Possibility of Increasing the Growth and Photosynthetic Properties of Precocious Walnut by Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Study Site
2.2. Graft Compatibility and Plant Characteristics
2.3. Leaf Gas Exchange and Chlorophyll Fluorescence Parameter Measurements
2.4. Statistical Analyses
3. Results
3.1. Growth Characteristics and Chlorophyll Fluorescence Parameters of Different Grafting Combinations
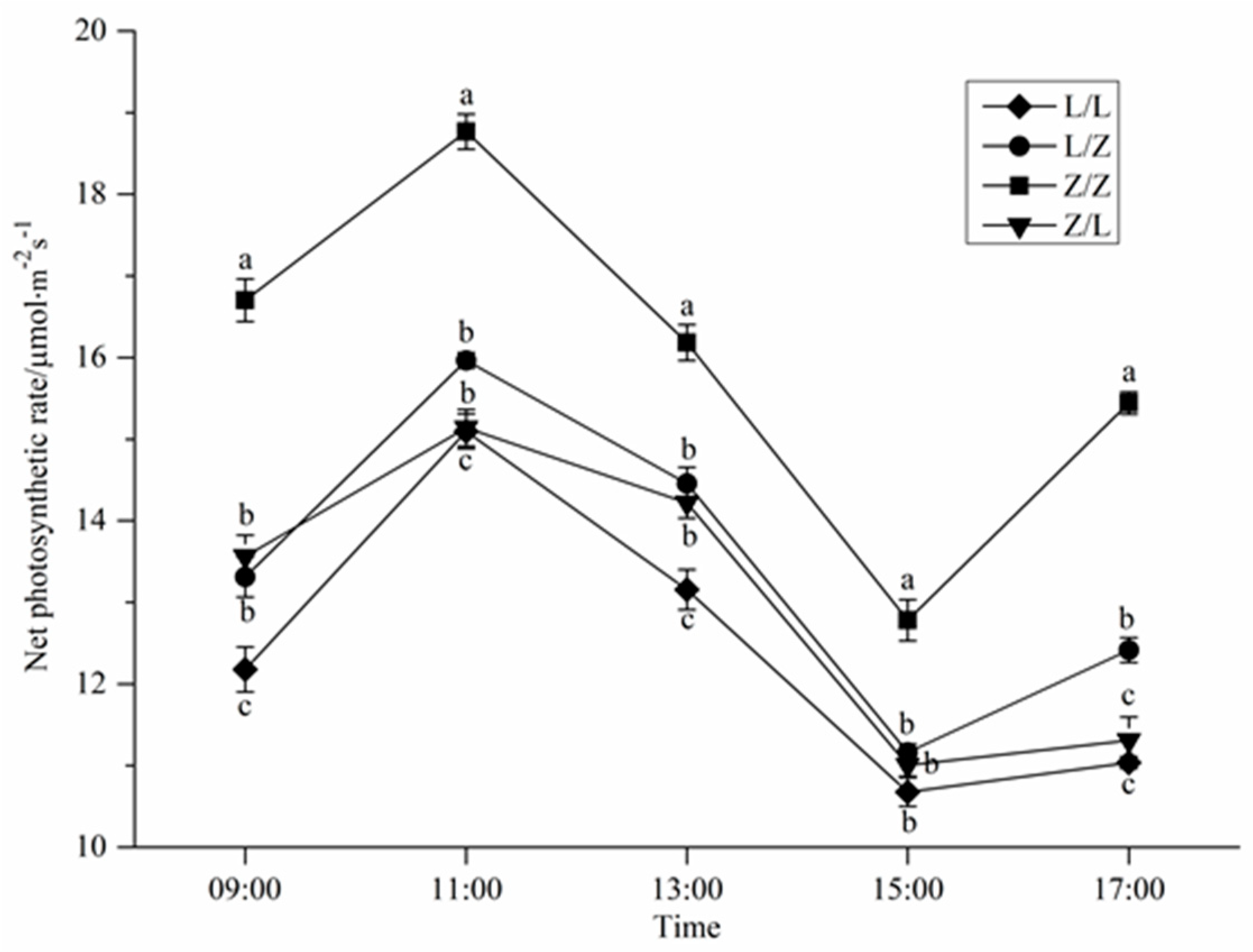
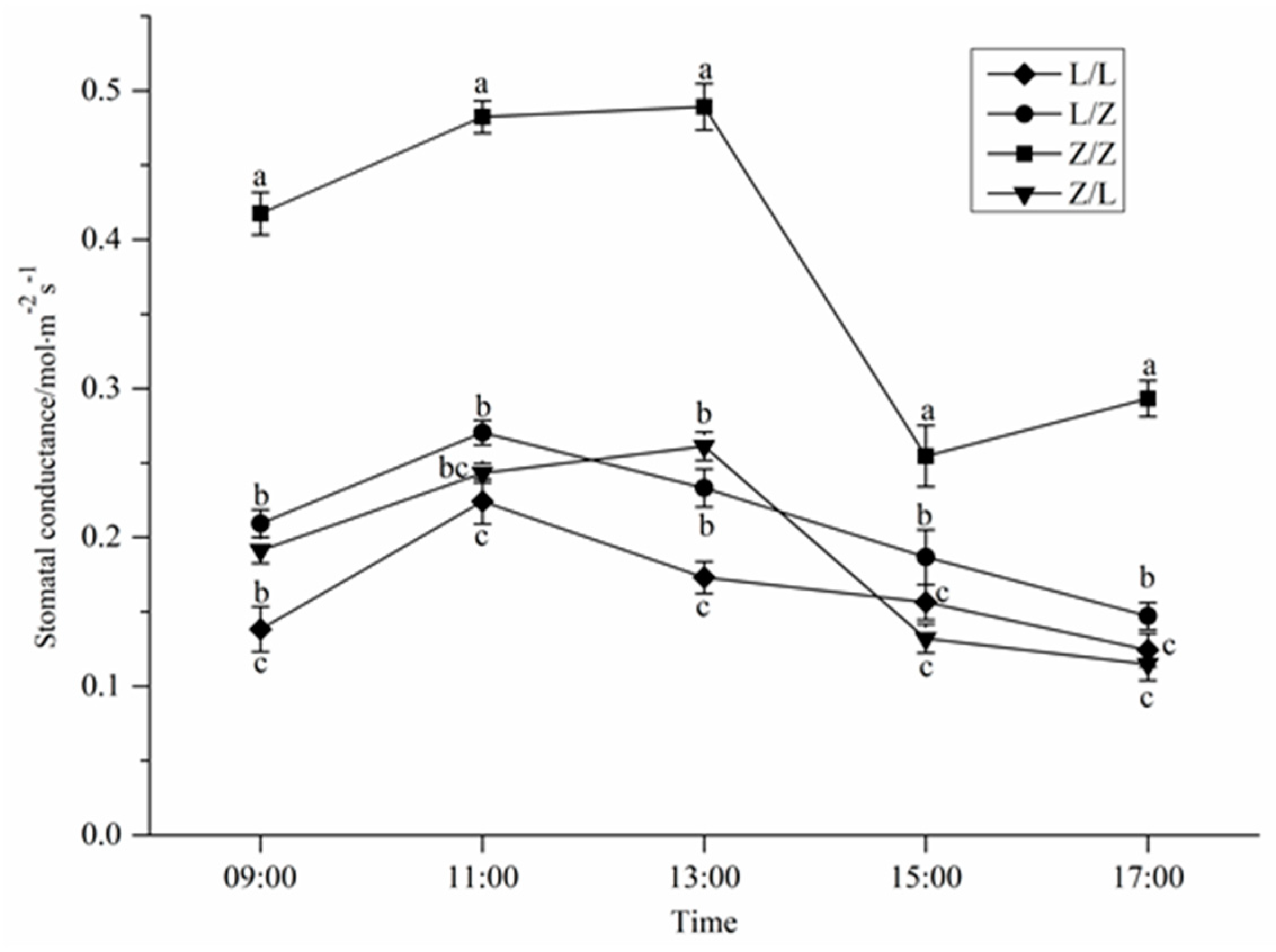
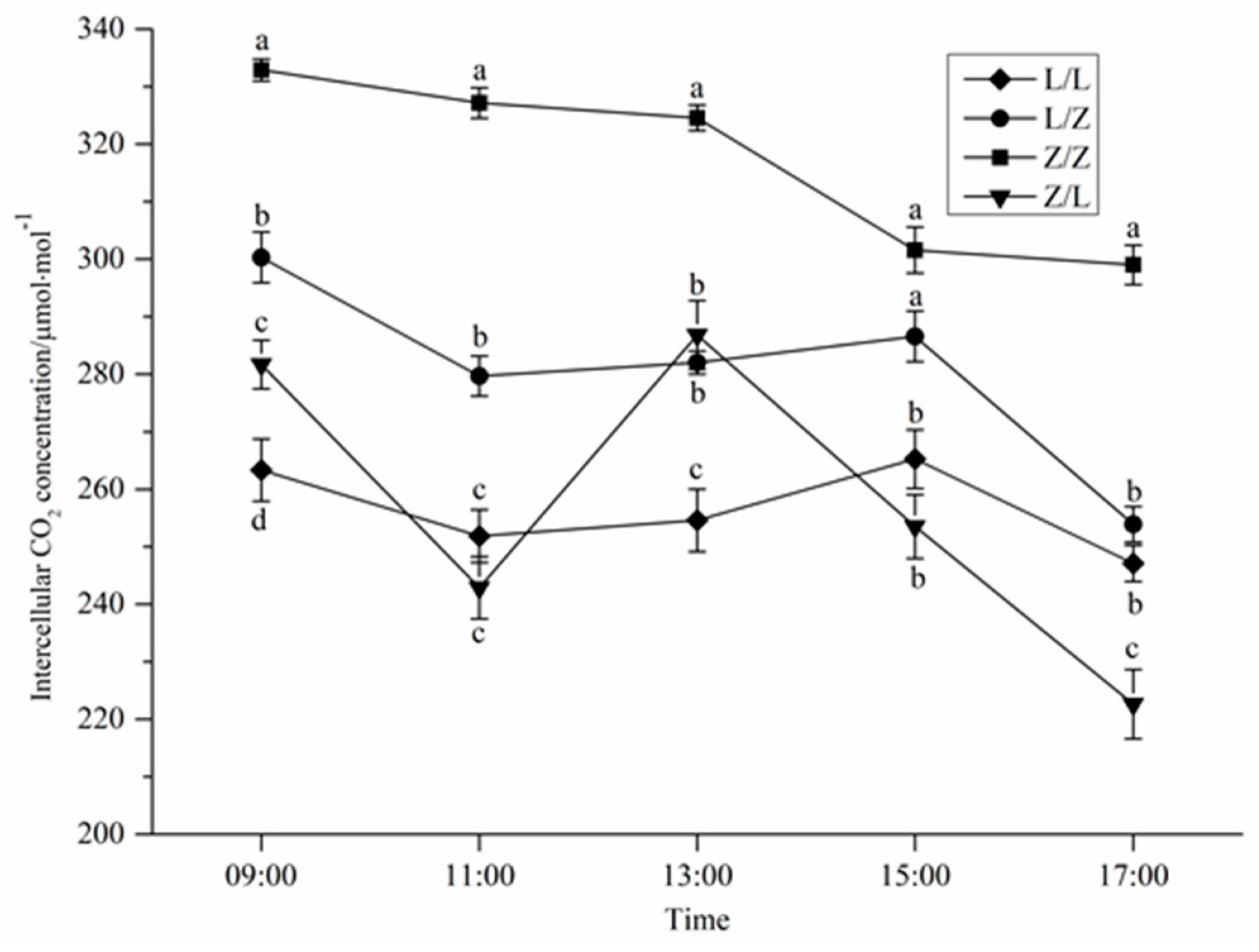
3.2. Photosynthetic Characteristics of Different Grafting Combinations
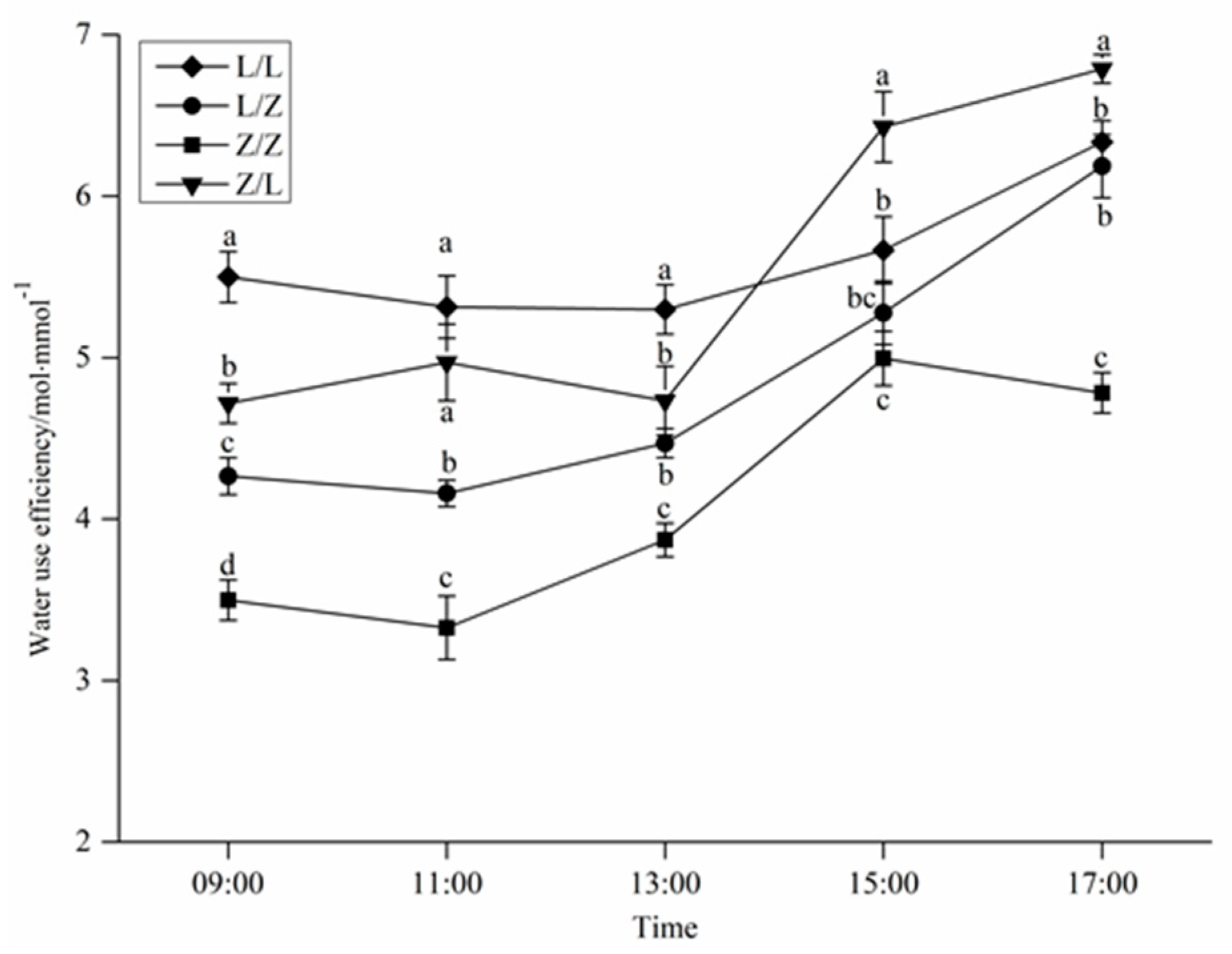
3.3. Water Use Efficiency of Different Grafting Combinations
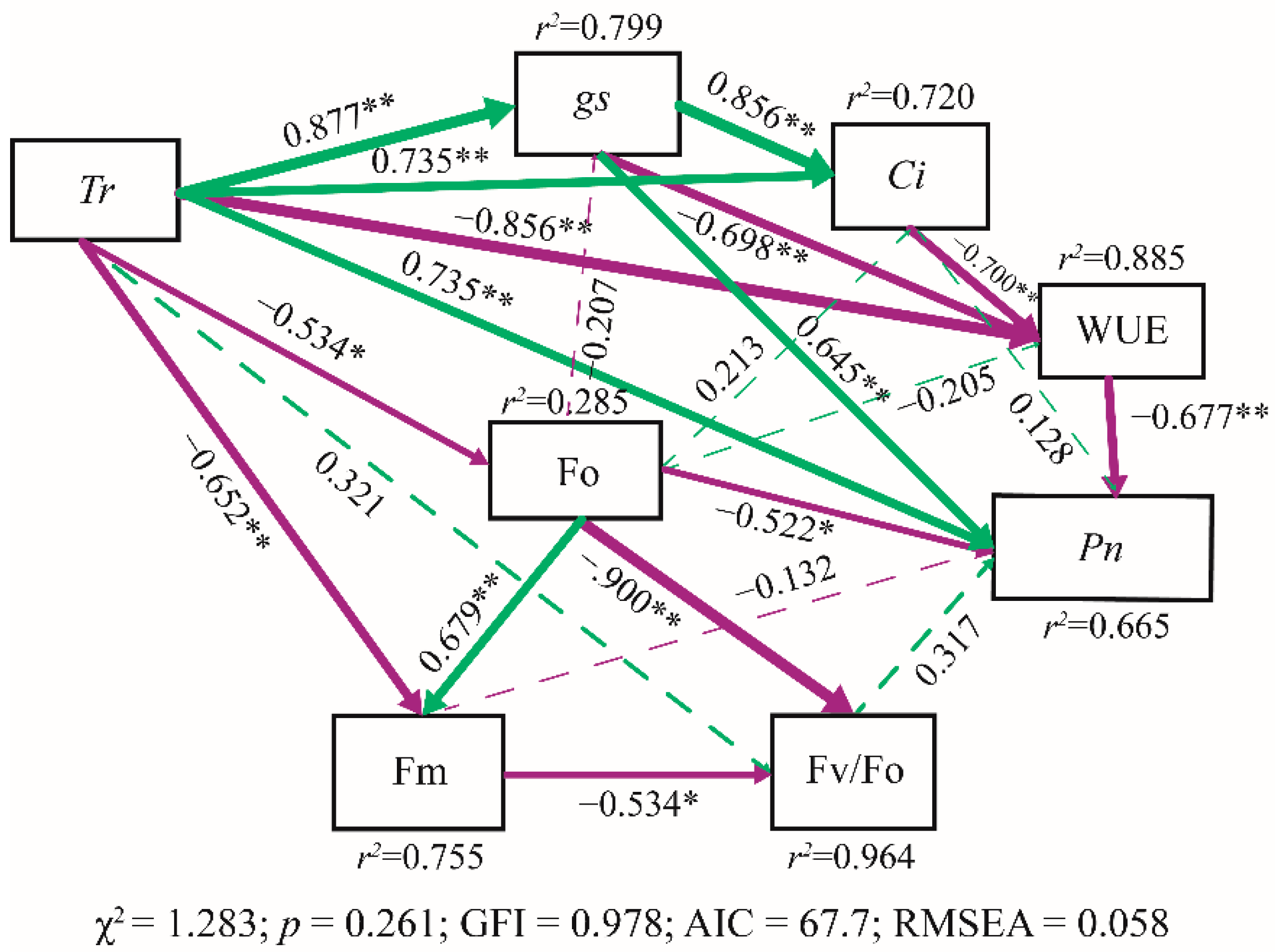
3.4. Influential Factors on Net Photosynthesis Rate of Grafted Seedling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pei, D.; Lu, X.-Z. (Eds.) Walnut Germplasm Resources in China; China Forestry Publishing House: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Zhang, W.-B.; Xu, L.-L.; Li, N.; Yan, P.-C.; Jiang, X.-H.; Woeste, K.E.; Lin, K.; Renner, S.S.; Zhang, D.-Y.; Bai, W.-N. Phylogenomics reveals an ancient hybrid origin of the Persian walnut. Mol. Biol. Evol. 2019, 36, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-S.; Wang, S.-H.; Chen, Q. Breeding of new walnut cultivars including ‘Liaoning 1’. China Fruits 1990, 2, 1–4. (In Chinese) [Google Scholar]
- Zhao, B.-S.; Liu, F.; Gong, Y.-H.; Li, D.-S.; Chang, Y.-H.; Wang, Y.-F. ‘Liaoning 1’ walnut cultivar. Hortscience 2020, 55, 392–394. [Google Scholar] [CrossRef] [Green Version]
