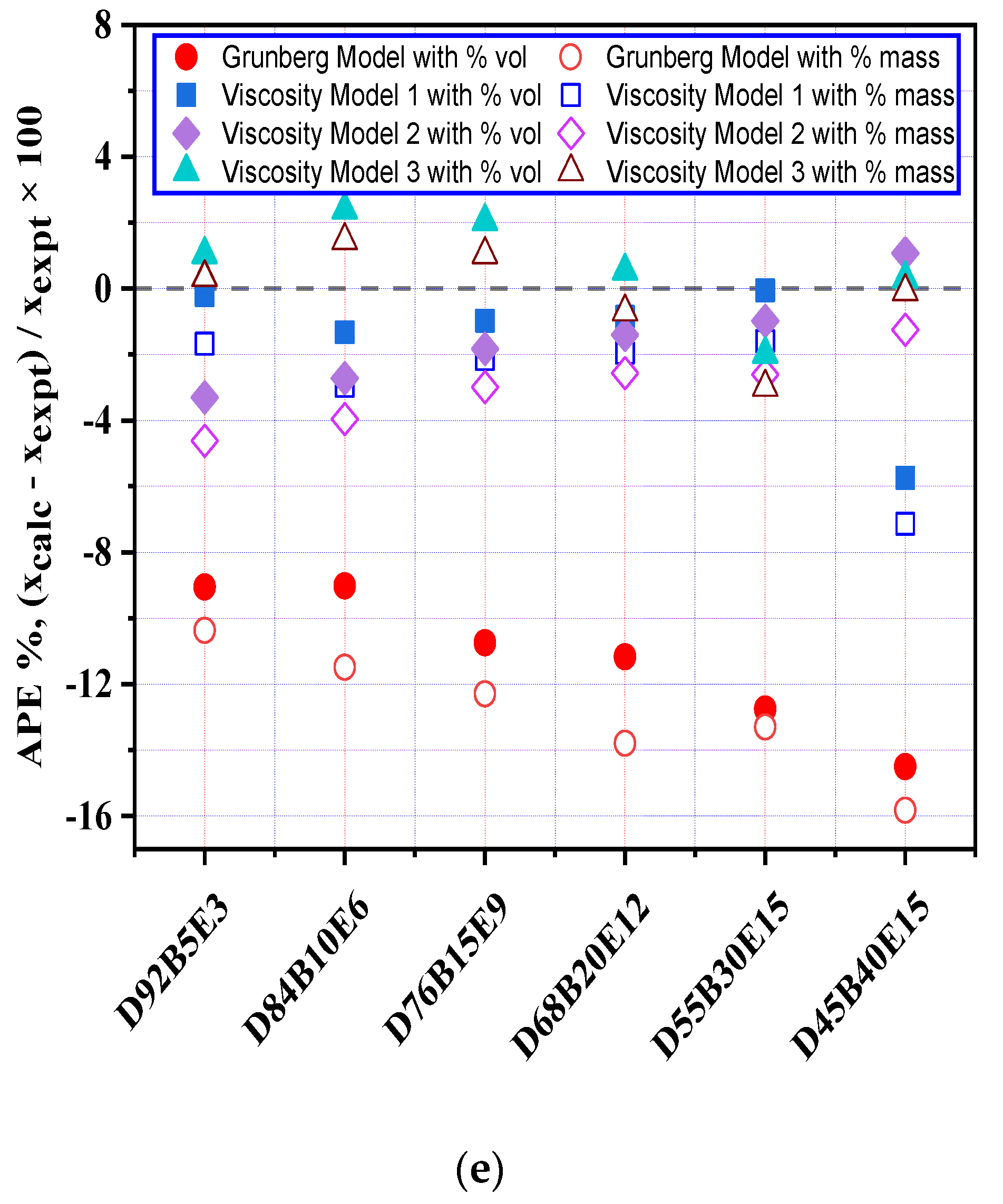
3.6. Evaluation of Viscosity Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| PBD | Palm biodiesel |
| CBD | Coconut biodiesel |
| SBD | Soybean biodiesel |
| MBD | Mustard biodiesel |
| CaBD | Calophyllum biodiesel |
| HSD | High-speed diesel |
| APE | Absolute percentage error (%) |
| MAPE | Mean absolute percentage error (%) |
| KED | Kay’s mixing rule |
| Kinematic viscosity of the ternary blend (mm2/s) | |
| Kinematic viscosity of the ternary blends (mm2/s) | |
| Mass or volume fraction of the individual components | |
| Density of the ternary blend (kg/m3) | |
| Density of biodiesel (kg/m3) | |
| D | Percentage mass or percentage volume portion of diesel in the ternary blend |
| BD | Percentage mass or percentage volume portion of biodiesel in the ternary blend |
| EtOH | Percentage mass or percentage volume portion of ethanol in the ternary blend |
| SD | Standard deviation |
| R2 | Coefficients of determinations |
| Xexpt | Density and viscosity values obtained from experiments (kg/m3), (mm2/s) |
| Xcalc | Density and viscosity values calculated using proposed models (kg/m3), (mm2/s) |
| Average value of the measured densities and viscosities (kg/m3), (mm2/s) | |
| N | Total number of observations |
References
- Ağbulut, Ü.; Sarıdemir, S. A general view to converting fossil fuels to cleaner energy source by adding nanoparticles. Int. J. Ambient Energy 2018, 1–6. [Google Scholar] [CrossRef]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M. Performance and emission characteristics of diesel engine fueled with rice bran biodiesel and n-butanol. Energy Rep. 2019, 5, 78–83. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Yunus Khan, T.M.; Badruddin, I.A.; Ankalgi, R.F.; Badarudin, A.; Hungund, B.S.; Ankalgi, F.R. Biodiesel Production by Direct Transesterification Process via Sequential Use of Acid–Base Catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Sung, J.H.; Ryu, Y.; Seo, S.B. Utilizing Bivariate Climate Forecasts to Update the Probabilities of Ensemble Streamflow Prediction. Sustainability 2020, 12, 2905. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Manzano-Agugliaro, F.; Hernandez-Escobedo, Q.; Perea-Moreno, A.J. Sustainable thermal energy generation at universities by using loquat seeds as biofuel. Sustainability 2020, 12, 2093. [Google Scholar] [CrossRef]
- Tucki, K.; Orynycz, O.; Mruk, R.; Świć, A.; Botwińska, K. Modeling of biofuel’s emissivity for fuel choice management. Sustainability 2019, 11, 6842. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Liu, F.; Fang, M.; Farooq, M.; Luo, Z. Enhanced CO2 absorption and desorption by monoethanolamine (MEA)-based nanoparticle suspensions. Ind. Eng. Chem. Res. 2016, 55, 7830–7838. [Google Scholar] [CrossRef]
- Arslan, M.; Farooq, M.; Naqvi, M.; Sultan, U.; Tahir, Z.-R.; Nawaz, S.; Waheed, N.; Naqvi, S.R.; Ali, Q.; Tariq, M.S. Impact of Varying Load Conditions and Cooling Energy Comparison of a Double-Inlet Pulse Tube Refrigerator. Processes 2020, 8, 352. [Google Scholar] [CrossRef]
- Zhang, Z.; Lis, M. Modeling green energy development based on sustainable economic growth in China. Sustainability 2020, 12, 1368. [Google Scholar] [CrossRef]
- Farooq, M.; Hamayoun, A.; Naqvi, M.; Nawaz, S.; Usman, M.; Naqvi, S.R.; Imran, M.; Nadeem, R.; Razi, A.; Turan, A.; et al. Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment. Processes 2020, 8, 187. [Google Scholar] [CrossRef]
- Uddin, G.M.; Niazi, S.G.; Arafat, S.M.; Kamran, M.S.; Farooq, M.; Hayat, N.; Malik, S.A.; Zeid, A.; Kamarthi, S.; Saqib, S.; et al. Neural networks assisted computational aero-acoustic analysis of an isolated tire. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2020, 0954407020. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-economic assessment of a scaled-up meat waste biorefinery system: A simulation study. Materials (Basel) 2019, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Arafat, S.M.; Ashraf, W.M.; Asim, M.; Bhutta, M.M.A.; Jatoi, H.U.K.; Niazi, S.G.; Jamil, A.; Farooq, M.; Ghufran, M. Artificial Intelligence-Based Emission Reduction Strategy for Limestone Forced Oxidation Flue Gas Desulfurization System. J. Energy Resour. Technol. 2020, 142, 092103. [Google Scholar] [CrossRef]
