Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts
Abstract
:1. Introduction
2. Methodology for Strain Improvement
2.1. Use of Random Mutagenesis
2.2. Use of Adaptive Lab Evolution
2.3. Screening Methods for Selecting Promising Mutants
3. Strain Improvement Results
3.1. Use of Random Mutagenesis
3.1.1. Microalga Mutants
3.1.2. Yeast Mutants
3.2. Use of Adaptive Laboratory Evolution
3.2.1. Microalga Evolved Strains
3.2.2. Yeast Evolved Strains
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| ACCase | Acetyl-CoA Carboxylase |
| ACH | Aconitase |
| ACP | Acyl Carrier Protein |
| AGPase | ADP-Glucose Pyrophosphorylase |
| ALE | Adaptive Laboratory Evolution |
| APX | Ascorbate Peroxidase |
| ARTP | Atmospheric and Room Temperature Plasma |
| AT | Acetyltransferase |
| ATP | Adenosine Triphosphate |
| CAM | Crassulacean Acid Metabolism |
| CAT | Catalase |
| CCM | Carbon Concentration Mechanism |
| CDS | Coding Sequences |
| CS | Citrate Synthase |
| DAG | Diacylglycerol |
| DCW | Dry Cell Weight |
| DES | Diethyl Sulphate |
| DGAT | Diacylglycerol Acyltransferase |
| DHA | Docosahexaenoic Acid |
| DNA | Deoxyribonucleic Acid |
| EMS | Ethyl Methane Sulfonate |
| ER | Enoyl ACP Reductase |
| FACS | Fluorescence-Activated Cell Sorting |
| FADH2 | Flavin Adenine Dinucleotide |
| FAMEs | Fatty Acid Methyl Esters |
| FAS | Fatty Acid Synthase |
| FAT | Fatty acyl-ACP Thioesterase |
| FUM | Fumarase |
| G-3-P | Glycerol-3-Phosphate |
| GADPH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GHG | Green House Gases |
| GPAT | Glycerol Phosphate Acyl Transferase |
| HD | 3-Hyroxyacyl ACP Dehydrase |
| HMF | 5-Hydroxy Methyl Furfural |
| HMP | Hexose Monophosphate Pathway |
| IDH | Isocitrate Dehydrogenase |
| KAR | 3-Ketoacyl ACP Reductase |
| KAS | β- Keto ACP Synthase |
| LHC | Light Harvesting Complex |
| LPAAT | Lyso-Phosphatidic Acid Acyltransferase |
| LPAT | Lyso-Phosphatidylcholine Acyltransferase |
| MDH | Malate Dehydrogenase |
| ME | Malic Enzyme |
| MGDG | Mono Galactosyl Diacyl Glycerol |
| MMS | Methyl Methane Sulfonate |
| MNNG | Methyl Nitro Nitroso Guanidine |
| MPT | Malonyl Transacylase |
| NADH | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NCBI | National Centre for Biotechnology Information |
| NPQ | Non-Photochemical Quenching |
| NR | Nitrate Reductase |
| NTG | N-Methyl-N-Nitro-N-Nitrosoguanidine |
| OGD | Oxoglutarate Dehydrogenase |
| PBR | Photo Bioreactor |
| PDAT | Phospholipid Diacylglycerol Acyl Transferase |
| PDC | Pyruvate Dehydrogenase Complex |
| PKS | Polyunsaturated Fatty Acid Synthase Pathway |
| PPi | Pyrophosphate |
| PPP | Pentose Phosphate Pathway |
| PPT | Phosphopantetheinyl Transferase |
| PSI | Photosystem I |
| PSII | Photosystem II |
| PUFA | Polyunsaturated Fatty acid |
| RF | Radio Frequency |
| ROS | Reactive Oxygen Species |
| RuBisCo | Ribulose-1,5-Bisphosphate Carboxylase |
| SAD | Stearoyl-ACP Desaturase |
| SBH | Sugarcane Bagasse Hydrolysate |
| SCD1 | Stearoyl-CoA Desaturase |
| SDH | Succinyl-CoA Dehydrogenase |
| Sis | Siroheme Synthase |
| SNPs | Single Nucleotide Polymorphisms |
| SOD | Super Oxide Dismutase |
| SP | Starch Phosphorylase |
| TAG | Triacylglycerol |
| TCA | Tricarboxylic Acid |
| UV | Ultraviolet |
| WT | Wild Type |
References
- Ratledge, C. Single Cell Oils for the 21st Century. In Single Cell Oils, 2nd ed.; AOCS Press: Urbana, IL, USA, 2010; pp. 3–26. ISBN 9781893997738. [Google Scholar]
- Ratledge, C. Single cell oils—Have they a biotechnological future? Trends. Biotechnol. 1993, 11, 278–284. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid. Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Beopoulos, A.; Chardot, T.; Nicaud, J.-M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Archanaa, S.; Jose, S.; Mukherjee, A.; Suraishkumar, G.K. Sustainable Diesel Feedstock: A Comparison of Oleaginous Bacterial and Microalgal Model Systems. Bioenerg. Res. 2019, 12, 205–216. [Google Scholar] [CrossRef]
- Arora, N.; Pienkos, P.T.; Pruthi, V.; Poluri, K.M.; Guarnieri, M.T. Leveraging algal omics to reveal potential targets for augmenting TAG accumulation. Biotechnol. Adv. 2018, 36, 1274–1292. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.L.; Maroto, F.G. Plants as ‘chemical factories’ for the production of polyunsaturated fatty acids. Biotechnol. Adv. 2000, 18, 481–497. [Google Scholar] [CrossRef]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Arora, N.; Laurens, L.M.L.; Sweeney, N.; Pruthi, V.; Poluri, K.M.; Pienkos, P.T. Elucidating the unique physiological responses of halotolerant Scenedesmus sp. cultivated in sea water for biofuel production. Algal Res. 2019, 37, 260–268. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Ho, S.-H.; Lee, D.-J.; Chang, J.-S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Gujjala, L.K.S.; Kumar, S.P.J.; Talukdar, B.; Dash, A.; Kumar, S.; Sherpa, K.C.; Banerjee, R. Biodiesel from oleaginous microbes: Opportunities and challenges. Biofuels 2019, 10, 45–59. [Google Scholar] [CrossRef]