- Hemery, G.E.; Russell, K. Advances in walnut breeding and culture in the United Kingdom. Acta Hortic. 2005, 705, 95–101. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhao, Y.-G.; Sun, M.-G.; Zhang, X.-S.; Yang, K.-Q.; Wang, J.-Y.; Guo, Q.-R. Studies on floral variation in walnut (Juglans regia L.). Acta Hortic. Sin. 2009, 36, 21–26. [Google Scholar]
- Wu, G.-L.; Meng, H.-J.; Hao, Y.-Y.; Liu, Q.-L.; Wang, D.; Tian, J.-B. Thirty years of breeding walnut in China, in: VI International Walnut Symposium. Acta Hortic. 2009, 861, 109–118. [Google Scholar]
- Wang, G.-A.; Zhang, Q.; Huang, M.M.; Yakup, A. The breeding of six Xinjiang dwarf walnut cultivars. Acta Hortic. 2014, 1050, 151–160. [Google Scholar] [CrossRef]
- Song, X.-B.; Zhang, J.-P.; Xu, H.-M.; Xu, H.-Z.; Pei, D. Cultivation mode of walnut in hilly area for nut and timber uses. J. Beijing For. Univ. 2016, 38, 60–66. [Google Scholar]
- Vahdati, K.; Mohseniazar, M. Precocious genotypes of walnut: A suitable material for breeding and higher density orchards. Acta Hortic. 2016, 1139, 101–105. [Google Scholar] [CrossRef]
- Chen, L.-N.; Dong, R.-Q.; Ma, Q.-G.; Zhang, Y.; Xu, S.-Z.; Ning, D.-L.; Chen, Q.; Pei, D. Precocious genotypes and homozygous tendency generated by self-pollination in walnut. BMC Plant Biol. 2018, 18, 323. [Google Scholar] [CrossRef]
- Lawson, T.; Kramer, D.M.; Raines, C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 2012, 23, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [Green Version]
- Éva, C.; Oszvald, M.; Tamás, L. Current and possible approaches for improving photosynthetic efficiency. Plant Sci. 2019, 280, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ort, D.R.; Zhu, X.-G.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Lopez-Calcagno, P.E.; Raines, C.A.; Ort, D.R. Optimizing photorespiration for improved crop productivity. J. Integr. Plant Biol. 2018, 60, 1217–1230. [Google Scholar] [CrossRef]