- Kashif, M.; Awan, M.B.; Nawaz, S.; Amjad, M.; Talib, B.; Farooq, M.; Nizami, A.S.; Rehan, M. Untapped renewable energy potential of crop residues in Pakistan: Challenges and future directions. J. Environ. Manag. 2020, 256, 109924. [Google Scholar] [CrossRef]
- Almustapha, M.N.; Farooq, M.; Mohammed, M.L.; Farhan, M.; Imran, M.; Andresen, J.M. Modification of acidic and textural properties of a sulphated zirconia catalyst for efficient conversion of high-density polyethylene into liquid fuel. Environ. Sci. Pollut. Res. 2019, 27, 55–65. [Google Scholar] [CrossRef]
- Farooq, M.; Asim, M.; Imran, M.; Imran, S.; Ahmad, J.; Younis, M.R. Mapping past, current and future energy research trend in Pakistan: A scientometric assessment. Scientometrics 2018, 117, 1733–1753. [Google Scholar] [CrossRef]
- Syed, M.S.; Farooq, M.; Qamar, A. Modeling and Forecasting of Energy Scenario in Pakistan with Application of Decentralized Energy Planning. J. Fac. Eng. Technol. 2014, 21, 11–24. [Google Scholar]
- Lenzen, M. Current state of development of electricity-generating technologies: A literature review. Energies 2010, 3, 462. [Google Scholar] [CrossRef]
- Imran, S.; Emberson, D.R.; Hussain, A.; Ali, H.; Ihracska, B.; Korakianitis, T. Performance and specific emissions contours throughout the operating range of hydrogen-fueled compression ignition engine with diesel and RME pilot fuels. Alex. Eng. J. 2015, 54, 303–314. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Farooq, M.; Saeed, M.A.; Imran, M.; Uddin, G.M.; Asim, M.; Bilal, H.; Younas, M.R.; Andresen, J.M. CO2 capture through electro-conductive adsorbent using physical adsorption system for sustainable development. Environ. Geochem. Health 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glensor, K.; María Rosa Muñoz, B. Life-cycle assessment of Brazilian transport biofuel and electrification pathways. Sustainability 2019, 11, 6332. [Google Scholar] [CrossRef]
- Kumar, S.; Abdul Salam, P.; Shrestha, P.; Ackom, E.K. An assessment of thailand’s biofuel development. Sustainability 2013, 5, 1577. [Google Scholar] [CrossRef]
- Lorenzo-s, E.; Oliver-villanueva, J.; Coll-aliaga, E. Energy E ffi ciency and GHG Emissions Mapping of Buildings for Decision-Making Processes against Climate Change at the Local Level. Sustainability 2020, 12, 2982. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Chen, G.; Gao, Q. Observed Characteristics and Modeled Emissions of Transit Buses on Ramps. Sustainability 2020, 12, 2770. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Naqvi, M.; Saleem, M.W.; Hussain, J.; Naqvi, S.R.; Jahangir, S.; Usama, J.; Muhammad, H.; Idrees, S. Use of Gasoline, LPG and LPG-HHO Blend in SI Engine: A Comparative Performance for Emission Control and Sustainable Environment. Processes 2020, 8, 74. [Google Scholar] [CrossRef]
- Saeed, M.A.; Farooq, M.; Andrews, G.E.; Phylaktou, H.N.; Gibbs, B.M. Ignition sensitivity of different compositional wood pellets and particle size dependence. J. Environ. Manag. 2019, 232, 789–795. [Google Scholar] [CrossRef]
- Yıldızhan, Ş. Hydrogen addition to tea seed oil biodiesel: Performance and emission characteristics. Int. J. Hydrogen Energy 2018, 43, 18020–18027. [Google Scholar]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M.; Abedin, M.J. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers. Manag. 2014, 79, 265–272. [Google Scholar] [CrossRef]