- Wang, S.; Sirbu, D.; Thomsen, L.; Kuhnert, N.; Ullrich, M.S.; Thomsen, C. Comparative lipidomic studies of Scenedesmus sp. (Chlorophyceae) and Cylindrotheca closterium (Bacillariophyceae) reveal their differences in lipid production under nitrogen starvation. J. Phycol. 2019, 55, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, R.V.; Mohan, S.V. Obscure yet Promising Oleaginous Yeasts for Fuel and Chemical Production. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Yen, H.-W.; Yang, Y.-C.; Yu, Y.-H. Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2012, 114, 453–456. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Higashiyama, K.; Fujikawa, S.; Park, E.Y.; Shimizu, S. Production of Arachidonic Acid by Mortierella Fungi. Biotechnol. Bioprocess Eng. 2002, 7, 252–262. [Google Scholar] [CrossRef]
- Jareonkitmongkol, S.; Shimizu, S.; Yamada, H. Production of an Eicosapentaenoic Acid-Containing Oil by a A12 Desaturase-Defective Mutant of Mortierella alpina 1S-4. JAOCS 1993, 70, 119–123. [Google Scholar] [CrossRef]
- Jareonkitmongkol, S.; Kawashima, H.; Shimizu, S.; Yamada, H. Production of Dihomo--y-Linolenic Acid by a A5-Desaturase- Defective Mutant of Mortierella alpina 1S-4. Appl. Environ. Microbiol. 1992, 2196–2200. [Google Scholar] [CrossRef] [Green Version]
- Jareonkitmongkol, S.; Sakuradani, E.; Shimizu, S. Isolation and Characterization of a ∆9-Desaturation- Defective Mutant of an Arachidonic Acid-Producing Fungus, Mortierella alpina 1S-4. JAOCS 2002, 79, 1021–1026. [Google Scholar] [CrossRef]
- Sakuradani, E.; Kobayashi, M.; Shimizu, S. D6-Fatty acid desaturase from an arachidonic acid-producing Mortierella fungus Gene cloning and its heterologous expression in a fungus, Aspergillus. Gene 1999, 9, 445–453. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total. Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Iribarren, D.; Dufour, J. How do methodological choices affect the carbon footprint of microalgal biodiesel? A harmonised life cycle assessment. J. Clean. Prod. 2019, 207, 560–568. [Google Scholar] [CrossRef]
- Kamineni, A.; Shaw, J. Engineering triacylglycerol production from sugars in oleaginous yeasts. Curr. Opin. Biotechnol. 2020, 62, 239–247. [Google Scholar] [CrossRef]
- Fajardo, C.; Donato, M.; Carrasco, R.; Martínez-Rodríguez, G.; Mancera, J.M.; Fernández-Acero, F.J. Advances and challenges in genetic engineering of microalgae. Rev. Aquacult. 2020, 12, 365–381. [Google Scholar] [CrossRef]
- Mahajan, D.; Sengupta, S.; Sen, S. Strategies to improve microbial lipid production: Optimization techniques. Biocatal. Agric. Biotechnol. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Saxena, S. Strategies of Strain Improvement of Industrial Microbes. In Applied Microbiology; Springer: New Delhi, India, 2015; pp. 155–171. ISBN 978-81-322-2258-3. [Google Scholar]
- Doan, T.T.Y.; Obbard, J.P. Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Algal Res. 2012, 1, 17–21. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J.C. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, M.; Selvaraj, B.; Palabhanvi, B.; Kumar, V.; Das, D. Enhanced lipid content in Chlorella sp. FC2 IITG via high energy irradiation mutagenesis. Korean J. Chem. Eng. 2019, 36, 63–70. [Google Scholar] [CrossRef]
- Hu, W.; Li, W.; Chen, J. Recent advances of microbial breeding via heavy-ion mutagenesis at IMP. Lett. Appl. Microbiol. 2017, 65, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Sun, Z.; Cui, G.; Song, X.; Cui, Q. A new strategy for strain improvement of Aurantiochytrium sp. based on heavy-ions mutagenesis and synergistic effects of cold stress and inhibitors of enoyl-ACP reductase. Enzyme Microb. Technol. 2016, 93–94, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Jin, W.; Zhou, X.; Wang, M.; Chen, C.; Tu, R.; Han, S.; He, Z.; Li, S. Nitrogen ion beam implantation for enhanced lipid accumulation of Scenedesmus obliquus in municipal wastewater. Biomass Bioenerg. 2020, 134, 1–7. [Google Scholar] [CrossRef]
- Khazaei, H.; Mäkelä, P.S.A.; Stoddard, F.L. Ion beam irradiation mutagenesis in rye (Secale cereale L.), linseed (Linum usitatissimum L.) and faba bean (Vicia faba L.). AFSci 2018, 27, 146–155. [Google Scholar] [CrossRef]
- Beyaz, R.; Yildiz, M. The Use of Gamma Irradiation in Plant Mutation Breeding. In Plant Engineering; Jurić, S., Ed.; InTech: London, UK, 2017; pp. 33–43. ISBN 978-953-51-3607-1. [Google Scholar]