- Singh, J.; Pandey, P.; James, D.; Chandrasekhar, K.; Achary, V.M.M.; Kaul, T.; Tripathy, B.C.; Reddy, M.K. Enhancing C3 photosynthesis: An outlook on feasible interventions for crop improvement. Plant Biotechnol. J. 2014, 12, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Loescher, W.H.; Tyson, R.H.; Everard, J.D.; Redgwell, R.J.; Bieleski, R.L. Mannitol synthesis in higher plants: Evidence for the role and characterization of a NADPH-dependent mannose 6-phosphate reductase. Plant Physiol. 1992, 98, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance-current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Fuentes, I.; Stegemann, S.; Golczyk, H.; Karcher, D.; Bock, R. Horizontal genome transfer as an asexual path to the formation of new species. Nature 2014, 511, 232–235. [Google Scholar] [CrossRef]
- Arzani, K.; Khoshghalb, H.; Karimzadeh, G. Scion/rootstock influence on grafting success, early performance, tree survival and efficiency of nutrient uptake of some Asian Pear (Pyrus serotina Rehd.) cultivars. Acta Hortic. 2005, 671, 477–480. [Google Scholar] [CrossRef]
- Irisarri, P.; Binczycki, P.; Errea, P.; Martens, H.J.; Pina, A. Oxidative stress associated with rootstock-scion interactions in pear/quince combinations during early stages of graft development. J. Plant Physiol. 2015, 176, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tsipouridis, C.; Thomidis, T. Effect of 14 peach rootstocks on the yield, fruit quality, mortality, girth expansion and resistance to frost damages of May Crest peach variety and their susceptibility on Phytophthora citrophthora. Sci. Hortic. 2005, 103, 421–428. [Google Scholar] [CrossRef]