- Ramalingam, S.; Rajendran, S.; Ganesan, P. Performance improvement and exhaust emissions reduction in biodiesel operated diesel engine through the use of operating parameters and catalytic converter: A review. Renew. Sustain. Energy Rev. 2018, 81, 3215–3222. [Google Scholar] [CrossRef]
- Can, Ö.; Öztürk, E.; Solmaz, H.; Aksoy, F.; Çinar, C.; Yücesu, H.S. Combined effects of soybean biodiesel fuel addition and EGR application on the combustion and exhaust emissions in a diesel engine. Appl. Therm. Eng. 2016, 95, 115–124. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Development, R. Impacts of International Commodity Trade on Conventional Biofuels Production. Sustainability 2020, 12, 2626. [Google Scholar]
- Samios, D.; Pedrotti, F.; Nicolau, A.; Reiznautt, Q.B.; Martini, D.D.; Dalcin, F.M. A Transesterification Double Step Process — TDSP for biodiesel preparation from fatty acids triglycerides. Fuel Process. Technol. 2009, 90, 599–605. [Google Scholar] [CrossRef]
- Mostafaei, M.; Javadikia, H.; Naderloo, L. Modeling the effects of ultrasound power and reactor dimension on the biodiesel production yield: Comparison of prediction abilities between response surface methodology (RSM) and adaptive neuro-fuzzy inference system (ANFIS). Energy 2016, 115, 626–636. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2018, 1–13. [Google Scholar] [CrossRef]
- Younis, M.R.; Farooq, M.; Imran, M.; Kazim, A.H.; Shabbir, A. Characterization of the viscosity of bio-oil produced by fast pyrolysis of the wheat straw. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–16. [Google Scholar] [CrossRef]
- Jeevanantham, A.K.; Nanthagopal, K.; Ashok, B.; Al-Muhtaseb, A.H.; Thiyagarajan, S.; Geo, V.E.; Ong, H.C.; Samuel, K.J. Impact of addition of two ether additives with high speed diesel-Calophyllum Inophyllum biodiesel blends on NOx reduction in CI engine. Energy 2019, 185, 39–54. [Google Scholar] [CrossRef]
- Suh, H.K.; Lee, C.S. A review on atomization and exhaust emissions of a biodiesel-fueled compression ignition engine. Renew. Sustain. Energy Rev. 2016, 58, 1601–1620. [Google Scholar] [CrossRef]
- Barabás, I. Liquid densities and excess molar volumes of ethanol + biodiesel binary system between the temperatures 273.15 K and 333.15 K. J. Mol. Liq. 2015, 204, 95–99. [Google Scholar] [CrossRef]
- Reddy, S.M.; Sharma, N.; Gupta, N.; Agarwal, A.K. Effect of non-edible oil and its biodiesel on wear of fuel injection equipment components of a genset engine. Fuel 2018, 222, 841–851. [Google Scholar] [CrossRef]
- Kegl, B. Biodiesel usage at low temperature. Fuel 2008, 87, 1306–1317. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Cold flow and filterability properties of n-butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2018, 224, 552–559. [Google Scholar] [CrossRef]
- Caligiuri, C.; Renzi, M.; Bietresato, M.; Baratieri, M. Experimental investigation on the effects of bioethanol addition in diesel-biodiesel blends on emissions and performances of a micro-cogeneration system. Energy Convers. Manag. 2019, 185, 55–65. [Google Scholar] [CrossRef]
- Todoruț, A.; Molea, A.; Barabás, I. Predicting the temperature and composition—Dependent density and viscosity of diesel fuel—Ethanol blends. Period. Polytech. Chem. Eng. 2020, 64, 213–220. [Google Scholar] [CrossRef]
- Emiroğlu, A.O.; Şen, M. Combustion, performance and exhaust emission characterizations of a diesel engine operating with a ternary blend (alcohol-biodiesel-diesel fuel). Appl. Therm. Eng. 2018, 133, 371–380. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Q.; Song, F.; Wang, X.; Zhang, L. Experimental investigations on the liquid thermal conductivity of five saturated fatty acid methyl esters components of biodiesel. J. Chem. Thermodyn. 2018, 125, 50–55. [Google Scholar] [CrossRef]
- Barabás, I.; Todoruţ, A.I. Key Fuel Properties of Biodiesel-Diesel Fuel-Ethanol Blends; SAE: Warrendale, PA, USA, 2009; Available online: https://www.sae.org/publications/technical-papers/content/2009-01-1810/ (accessed on 24 June 2020).