- Cao, S.; Zhou, X.; Jin, W.; Wang, F.; Tu, R.; Han, S.; Chen, H.; Chen, C.; Xie, G.-J.; Ma, F. Improving of lipid productivity of the oleaginous microalgae Chlorella pyrenoidosa via atmospheric and room temperature plasma (ARTP). Bioresour. Technol. 2017, 244, 1400–1406. [Google Scholar] [CrossRef]
- Fang, M.; Jin, L.; Zhang, C.; Tan, Y.; Jiang, P.; Ge, N.; Heping, L.; Xing, X. Rapid Mutation of Spirulina platensis by a New Mutagenesis System of Atmospheric and Room Temperature Plasmas (ARTP) and Generation of a Mutant Library with Diverse Phenotypes. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.-F.; Li, H.-P.; Wang, L.-Y.; Zhang, C.; Xing, X.-H.; Bao, C.-Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef]
- Singer, B.; Kusmierek, J.T. Chemical mutagenesis. Ann. Rev. Biochem. 1982, 52, 655–693. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Lin, T.-H.; Yang, Y.-C.; Zhang, W.-X.; Lai, J.-T.; Wu, H.-T.; Chang, J.-S.; Lin, C.-S. Ability of an alkali-tolerant mutant strain of the microalga Chlorella sp. AT1 to capture carbon dioxide for increasing carbon dioxide utilization efficiency. Bioresour. Technol. 2017, 244, 243–251. [Google Scholar] [CrossRef]
- Sarayloo, E.; Tardu, M.; Unlu, Y.S.; Simsek, S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Understanding lipid metabolism in high-lipid-producing Chlorella vulgaris mutants at the genome-wide level. Algal Res. 2017, 28, 244–252. [Google Scholar] [CrossRef]
- Dent, R.M.; Sharifi, M.N.; Malnoë, A.; Haglund, C.; Calderon, R.H.; Wakao, S.; Niyogi, K.K. Large-scale insertional mutagenesis of Chlamydomonas supports phylogenomic functional prediction of photosynthetic genes and analysis of classical acetate-requiring mutants. Plant J. 2015, 82, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.J.; Kang, N.K.; Jeon, S.; Hur, D.H.; Lee, E.M.; Lee, D.Y.; Jeong, B.; Chang, Y.K.; Jeong, K.J. Development and characterization of a Nannochloropsis mutant with simultaneously enhanced growth and lipid production. Biotechnol. Biofuels 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, V.A.; Bezdan, D.; Zengler, K. Adaptive laboratory evolution—harnessing the power of biology for metabolic engineering. Curr. Opin. Biotechnol. 2011, 22, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, Y.; Cheng, D.; Gao, J.; Kong, L.; Zhao, Q.; Wei, W.; Sun, Y. Exploring stress tolerance mechanism of evolved freshwater strain Chlorella sp. S30 under 30 g/L salt. Bioresour. Technol. 2018, 250, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution—Principles and applications for biotechnology. Microb. Cell. Fact. 2013, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Li, X.; Yuan, Y.; Yang, C.; Tang, T.; Zhao, Q.; Sun, Y. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Sci. Total. Environ. 2019, 650, 2931–2938. [Google Scholar] [CrossRef]
- LaCroix, R.A.; Palsson, B.O.; Feist, A.M. A Model for designing adaptive laboratory evolution experiments. Appl. Environ. Microbiol. 2017, 83, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J.; Reyes, L.H.; Kao, K.C. Adaptive Laboratory Evolution for Strain Engineering. In Systems Metabolic Engineering; Alper, H.S., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 985, pp. 211–222. ISBN 978-1-62703-298-8. [Google Scholar]
- Yu, Q.; Li, Y.; Wu, B.; Hu, W.; He, M.; Hu, G. Novel mutagenesis and screening technologies for food microorganisms: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 1517–1531. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.-P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Suzuki, H.; Takeuchi, T.; Kazama, Y.; Mitra, S.; Abe, T.; Goda, K.; Suzuki, K.; Iwata, O. Efficient selective breeding of live oil-rich Euglena gracilis with fluorescence-activated cell sorting. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, H.; Kobayashi, S.; Ebina, S.; Abe, S.; Ara, S.; Shida, Y.; Ogasawara, W.; Yaoi, K.; Araki, H.; Takaku, H. Highly selective isolation and characterization of Lipomyces starkeyi mutants with increased production of triacylglycerol. Appl. Microbiol. Biotechnol. 2019, 103, 6297–6308. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.J.; Choi, H.I.; Lee, J.S.; Hong, M.E.; Sim, S.J. Screening of oleaginous algal strains from Chlamydomonas reinhardtii mutant libraries via density gradient centrifugation. Biotechnol. Bioeng. 2019, 116, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, L.; Wang, S.; Zheng, R.; Jin, S.; Huang, S.; Huang, Y. Toxicity of 40 Herbicides to the Green Alga Chlorella vulgaris. Ecotoxcol. Environ. Saf. 2002, 51, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.D.; Gronwald, J.W.; Somers, D.A.; Connelly, J.A.; Gengenbach, B.G.; Wyse, D.L. Inhibition of plant acetyl-coenzyme a carboxylase by the herbicides sethoxydim and haloxyfop. Biochem. Biophy. Res. Commun. 1987, 148, 1039–1044. [Google Scholar] [CrossRef]