- Al-Hinai, Y.K.; Roper, T.R. Rootstock effects on growth and quality of “Gala” apples. HortScience 2004, 39, 1231–1233. [Google Scholar] [CrossRef] [Green Version]
- Russo, N.L.; Robinson, T.L.; Fazio, G.; Aldwinckle, H.S. Field evaluation of 64 apple rootstocks for orchard performance and fire blight resistance. HortScience 2007, 42, 1517–1525. [Google Scholar] [CrossRef] [Green Version]
- Mademba-Sy, F.; Lemerre-Desprez, Z.; Lebegin, S. Use of flying dragon trifoliate orange as dwarfing rootstock for citrus under tropical climatic conditions. HortScience 2012, 47, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Vršič, S.; Pulko, B.; Kocsis, L. Factors influencing grafting success and compatibility of grape rootstocks. Sci. Hortic. 2015, 181, 168–173. [Google Scholar] [CrossRef]
- Chitarra, W.; Perrone, I.; Avanzato, C.G.; Minio, A.; Boccacci, P.; Santini, D.; Gilardi, G.; Siciliano, I.; Gullino, M.L.; Delledonne, M.; et al. Grapevine grafting: Scion transcript profiling and defense-related metabolites induced by rootstocks. Front. Plant Sci. 2017, 8, 654. [Google Scholar] [CrossRef]
- Buzo, T.; Mckenna, J.; Kaku, S.; Anwar, S.A.; Mckenry, M.V. VX211, A vigorous new walnut hybrid clone with nematode tolerance and a useful resistance mechanism. J. Nematol. 2009, 41, 211–216. [Google Scholar]
- Song, X.-B.; Xi, S.-K.; Zhang, J.-P.; Ma, Q.-G.; Zhou, Y.; Pei, D.; Xu, H.-Z.; Zhang, J.-W. ‘Zhong Ning Sheng’: A new distant hybrid cultivar of walnut. HortScience 2019, 12, 2257–2259. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Jiang, L.-B.; Wu, R.-L. Plant grafting: How genetic exchange promotes vascular reconnection. New Phytol. 2017, 214, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-P.; Zhang, W.-T.; Ji, F.