- Na, M.M.; Aziz, A.; Rashid, A.; Hagos, F.Y.; Noor, M.M.; Kadirgama, K.; Mamat, R.; Abdullah, A.A. The influence of formulation ratio and emulsifying settings on tri-fuel (Diesel–Ethanol–Biodiesel) emulsion properties. Energies 2019, 12, 1708. [Google Scholar] [CrossRef]
- Ramírez Verduzco, L.F. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sustain. Energy Rev. 2013, 19, 652–665. [Google Scholar] [CrossRef]
- Dong, N.H.; Duong, N.H. An empirical approach for predicting biodiesel fuels dynamic viscosity from their fatty acid ester composition at various temperatures. Petrovietnal J. 2015, 6, 33–42. [Google Scholar]
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Modeling viscosity of butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2017, 199, 332–338. [Google Scholar] [CrossRef]
- Hurtado, B.; Posadillo, A.; Luna, D.; Bautista, F.M.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.A.; Estevez, R. Synthesis, performance and emission quality assessment of ecodiesel from castor oil in diesel/biofuel/alcohol triple blends in a diesel engine. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef]
- Barabás, I.; Todoruţ, I.-A. Predicting the temperature dependent viscosity of biodiesel–diesel–bioethanol blends. Energy Fuels 2011, 25, 5767–5774. [Google Scholar] [CrossRef]
- Barabás, I. Predicting the temperature dependent density of biodiesel–diesel–bioethanol blends. Fuel 2013, 109, 563–574. [Google Scholar] [CrossRef]
- Chavarria-Hernandez, J.C.; Pacheco-Catalán, D.E. Predicting the kinematic viscosity of FAMEs and biodiesel: Empirical models. Fuel 2014, 124, 212–220. [Google Scholar] [CrossRef]