- Xia, X.; Tang, W.; He, S.; Kang, J.; Ma, H.; Li, J. Mechanism of metamifop inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase in Echinochloa crus-galli. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lomakin, I.B.; Xiong, Y.; Steitz, T.A. The crystal structure of yeast fatty acid synthase, a cellular machine with eight active sites working together. Cell 2007, 129, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Beld, J.; Lee, D.J.; Burkart, M.D. Fatty acid biosynthesis revisited: Structure elucidation and metabolic engineering. Mol. BioSyst. 2015, 11, 38–59. [Google Scholar] [CrossRef] [Green Version]
- Baba, M.; Shiraiw, Y. Biosynthesis of Lipids and Hydrocarbons in Algae. In Photosynthesis; Dubinsky, Z., Ed.; InTech: London, UK, 2013; pp. 331–356. ISBN 978-953-51-1161-0. [Google Scholar]
- Tapia, V.E.; Anschau, A.; Coradini, A.L.T.; Franco, T.; Deckmann, A. Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express 2012, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Liu, J.; Sun, P.; Ma, X.; Jiang, Y.; Chen, F. Sesamol Enhances Cell Growth and the Biosynthesis and Accumulation of Docosahexaenoic Acid in the Microalga Crypthecodinium cohnii. J. Agric. Food Chem. 2015, 63, 5640–5645. [Google Scholar] [CrossRef]
- Xue, J.; Wang, L.; Zhang, L.; Balamurugan, S.; Li, D.-W.; Zeng, H.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. The pivotal role of malic enzyme in enhancing oil accumulation in green microalga Chlorella pyrenoidosa. Microb. Cell Fact. 2016, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pascuala, A.; de Lorenzo, V.; Nikel, P.I. Refactoring the Embden–Meyerhof–Parnas pathway as a whole of portable glucobricks for implantation of glycolytic modules in gram-negative bacteria. ACS Synth. Biol. 2017, 6, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Huang, H.; Ren, L.; Ji, X.; Zhu, J.; Jin, L. Increase of docosahexaenoic acid production by Schizochytrium sp. through mutagenesis and enzyme assay. Appl. Biochem. Biotechnol. 2010, 162, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Nie, X.; Liu, W.; Snoeijs, P.; Guan, C.; Tsui, M.T.K. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxcol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Fujita, Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycological. Res. 2006, 54, 208–219. [Google Scholar] [CrossRef]
- Sarayloo, E.; Simsek, S.; Unlu, Y.S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour. Technol. 2018, 250, 764–769. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yang, G.; Han, J.; Thomsen, L. Kehou Pan Breeding 3 elite strains of Nannochloropsis oceanica by nitrosoguanidine mutagenesis and robust screening. Algal Res. 2016, 19, 104–108. [Google Scholar] [CrossRef]
- Sun, X.; Li, P.; Liu, X.; Wang, X.; Liu, Y.; Turaib, A.; Cheng, Z. Strategies for enhanced lipid production of Desmodesmus sp. mutated by atmospheric and room temperature plasma with a new efficient screening method. J. Clean. Prod. 2020, 250, 1–11. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, Y.; Feng, J.; Sun, J.; Zhou, J.; Cen, K. Mutate Chlorella sp. by nuclear irradiation to fix high concentrations of CO2. Bioresour. Technol. 2013, 136, 496–501. [Google Scholar] [CrossRef]
- Shin, W.-S.; Lee, B.; Jeong, B.; Chang, Y.K.; Kwon, J.-H. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J Appl. Phycol. 2016, 28, 3193–3202. [Google Scholar] [CrossRef]
- Kato, Y.; Ho, S.-H.; Vavricka, C.J.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Evolutionary engineering of salt-resistant Chlamydomonas sp. strains reveals salinity stress-activated starch-to-lipid biosynthesis switching. Bioresour. Technol. 2017, 245, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Choi, G.-G.; Choi, Y.-E.; Sung, M.; Park, M.S.; Yang, J.-W. Enhancement of lipid productivity by ethyl methane sulfonate-mediated random mutagenesis and proteomic analysis in Chlamydomonas reinhardtii. Korean J. Chem. Eng. 2014, 31, 1036–1042. [Google Scholar] [CrossRef]
- Xie, B.; Stessman, D.; Hart, J.H.; Dong, H.; Wang, Y.; Wright, D.A.; Nikolau, B.J.; Spalding, M.H.; Halverson, L.J. High-throughput fluorescence-activated cell sorting for lipid hyperaccumulating Chlamydomonas reinhardtii mutants. Plant Biotechnol. J. 2014, 12, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ma, C.; Xiao, R.; Xing, D.; Ren, H.; Ren, N. The screening of microalgae mutant strain Scenedesmus sp. Z-4 with a rich lipid content obtained by 60 Co γ-ray mutation. RSC Adv. 2015, 5, 52057–52061. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, C.; Bai, J.; Fang, S.; Zhuang, X.; Yuan, Z. Mutants of Scenedesmus sp. for purifying highly concentrated cellulosic ethanol wastewater and producing biomass simultaneously. J. Appl. Phycol. 2018, 30, 969–978. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment. Bioresour. Technol. 2017, 235, 366–370. [Google Scholar] [CrossRef]