-Y.; Qiu, J.; Song, X.-B.; Bu, D.-C.; Pan, G.; Ma, Q.-G.; Chen, J.-X.; Huang, R.-M.; et al. A high-quality walnut genome assembly reveals extensive gene expression divergences after whole-genome duplication. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2006. [Google Scholar]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Hammer, G.L.; Doherty, A.; Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Judy, L.H.; Shimon, R.; Ron, M.; Yehouda, M.; Aaron, K. Enhanced photosynthesis and growth of transgenic plants that express ictB, a gene involved in HCO3− accumulation in cyanobacteria. Plant Biotechnol. J. 2003, 1, 43–50. [Google Scholar]
- Kubis, A.; Bar-Even, A. Synthetic biology approaches for improving photosynthesis. J. Exp. Bot. 2019, 70, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Achim, G.H.; Botu, I. Results in walnut propagation by using different methods, in: IV International Walnut Symposium. Acta Hortic. 1999, 544, 503–509. [Google Scholar]
- Grandgirard, J.; Poinsot, D.; Krespi, L.; Nénon, J.P.; Cortesero, A.M. Costs of secondary parasitism in the facultative hyperparasitoid Pachycrepoideus dubius: Does host size matter? Entomol. Exp. Appl. 2002, 103, 239–248. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-L.; Guo, L.-S.; Tian, Y.-L. Photosynthetic rates and chlorophyll fluorescence of Nitraria tangutorum at different leaf water potentials. Acta Bot. Boreali Occident. Sin. 2005, 25, 2226–2233. [Google Scholar]
- Dehghan, B.; Vahdati, K.; Rezaee, R.; Hassani, D. Persian walnut (Juglans regia L.) grafting as influenced by different bench grafting methods and scion cultivars. J. Appl. Hortic. 2009, 11, 56–58. [Google Scholar] [CrossRef]