- Allen, C.A.W.; Watts, K.C.; Ackman, R.G.; Pegg, M.J. Predicting the viscosity of biodiesel fuels from their fatty acid ester composition. Fuel 1999, 78, 1319–1326. [Google Scholar] [CrossRef]
- Pereira, E.; Meirelles, A.J.A.; Maximo, G.J. Predictive models for physical properties of fats, oils, and biodiesel fuels. Fluid Phase Equilib. 2020, 508, 112440. [Google Scholar] [CrossRef]
- Mallah, T.A.; Sahito, A.R. Optimization of castor and neem biodiesel blends and development of empirical models to predicts its characteristics. Fuel 2020, 262, 116341. [Google Scholar] [CrossRef]
- Atabani, A.E.; Shobana, S.; Mohammed, M.N.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Al-Muhtaseb, A.H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Adu-Mensah, D.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel properties. Fuel 2019, 251, 660–668. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Kusumo, F.; Mahlia, T.M.I.; Bahar, A.H. Pilot-scale production and the physicochemical properties of palm and Calophyllum inophyllum biodiesels and their blends. J. Clean. Prod. 2016, 126, 654–666. [Google Scholar] [CrossRef]
- Jiang, S.; Qi, J.; Hu, Y.; Ren, C.; Jiang, Y.; Zhao, Z. Predicting the Density and Viscosity of Biodiesels and Biodiesel Blends by the Regular-Solution Theory. Ind. Eng. Chem. Res. 2019, 58, 17038–17048. [Google Scholar] [CrossRef]
- Corral-Gómez, L.; Rubio-Gómez, G.; Martínez-Martínez, S.; Sánchez-Cruz, F.A. Effect of diesel-biodiesel-ethanol blends on the spray macroscopic parameters in a common-rail diesel injection system. Fuel 2019, 241, 876–883. [Google Scholar] [CrossRef]