- De Jaeger, L.; Verbeek, R.E.; Draaisma, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; He, M.; Zou, S.; Fei, C.; Yan, Y.; Zheng, S.; Rajper, A.A.; Wang, C. Breeding of high biomass and lipid producing Desmodesmus sp. by Ethylmethane sulfonate-induced mutation. Bioresour. Technol. 2016, 207, 268–275. [Google Scholar] [CrossRef]
- Hu, G.; Fan, Y.; Zhang, L.; Yuan, C.; Wang, J.; Li, W.; Hu, Q.; Li, F. Enhanced lipid productivity and photosynthesis efficiency in a Desmodesmus sp. mutant induced by heavy carbon ions. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Beacham, T.A.; Macia, V.M.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following EMS and UV induced mutagenesis. Biotechnol. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.; Ryu, S.; Trinh, C.T. Exceptional solvent tolerance in Yarrowia lipolytica is enhanced by sterols. Metab. Eng. 2019, 54, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kawaroe, M.; Prartono, T.; Hwangbo, J.; Sunuddin, A.; Augustine, D.; Gustina, A.S. Effect of ethyl methane sulfonate (EMS) on cell size, fatty acid content, growth rate, and antioxidant activities of microalgae Dunaliella sp. AACL Bioflux 2015, 8, 924–932. [Google Scholar]
- Moha-León, J.D.; Pérez-Legaspi, I.A.; Ortega-Clemente, L.A.; Rubio-Franchini, I.; Ríos-Leal, E. Improving the lipid content of Nannochloropsis oculata by a mutation-selection program using UV radiation and quizalofop. J. Appl. Phycol. 2019, 31, 191–199. [Google Scholar] [CrossRef]
- Perin, G.; Bellan, A.; Segalla, A.; Meneghesso, A.; Alboresi, A.; Morosinotto, T. Generation of random mutants to improve light-use efficiency of Nannochloropsis gaditana cultures for biofuel production. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.-C.; Kao, C.-Y.; Chiu, S.-Y.; Tsai, M.-T.; Lin, C.-S. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour. Technol. 2010, 101, 2880–2883. [Google Scholar] [CrossRef]
- Sachdeva, N.; Gupta, R.P.; Mathur, A.S.; Tuli, D.K. Enhanced lipid production in thermo-tolerant mutants of Chlorella pyrenoidosa NCIM 2738. Bioresour. Technol. 2016, 221, 576–587. [Google Scholar] [CrossRef]
- Tanadul, O.; Noochanong, W.; Jirakranwong, P.; Chanprame, S. EMS-induced mutation followed by quizalofop-screening increased lipid productivity in Chlorella sp. Bioprocess Biosyst. Eng. 2018, 41, 613–619. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Sun, W.; Li, Y.; Hu, Q.; Zhou, H.; Han, D. Metabolic plasticity of the starchless mutant of Chlorella sorokiniana and mechanisms underlying its enhanced lipid production revealed by comparative metabolomics analysis. Algal Res. 2019, 42, 1–13. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Dall’Osto, L.; Szaub, J.; Scibilia, L.; Ballottari, M.; Purton, S.; Bassi, R. Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light-use efficiency in a photobioreactor. Biotechnol. Biofuels 2014, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mehtani, J.; Arora, N.; Patel, A.; Jain, P.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. Augmented lipid accumulation in ethyl methyl sulphonate mutants of oleaginous microalga for biodiesel production. Bioresour. Technol. 2017, 242, 121–127. [Google Scholar] [CrossRef]
- Dinesh Kumar, S.; Sojin, K.; Santhanam, P.; Dhanalakshmi, B.; Latha, S.; Park, M.S.; Kim, M.-K. Triggering of fatty acids on Tetraselmis sp. by ethyl methanesulfonate mutagenic treatment. Bioresour. Technol. Rep. 2018, 2, 21–28. [Google Scholar] [CrossRef]
- Thurakit, T.; Pekkoh, J. Enhancement of Biomass, Lipid and Hydrocarbon Production from Green Microalga, Botryococcus braunii AARL G037, by UV-C Induction. Chiang Mai J. Sci. 2018, 45, 2637–2651. [Google Scholar]
- Hu, C.-W.; Chuang, L.-T.; Yu, P.-C.; Chen, C.-N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pei, G.; Liu, L.; Chen, L.; Zhang, W. Metabolomic analysis and lipid accumulation in a glucose tolerant Crypthecodinium cohnii strain obtained by adaptive laboratory evolution. Bioresour. Technol. 2017, 235, 87–95. [Google Scholar] [CrossRef]
- Wang, H.; Nche-Fambo, F.A.; Yu, Z.; Chen, F. Using microalgal communities for high CO2-tolerant strain selection. Algal Res. 2018, 35, 253–261. [Google Scholar] [CrossRef]
- Watanabe, K.; Fujii, K. Isolation of high-level-CO2 -preferring Picochlorum sp. strains and their biotechnological potential. Algal Res. 2016, 18, 135–143. [Google Scholar] [CrossRef]
- Yue, L.; Chen, W. Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Convers. Manag. 2005, 46, 1868–1876. [Google Scholar] [CrossRef]