- Xu, R.-T.; Ding, P.-H. Theory and practice of walnut grafting, in: I International Symposium on Walnut Production. Acta Hortic. 1989, 284, 69–90. [Google Scholar]
- Rezaee, R.; Vahdati, K.; Grigoorian, V.; Valizadeh, M. Walnut grafting success and bleeding rate as affected by different grafting methods and seedling vigour. J. Hortic. Sci. Biotechnol. 2008, 83, 94–99. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munjonji, L.; Ayisi, K.K.; Boeckx, P.; Haesaert, G. Stomatal behavior of cowpea genotypes grown under varying moisture levels. Sustainability 2018, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, R.E.; Chen, M. Spectral expansion and antenna reduction can enhance photosynthesis for energy production. Curr. Opin. Chem. Biol. 2013, 17, 457–461. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soni, D.K.; Singh, R.; Dwivedi, U.N.; Pathre, U.V.; Nath, P.; Sane, A.P. SlERF36, an EAR-motif-containing ERF gene from tomato, alters stomatal density and modulates photosynthesis and growth. J. Exp. Bot. 2013, 64, 3237–3247. [Google Scholar] [CrossRef] [Green Version]
- Tinklin, R.; Weatherley, P.E. On the relationship between transpiration rate and leaf water potential. New Phytol. 1966, 65, 509–517. [Google Scholar] [CrossRef]
- Ouyang, W.J.; Struik, P.C.; Yin, X.-Y.; Yang, J.-C. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [Green Version]
- Bauerle, W.L.; Bowden, J.D.; Wang, G.G. The influence of temperature on within-canopy acclimation and variation in leaf photosynthesis: Spatial acclimation to microclimate gradients among climatically divergent Acer rubrum L. genotypes. J. Exp. Bot. 2007, 58, 3285–3298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, M.L.S.; Erismann, N.M.; Machado, E.C.; Ribeiro, R.V. Diurnal and seasonal variation in photosynthesis of peach palms grown under subtropical conditions. Photosynthetica 2010, 48, 421–429. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Wang, S.-Q.; Chi, Y.-G.; Chen, J.-H. Leaf temperature and vapour pressure deficit (VPD) driving stomatal conductance and biochemical processes of leaf photosynthetic rate in a subtropical evergreen coniferous plantation. Sustainability 2018, 10, 4063. [Google Scholar] [CrossRef] [Green Version]
- Germon, A.; Cardinael, R.; Prieto, I.; Mao, Z.; Kim, J.; Stokes, A.; Dupraz, C.; Laclau, J.P.; Jourdan, C. Unexpected phenology and lifespan of shallow and deep fine roots of walnut trees grown in a silvoarable Mediterranean agroforestry system. Plant Soil 2016, 401, 409–426. [Google Scholar] [CrossRef]
- Mohamed, A.; Stokes, A.; Mao, Z.; Jourdan, C.; Sabatier, S.; Pailler, F.; Fourtier, S.; Dufour, L.; Monnier, Y. Linking above- and belowground phenology of hybrid walnut growing along a climatic gradient in temperate agroforestry systems. Plant Soil 2018, 424, 103–122. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M. Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant 2002, 116, 563–572. [Google Scholar] [CrossRef]
- Peng, Y.F.; Li, C.J.; Fritschi, F.B. Diurnal dynamics of maize leaf photosynthesis and carbohydrate concentrations in response to differential N availability. Environ. Exp. Bot. 2014, 99, 18–27. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Field, T.S. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytol. 2009, 183, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.D.; Berlyn, G.P. Spectral reflectance and photosynthetic properties of Betula papyrifera (Betulaceae) leaves along an elevational gradient on Mt. Mansfield, Vermont, USA. Am. J. Bot. 2002, 89, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Wen, T.; Tang, Y.