- Saleh, H.E.; Selim, M.Y.E. Improving the performance and emission characteristics of a diesel engine fueled by jojoba methyl ester-diesel-ethanol ternary blends. Fuel 2017, 207, 690–701. [Google Scholar] [CrossRef]
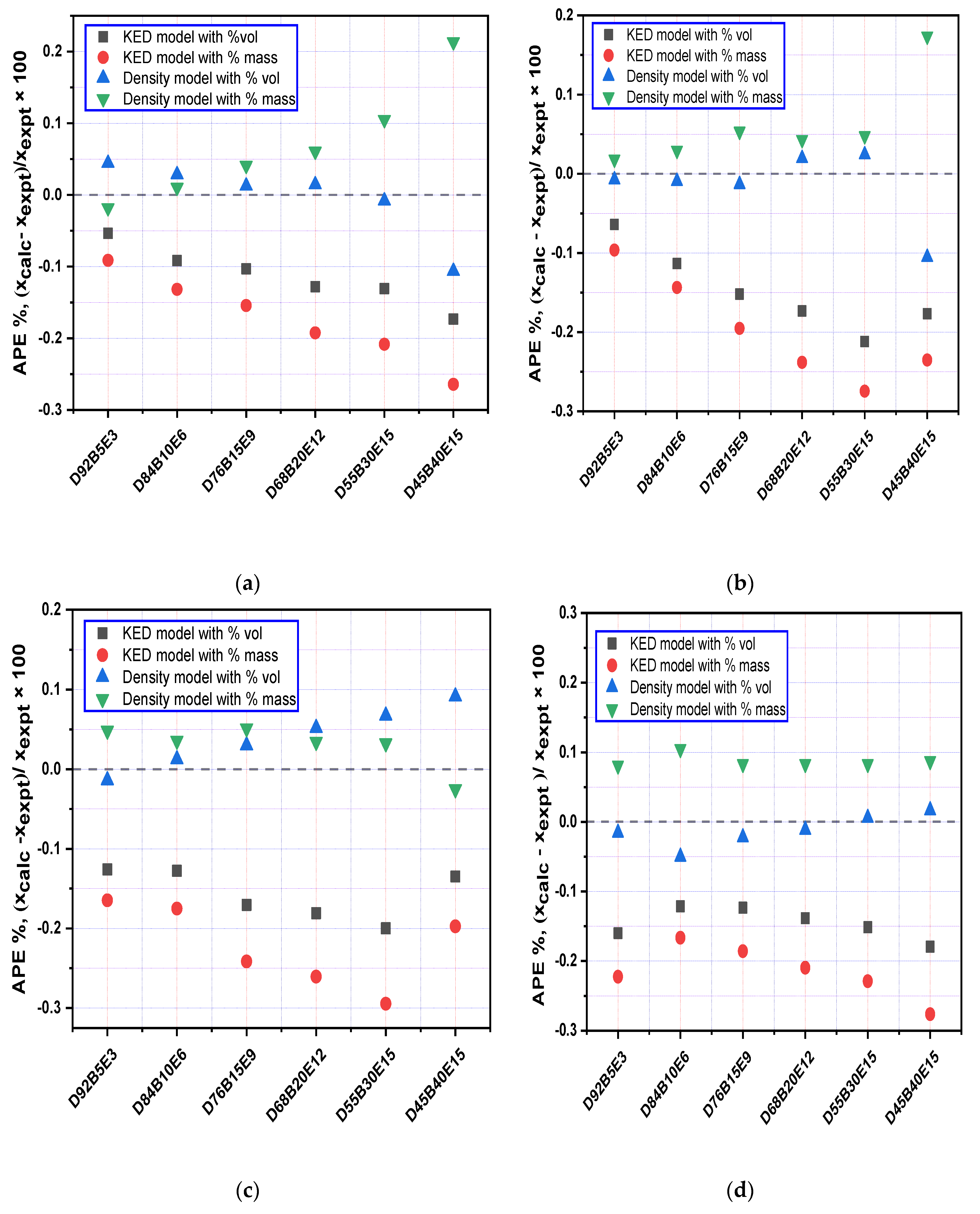
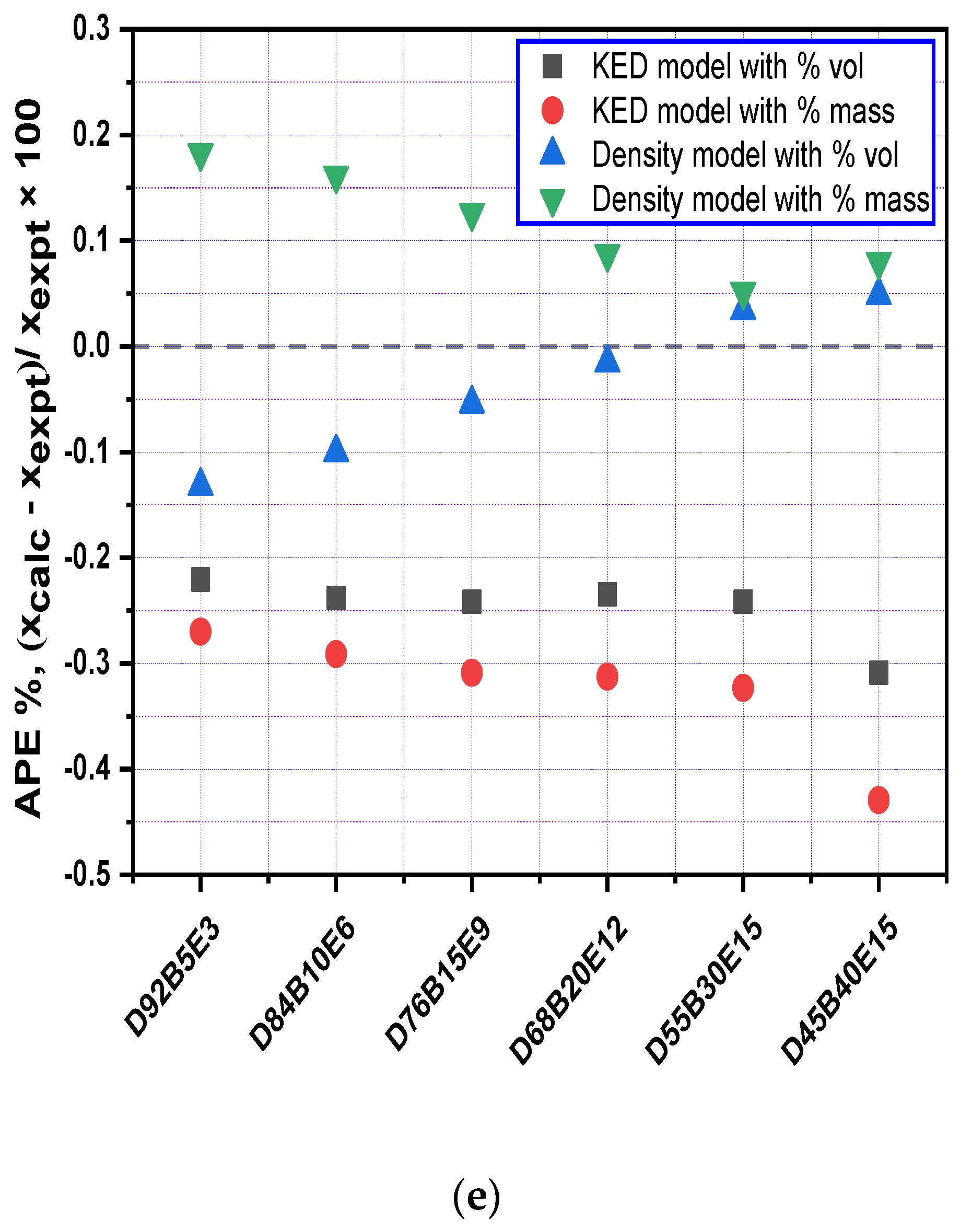

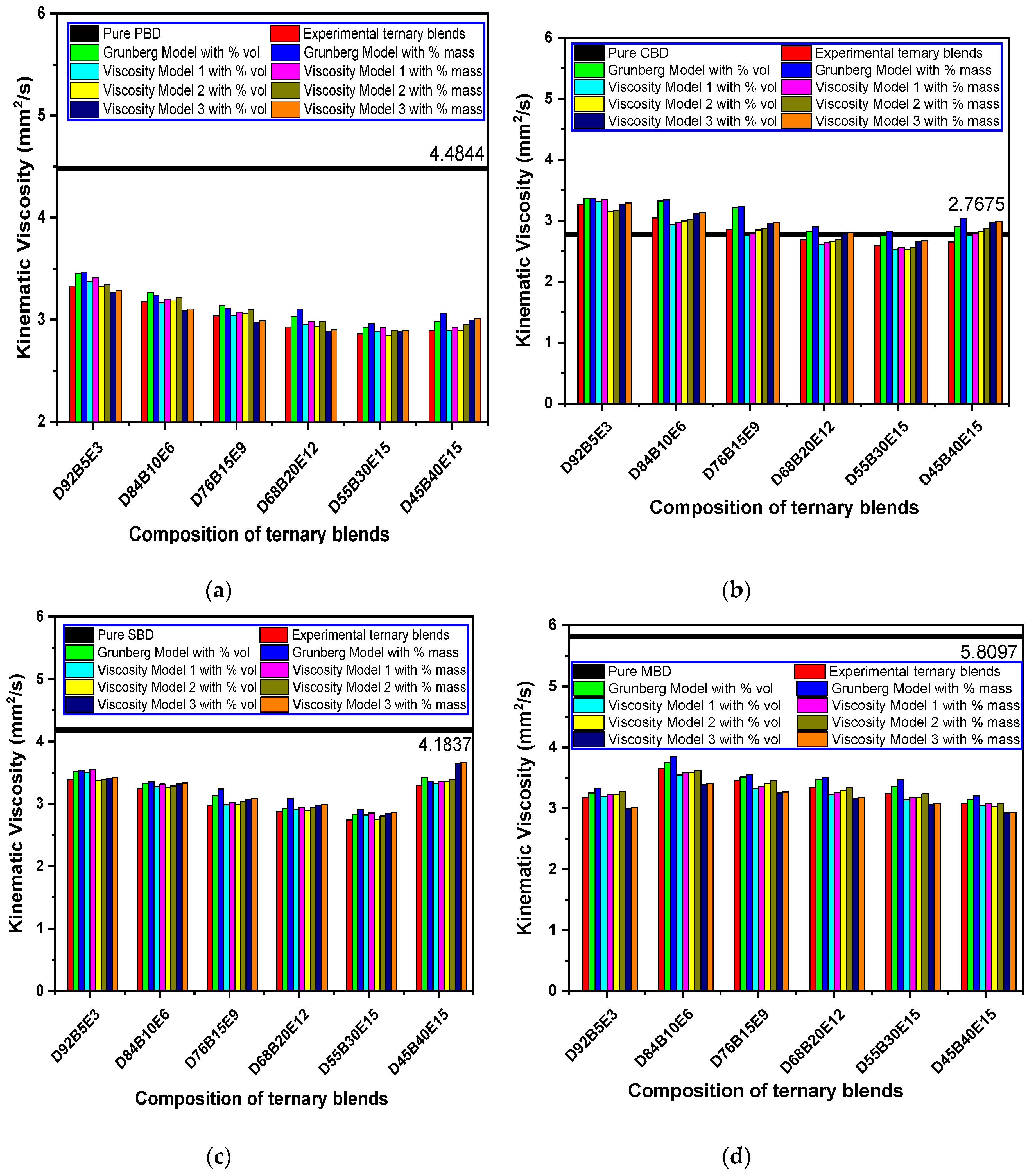
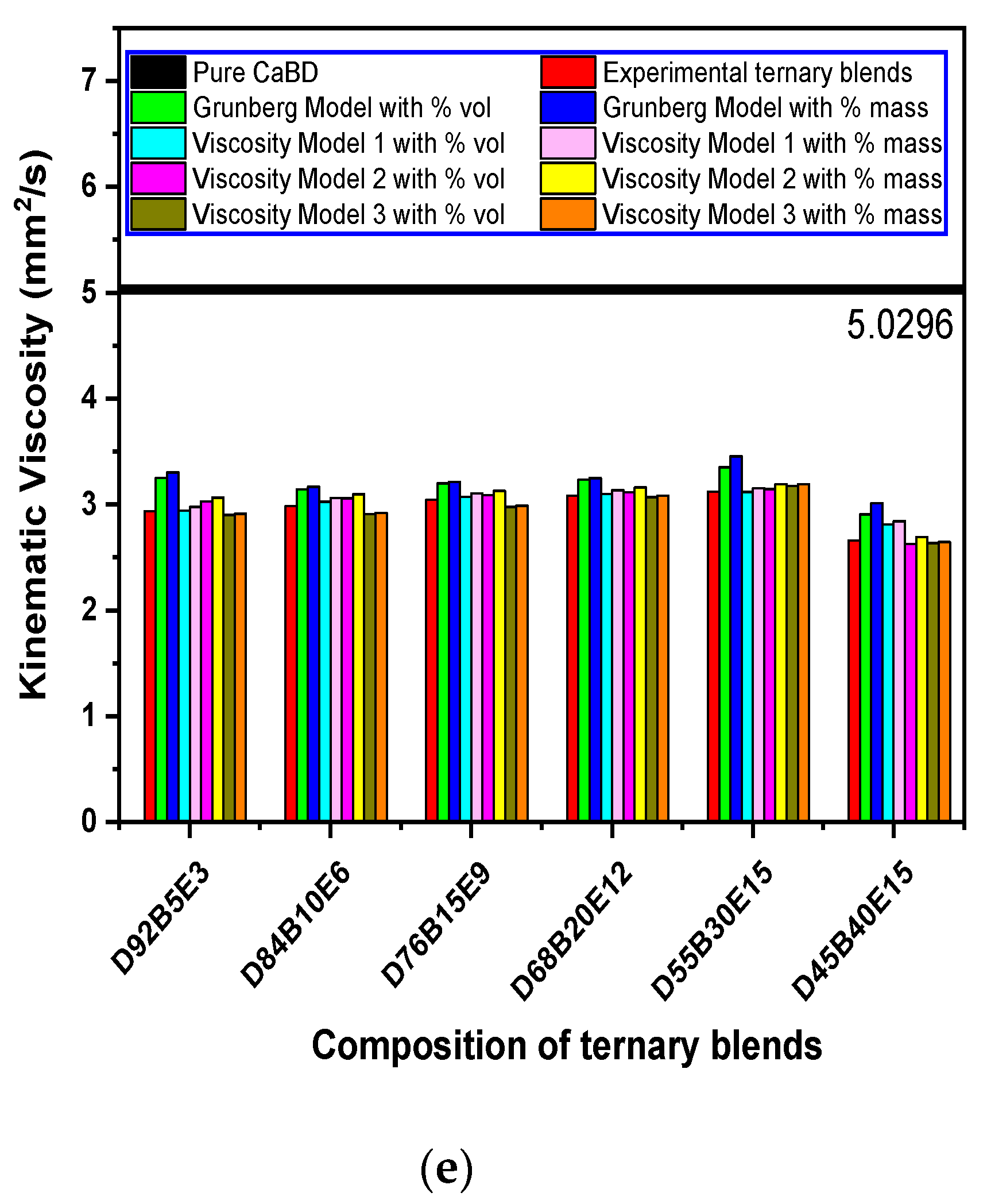
| Name | Diesel (%) | Biodiesel (%) | Ethanol (%) |
|---|---|---|---|
| D100 | 100 | 0 | 0 |
| D92B5E3 | 92 | 5 | 3 |
| D84B10E6 | 84 | 10 | 6 |
| D76B15E9 | 76 | 15 | 9 |
| D68B20E12 | 68 | 20 | 12 |
| D55B30E15 | 55 | 30 | 15 |
| D45B40E15 | 45 | 40 | 15 |
| 5 | Properties | Test Standard | Measurement Uncertainty | Diesel | PBD | CBD | SBD | MBD | CaBD | Ethanol |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Density at 15 °С (kg/m3) | ASTM D4052 | ±0.1 kg/m3 | 836 | 859 | 856 | 866 | 862 | 872 | 776 |
| 2 | Viscosity at 40 °С (mm2/s) | ASTM D7042 | ±0.30% | 3.70 | 4.5 | 2.79 | 4.45 | 5.92 | 5.25 | 1.14 |
| 3 | Calorific value (MJ/kg) | ASTM 4809 | ±0.1% | 44.54 | 40.90 | 39.10 | 39.88 | 40.71 | 39.91 | 28.85 |
| 4 | Flash point (°С) | ASTM D93 | ±0.1 °С | 78 | 183 | 167 | 159 | 150 | 173 | 12.2 |
| 5 | Pour point (°С) | ASTM D97 | ±0.1 °С | 8 | 16 | −3.5 | −4 | −17 | 9 | −114 |
| 6 | Cloud point (°С) | ASTM D2500 | ±0.1 °С | 9 | 12 | 2 | 3 | 5 | 10 | - |
| 7 | Oxygen stability (h) | ASTM D7462 | ±0.01 h | 59.1 | 3.92 | 8.14 | 3 | 15.92 | 3.18 | 1.5 |
| 8 | Cetane index | ASTM D4737 | 48 | 61 | 63.52 | 51 | 76 | 56.3 | 8 | |
| 9 | Iodine value (g I/100 g) | ASTM D1959 | - | 61 | 15.76 | 129.8 | 102 | 82.1 | - |