- Ho, S.; Lai, Y.-Y.; Chiang, C.-Y.; Chen, C.-N.N.; Chang, J.-S. Selection of elite microalgae for biodiesel production in tropical conditions using a standardized platform. Bioresour. Technol. 2013, 147, 135–142. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Polle, J. Truncated chlorophyll antenna size of the photosystems: A practical method to improve microalgal productivity and hydrogen production in mass culture. Int. J. Hydrogen Energy 2002, 27, 1257–1264. [Google Scholar] [CrossRef]
- Neidhardt, J.; Benemann, J.R.; Zhang, L.; Melis, A. Photosystem-II repair and chloroplast recovery from irradiance stress: Relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae). Photosynth. Res. 1998, 56, 175–184. [Google Scholar] [CrossRef]
- He, M.; Song, H.; Chen, W.; Zhang, Y.; Wang, T.; Wang, C.; Du, W. Comparative transcriptome analysis of wild type and an oleaginous mutant strain of Desmodesmus sp. reveals a unique reprogramming of lipid metabolism under high light. J. Appl. Phycol. 2019, 31, 2895–2910. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Wang, J.-H.; Cheng, L.-Y.; Tang, Y.-J.; Chi, Z.-Y. Selection of microalgae strains for bicarbonate-based integrated carbon capture and algal production system to produce lipid. Int. J. Green Energy 2019, 16, 825–833. [Google Scholar] [CrossRef]
- Ykema, A.; Verbree, E.C.; Verwoert, I.I.G.S.; van der Linden, K.H.; Nijkamp, H.J.J.; Smit, H. Lipid production of revertants of Ufa mutants from the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1990, 33, 176–182. [Google Scholar] [CrossRef]
- Ykema, A.; Verbree, E.C.; Nijkamp, H.J.J.; Smit, H. Isolation and characterization of fatty acid auxotrophs from the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1989, 32, 76–84. [Google Scholar] [CrossRef]
- Hassan, M.; Blanc, P.J.; Granger, L.-M.; Pareilleux, A.; Goma, G. Lipid production by an unsaturated fatty acid auxotroph of the oleaginous yeast Apiotrichum curvature grown in single-stage continuous culture. Appl. Microbiol. Biotechnol. 1993, 40, 483–488. [Google Scholar] [CrossRef]
- Dias, C.; Santos, J.; Reis, A.; Lopes da Silva, T. Yeast and microalgal symbiotic cultures using low-cost substrates for lipid production. Bioresour. Technol. Rep. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Kitahara, Y.; Yin, T.; Zhao, X.; Wachi, M.; Du, W.; Liu, D. Isolation of oleaginous yeast (Rhodosporidium toruloides) mutants tolerant of sugarcane bagasse hydrolysate. Biosci. Biotechnol. Biochem. 2014, 78, 336–342. [Google Scholar] [CrossRef]
- Qi, F.; Kitahara, Y.; Wang, Z.; Zhao, X.; Du, W.; Liu, D. Novel mutant strains of Rhodosporidium toruloides by plasma mutagenesis approach and their tolerance for inhibitors in lignocellulosic hydrolyzate: Novel mutant R. toruloides strains and their tolerance for hydrolyzate. J. Chem. Technol. Biotechnol. 2014, 89, 735–742. [Google Scholar] [CrossRef]
- Guo, M.; Cheng, S.; Chen, G.; Chen, J. Improvement of lipid production in oleaginous yeast Rhodosporidium toruloides by ultraviolet mutagenesis. Eng. Life Sci. 2019, 19, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Yamada, R.; Yamauchi, A.; Kashihara, T.; Ogino, H. Evaluation of lipid production from xylose and glucose/xylose mixed sugar in various oleaginous yeasts and improvement of lipid production by UV mutagenesis. Biochem. Eng. J. 2017, 128, 76–82. [Google Scholar] [CrossRef]
- Yamada, R.; Kashihara, T.; Ogino, H. Improvement of lipid production by the oleaginous yeast Rhodosporidium toruloides through UV mutagenesis. World J. Microbiol. Biotechnol. 2017, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Lu, D.; Ma, S.; Yan, Y.; Li, W. A quick isolation method for mutants with high lipid yield in oleaginous yeast. World J. Microbiol. Biotechnol. 2009, 25, 921–925. [Google Scholar] [CrossRef]
- Bessadok, B.; Santulli, A.; Brück, T.; Sadok, S. Species disparity response to mutagenesis of marine yeasts for the potential production of biodiesel. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar]
- Mussgnug, J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015, 99, 5407–5418. [Google Scholar] [CrossRef]
- Shin, S.-E.; Koh, H.G.; Kang, N.K.; Suh, W.I.; Jeong, B.; Lee, B.; Chang, Y.K. Isolation, phenotypic characterization and genome wide analysis of a Chlamydomonas reinhardtii strain naturally modified under laboratory conditions: Towards enhanced microalgal biomass and lipid production for biofuels. Biotechnol. Biofuels 2017, 10, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zhao, Q.; Miao, X.; Shi, J. Enhancement of lipid production in low-starch mutants Chlamydomonas reinhardtii by adaptive laboratory evolution. Bioresour. Technol. 2013, 147, 499–507. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving high carbon dioxide tolerance and carbon dioxide fixation capability of Chlorella sp. by adaptive laboratory evolution. Bioresour. Technol. 2015, 185, 269–275. [Google Scholar] [CrossRef]