-P.; Sun, X.; Xia, C. Effect of shading on photosynthetic and chlorophyll fluorescence characteristics of soybean. Acta Pratacult. Sin. 2014, 23, 198–206. [Google Scholar]

| Grafting Combination | ||||
|---|---|---|---|---|
| L/L | L/Z | Z/Z | Z/L | |
| Healed | good | good | good | good |
| Survival rate (%) | 95 ± 2.1a | 88 ± 3.8bc | 90 ± 1.6ab | 86 ± 2.4c |
| Overwintering survival rate (%) | 100 | 100 | 100 | 100 |
| New shoots’ lengths (cm) | 63.37 ± 1.1c | 67.62 ± 2.2b | 76.87 ± 2.7a | 69.16 ± 1.5b |
| Branching numbers (twig) | 11 ± 2b | 12 ± 2b | 16 ± 2a | 15 ± 2a |
| Fo | 14,531a | 14,792a | 12,589c | 13,158b |
| Fm | 67,061a | 67,086a | 62,900b | 65,615a |
| Fv/Fm | 0.791a | 0.799a | 0.800a | 0.801a |
| Fv/Fo | 3.615b | 3.535b | 3.997a | 4.039a |
| NPQ | 0.973a | 1.018a | 1.053a | 0.900a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Zhang, J.; Wu, Y.; Huang, R.; Chang, Y.; Lei, X.; Song, X.; Pei, D. Possibility of Increasing the Growth and Photosynthetic Properties of Precocious Walnut by Grafting. Sustainability 2020, 12, 5178. https://doi.org/10.3390/su12125178
Bai Y, Zhang J, Wu Y, Huang R, Chang Y, Lei X, Song X, Pei D. Possibility of Increasing the Growth and Photosynthetic Properties of Precocious Walnut by Grafting. Sustainability. 2020; 12(12):5178. https://doi.org/10.3390/su12125178
Chicago/Turabian StyleBai, Yongchao, Junpei Zhang, Yue Wu, Ruimin Huang, Yingying Chang, Xiashuo Lei, Xiaobo Song, and Dong Pei. 2020. "Possibility of Increasing the Growth and Photosynthetic Properties of Precocious Walnut by Grafting" Sustainability 12, no. 12: 5178. https://doi.org/10.3390/su12125178