| Common Name | Structure | PBD | CBD | SBD | MBD | CaBD |
|---|---|---|---|---|---|---|
| Methyl Caproate | C6:0 | 0.3 | 0.1 | |||
| Methyl Caprylate | C8:0 | 6.5 | 0.1 | |||
| Methyl Caprate | C10:0 | 6 | 0.1 | |||
| Methyl Laurate | C12:0 | 0.14 | 42.1 | 0.1 | ||
| Methyl Myristate | C14:0 | 1.05 | 17.4 | 0.1 | 0.1 | |
| Methyl Palmitate | C16:0 | 38.84 | 11.3 | 10.5 | 1.9 | 14.9 |
| Methyl Palmitoleate | C16:1 | 0.22 | 0.2 | 0.1 | 0.2 | 0.2 |
| Methyl Stearate | C18:0 | 4.08 | 3.8 | 4.3 | 1.2 | 17.2 |
| Methyl Oleate | C18:1 | 42.21 | 9.2 | 25 | 12.7 | 38.2 |
| Methyl Linoleate | C18:2 | 12.81 | 3 | 51.5 | 12.3 | 27.6 |
| Methyl Linolenate | C18:3 | 0.25 | <0.1 | 6.8 | 7.2 | 0.3 |
| Methyl Arachidate | C20:0 | 0.4 | 0.2 | 0.4 | 1 | 0.9 |
| Methyl Eicosenoate | C20:1 | <0.1 | 0.2 | 6.4 | 0.2 | |
| Methyl Eicosadienoate | C20:2 | 0.4 | ||||
| Methyl Eicosatrienoate | C20:3 | 0.1 | ||||
| Methyl Behenate | C22:0 | <0.1 | 0.4 | 0.9 | 0.3 | |
| Methyl Erucate | C22:1 | <0.1 | 0.1 | 53.7 | ||
| Methyl Docosadienoate | C22:2 | 0.8 | ||||
| Methyl Lignocerate | C24:0 | <0.1 | 0.2 | 0.1 | ||
| Methyl Nervonate | C24:1 | 1.3 |
| Equation | a | b | c | d | e | X | SD | R2 |
|---|---|---|---|---|---|---|---|---|
| Equation (2) | 0.6554 | 0.9397 | 0.1180 | - | - | 682.825 | 0.4535 | 0.9823 |
| Equation (4) | 0.0521 | 0.0448 | 0.2060 | 0.13 | - | 0.7423 | 0.0738 | 0.9202 |
| Equation (5) | 0.0171 | 0.0190 | 0.0469 | - | - | 0.6761 | 0.0589 | 0.9468 |
| Equation (6) | 0.246 | 0.0610 | 0.0006 | 0.3268 | 21.8024 | 143.4953 | 0.143 | 0.7207 |
| Density | f | Y | R2 | Density | f | Y | R2 |
|---|---|---|---|---|---|---|---|