- Arora, N.; Kumari, P.; Kumar, A.; Gangwar, R.; Gulati, K.; Pruthi, P.A.; Prasad, R.; Kumar, D.; Pruthi, V.; Poluri, K.M. Delineating the molecular responses of a halotolerant microalga using integrated omics approach to identify genetic engineering targets for enhanced TAG production. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xue, C.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Strain improvement of Chlorella sp. for phenol biodegradation by adaptive laboratory evolution. Bioresour. Technol. 2016, 205, 264–268. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, Y.; Li, X.; Mei, S.; Gao, J.; Zhao, Q.; Wei, W.; Sun, Y. Exploration of phenol tolerance mechanism through antioxidative responses of an evolved strain, Chlorella sp. L5. J. Appl. Phycol. 2018, 30, 2379–2385. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, M.; Chen, Y.; Jiang, X.; Lin, J.; Cao, X.; Huang, J. A lignocellulosic hydrolysate-tolerant Aurantiochytrium sp. mutant strain for docosahexaenoic acid production. Bioresour. Technol. 2017, 227, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Song, X.; Cui, J.; Liu, L.; Shi, M.; Wang, F.; Zhang, W. Rewiring metabolic network by chemical modulator based laboratory evolution doubles lipid production in Crypthecodinium cohnii. Metabol. Eng. 2019, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol. Biofuels 2017, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Huang, H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresour. Technol. 2018, 267, 438–444. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Jiang, L.; Huang, H. Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-M.; Ren, L.-J.; Ji, X.-J.; Chen, S.-L.; Guo, D.-S.; Huang, H. Adaptive evolution of Schizochytrium sp. by continuous high oxygen stimulations to enhance docosahexaenoic acid synthesis. Bioresour. Technol. 2016, 211, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Perrineau, M.-M.; Zelzion, E.; Gross, J.; Price, D.C.; Boyd, J.; Bhattacharya, D. Evolution of salt tolerance in a laboratory reared population of Chlamydomonas reinhardtii. Environ. Microbiol. 2014, 16, 1755–1766. [Google Scholar] [CrossRef]
- Fu, W.; Gudmundsson, O.; Feist, A.M.; Herjolfsson, G.; Brynjolfsson, S.; Palsson, B.Ø. Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. J. Biotechnol. 2012, 161, 242–249. [Google Scholar] [CrossRef]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Díaz, T.; Fillet, S.; Campoy, S.; Vázquez, R.; Viña, J.; Murillo, J.; Adrio, J.L. Combining evolutionary and metabolic engineering in Rhodosporidium toruloides for lipid production with non-detoxified wheat straw hydrolysates. Appl. Microbiol. Biotechnol. 2018, 102, 3287–3300. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.H.; Sze, Y.; Chuck, C.J.; Henk, D.A. Enhanced inhibitor tolerance and increased lipid productivity through adaptive laboratory evolution in the oleaginous yeast Metshnikowia pulcherrima. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.A.; Mituła, P.; Mirończuk, A.M. Lipid production from waste materials in seawater-based medium by the Yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.; Ryu, S.; Haridas, S.; Na, H.; Zane, M.; LaButti, K.; Barry, K.; Grigoriev, I.V.; Trinh, C.T. Draft Genome Assemblies of Ionic Liquid-Resistant Yarrowia lipolytica PO1f and Its Superior Evolved Strain, YlCW001. Microbiol. Resour. Announc. 2020, 9, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randez-Gil, F.; Prieto, J.A.; Rodríguez-Puchades, A.; Casas, J.; Sentandreu, V.; Estruch, F. Myriocin-induced adaptive laboratory evolution of an industrial strain of Saccharomyces cerevisiae reveals its potential to remodel lipid composition and heat tolerance. Microb. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutada, G.; Kavšček, M.; Hofer, F.; Gogg-Fassolter, G.; Schweiger, M.; Darnhofer, B.; Kordiš, D.; Birner-Gruenberger, R.; Natter, K. Characterization of a lipid droplet protein from Yarrowia lipolytica that is required for its oleaginous phenotype. BBA Mol. Cell Biol. L 2018, 1863, 1193–1205. [Google Scholar] [CrossRef]
- Meyers, A.; Chourey, K.; Weiskittel, T.M.; Pfiffner, S.; Dunlap, J.R.; Hettich, R.L.; Dalhaimer, P. The protein and neutral lipid composition of lipid droplets isolated from the fission yeast, Schizosaccharomyces pombe. J. Microbiol. 2017, 55, 112–122. [Google Scholar] [CrossRef]