| 0.697 | 863.10 | 0.9999 | 0.708 | 890.13 | 0.9999 | ||
| 0.729 | 888.40 | 0.9999 | 0.720 | 899.90 | 0.9999 | ||
| 0.778 | 888.12 | 0.9999 | 0.897 | 812.10 | 0.9999 | ||
| 0.731 | 897.73 | 0.9999 |
| Viscosity | f | Y | R2 | Viscosity | f | Y | R2 |
|---|---|---|---|---|---|---|---|
| 0.018 | 2.092 | 0.9829 | 0.019 | 2.574 | 0.9846 | ||
| 0.018 | 2.280 | 0.9850 | 0.019 | 2.423 | 0.9843 | ||
| 0.017 | 1.712 | 0.9863 | 0.018 | 0.809 | 0.9882 | ||
| 0.017 | 2.174 | 0.9857 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaq, L.; Farooq, M.; Mujtaba, M.A.; Sher, F.; Farhan, M.; Hassan, M.T.; Soudagar, M.E.M.; Atabani, A.E.; Kalam, M.A.; Imran, M. Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment. Sustainability 2020, 12, 5186. https://doi.org/10.3390/su12125186
Razzaq L, Farooq M, Mujtaba MA, Sher F, Farhan M, Hassan MT, Soudagar MEM, Atabani AE, Kalam MA, Imran M. Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment. Sustainability. 2020; 12(12):5186. https://doi.org/10.3390/su12125186
Chicago/Turabian StyleRazzaq, Luqman, Muhammad Farooq, M. A. Mujtaba, Farooq Sher, Muhammad Farhan, Muhammad Tahir Hassan, Manzoore Elahi M. Soudagar, A. E. Atabani, M. A. Kalam, and Muhammad Imran. 2020. "Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment" Sustainability 12, no. 12: 5186. https://doi.org/10.3390/su12125186
APA StyleRazzaq, L., Farooq, M., Mujtaba, M. A., Sher, F., Farhan, M., Hassan, M. T., Soudagar, M. E. M., Atabani, A. E., Kalam, M. A., & Imran, M. (2020). Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment. Sustainability, 12(12), 5186. https://doi.org/10.3390/su12125186