- Borkiewicz, L.; Mołoń, M.; Molestak, E.; Grela, P.; Horbowicz-Drożdżal, P.; Wawiórka, L.; Tchórzewski, M. Functional Analysis of the Ribosomal uL6 Protein of Saccharomyces cerevisiae. Cells 2019, 8, 718. [Google Scholar] [CrossRef] [Green Version]
- Vizoso-Vázquez, Á.; Lamas-Maceiras, M.; González-Siso, M.I.; Cerdán, M.E. Ixr1 Regulates Ribosomal Gene Transcription and Yeast Response to Cisplatin. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Picazo, C.; McDonagh, B.; Peinado, J.; Bárcena, J.A.; Matallana, E.; Aranda, A. Saccharomyces cerevisiae Cytosolic Thioredoxins Control Glycolysis, Lipid Metabolism, and Protein Biosynthesis under Wine-Making Conditions. Appl. Environ. Microbiol. 2019, 85, 1–16. [Google Scholar] [CrossRef] [Green Version]
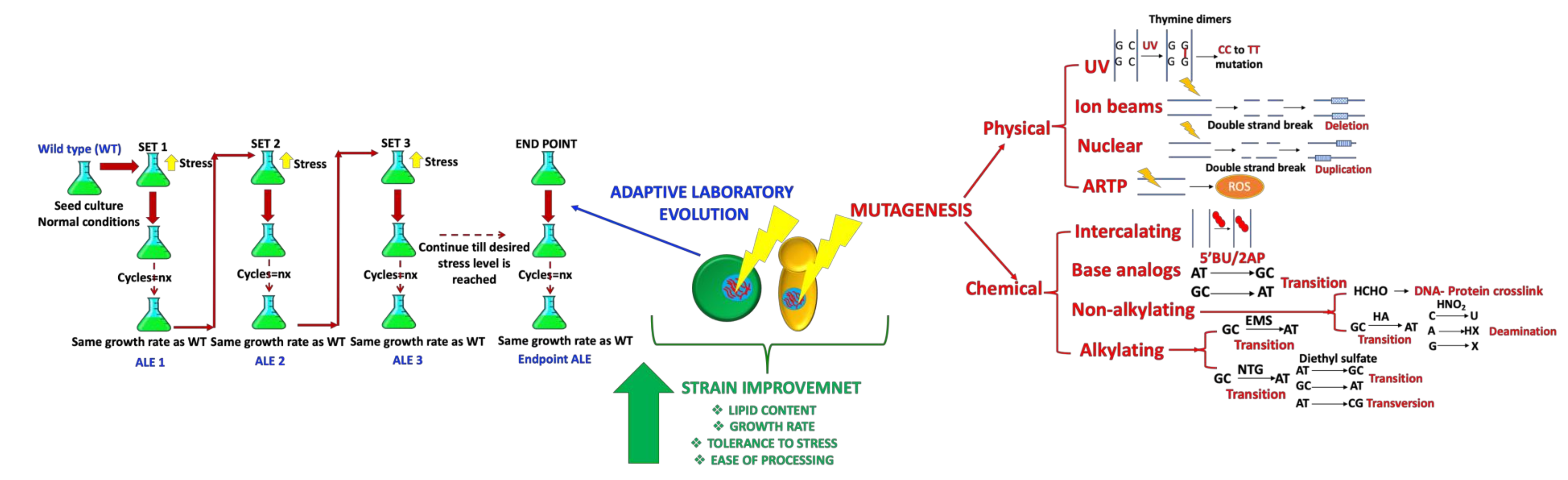
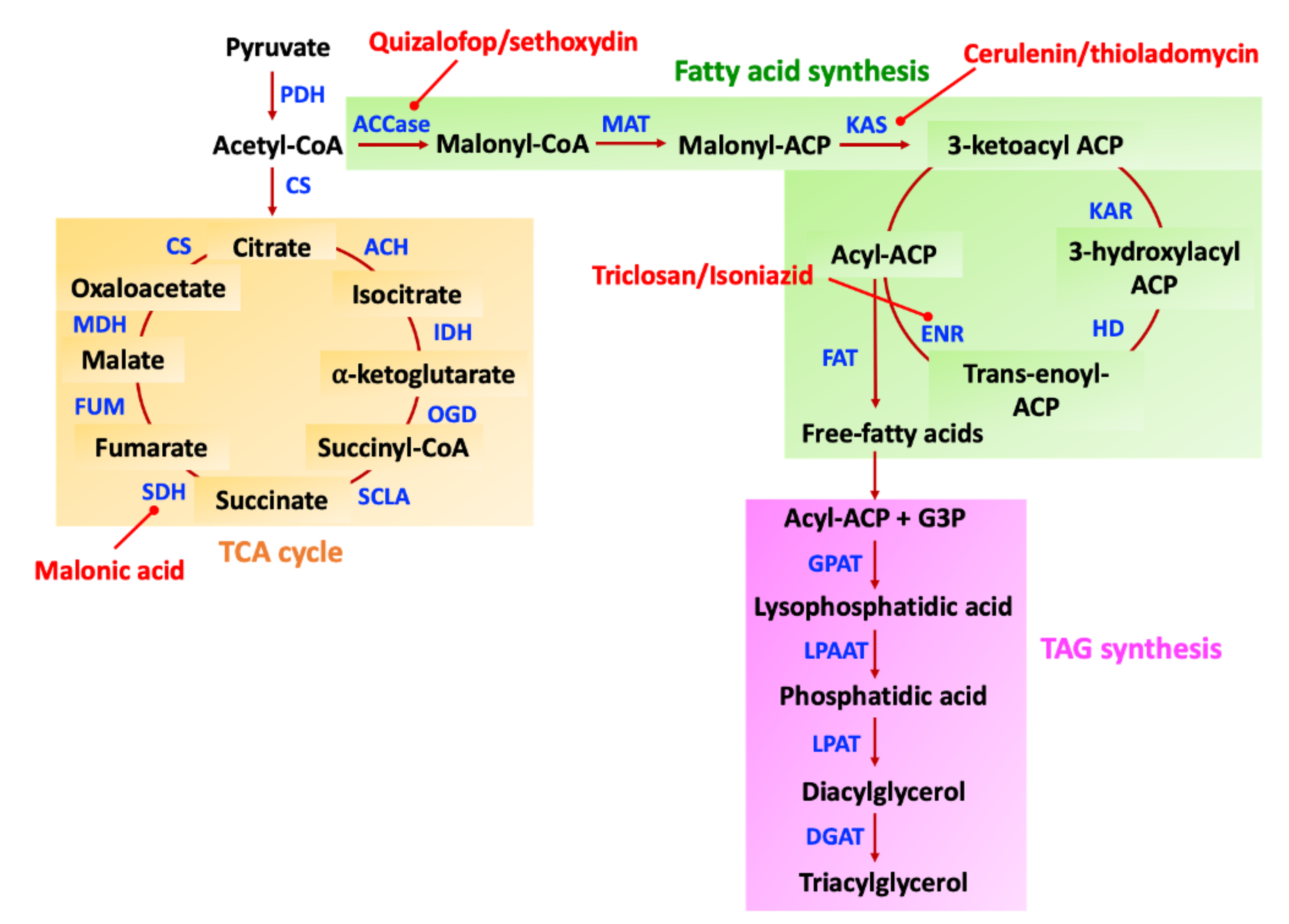
| Microalga | Mutagen | Survival Rate (%) | Screening Method | Mode of Cultivation | Cultivation Time (days) | DCW(g/L) | Lipid Yield (%) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | M | WT | M | |||||||
| Aurantiochytrium sp. | Heavy ion (120 Gy) | 50 | Triclosan and Isoniazid | 5 L fed batch culture | 4 | 32.1 | 34.2 | 41.38 | 46.82 | [34] |
| Nannochloroposis oceanica | Heavy ion (160 Gy) | n.r. | n.r. | Flask | 18 | 6 | 9.36 | 40.57 | 43.48 | [71] |
| NTG | 10 | 7 | 0.19 | 0.18 | 24.14 | 31.23 | [72] | |||
| Desmodesmus sp. | ARTP | 4 | 31 | 0.59 | 0.54 | 26.64 | 61.75 | [73] | ||
| Chlorella pyrenoidosa | 1 | 7 | 0.4 | 0.52 | 6.12 | 5.38 | [38] | |||
| 60Coγ (500Gy) | n.r. | 0.58 | 1.12 | n.r. | n.r. | [74] | ||||
| Chlorella vulgaris | 0.46 | 1.05 | n.r. | n.r. | ||||||
| UV + EMS (25 mM for 60 min) | 25 L Flat panel bioreactors, airlift | 10 | 0.96 | 1.3 | 25 | 41 | [70] | |||
| UV (254 nm) | 2.5 | Flask | 14 | 0.62 | 0.68 | 15.35 | 21.94 | [43] | ||
| EMS (25 mM for 60 min) | 10 | 0.64 | 15.35 | 22.46 | ||||||
| EMS (0.24 mM for 120 mins) | 0.56 | Decreased pigments | 2 L Flat panel PBR | 13 | 2.01 | 2.9 | n.r. | n.r. | [75] | |
| Schizochytrium sp. | NTG (2 mg/mL for 30 min) +UV (30 W for 3 min) | 2.5 | Iodoacetate and Malonic acid | Flask | 3 | 45.44 | 45.24 | 29.51 | 39.41 | [67] |
| Chlamydomonas sp. JSC4 | Heavy ion (100 Gy) | n.r. | Salinity (7%) | 9 | 6.09 | 4.08 | 18.3 | 34.7 | [76] | |
| Chlamydomonas reinhardtii acc-124 | EMS (40 µL/mL for 120 min) | n.r. | n.r. | 7 | 0.859 | 0.933 | 8 | 12 | [77] | |
| Chlamydomonas reinhardtii | FACS | 5.5 | 6.5 | 9.09 | 8 | [78] | ||||
| Scenedesmus sp. | N+ ion beam (1.8 × 105 ions/cm) | 5.20 | n.r. | 7 | 0.88 | 0.89 | 46.92 | 47.77 | [35] | |
| 60Coγ (500Gy) | 2 | 7 | 2.31 | 2.66 | 16.8 | 28.9 | [79] | |||
| UV (254 nm for 60 s) | 56 | Highly concentrated cellulosic ethanol wastewater | 27 | n.g. | 1.07 | n.g. | 21.4 | [80] | ||
| UV (3.4 W/m2 for 10 min) | n.r. | n.r. | 12 | 1 | 2.56 | 40 | 60 | [81] | ||
| Scenedesmus obliquus | UV (30 min) | 5–10 | Starchless | 12 | 4 | 12.6 | 4.8 | 11.5 | [82] | |
| Desmodesmus sp. | EMS (0.8 M) for 60 min | 9 | High light tolerance | 0.61 | 0.73 | 35.66 | 46.01 | [83] | ||
| Heavy ion (12C6+, 120 Gy) | 20 | n.r. | 8 | 4.95 | 5.23 | 48 | 37.78 | [84] | ||
| Nannochloroposis salina | EMS (0.24 mol/L for 30 min) | 3 | FACS | 22 | n.r. | n.r. | 17.5 | 34.1 (FAME content) | [85] | |
| EMS (0.24 mol/L for 30 min) + UV (45 s) | 27 | n.r. | n.r. | 17.5 | 78.7 (FAME content) | |||||
| InDels | n.r. | Flask | 12 | 2.2 | 3 | 24.9 | 32.8 | [86] | ||
| Nannochloroposis sp. | EMS (1 M) | 8 | 18 | 0.20 | 0.22 | 34 | 50.8 | [30] | ||
| EMS (0.5 M) | n.r. | n.r. | 11 | 0.79 | 1.08 | 8.32 | 11.30 | [87] | ||
| UV (354 nm for 120 min) | Cerulenin and Quizalofop | 8 | 1.03 | 1.40 | 49 | 57.05 | [88] | |||
| Nannochloroposis gaditana | EMS (70 mM for 60 min) + InDels | 10 | - | Decreased pigments | 7 | 2.59 | 3.29 | n.r. | n.r. | [89] |
| Chlorella sp. | EMS (100 mM for 60 min) | n.r. | Thermo tolerance | 40 L PBR outdoor | 8 | 0.90 | 1.8 | 16.2 | 12.0 | [90] |
| Chlorella pyrenoidosa | EMS (2% for 60 min) | 10 | Outdoor (10 L bottles) | 5 | n.g. | 1.97 | n.g. | 44.5 | [91] | |
| Chlorella sp. | NTG (5 µg/mL for 60 min) | 23 | Alkali tolerance | PBR (1 L) | 7 | 0.06 | 0.35 | n.r. | n.r. | [42] |
| EMS (100 mM for 30 min) | n.r. | Quizalofop | 6 | 0.30 | 0.64 | 10.07 | 12.44 | [92] | ||
| Chlorella sp. FC2 IITG | UV-C (30 W for 8 min) | 2 | n.r. | Flask | 10 | 0.5 | 0.67 | 56 | 68 | [32] |
| Chlorella sorokiniana | EMS (0.5%, w/v for 4 h) | n.r. | Starchless | 4 | 5.5 | 6.1 | n.r. | 11.2 | [93] | |
| UV | 10 | Reduced antenna size | 7-L outdoor hanging bags | 10 | 2.2 | 2.8 | n.r. | n.r. | [94] | |
| Chlorella minutissima | EMS (2 M for 30 min) | 5 | n.r. | Flask | 10 | 1.5 | 2.4 | 27 | 42 | [95] |
| Tetraselmis sp. | EMS (50 µ mol/mL 30 min) | 1.9 | 21 | 0.27 | 0.58 | 32 | 40 | [96] | ||
| Brotryococcus braunii | UV (15 W/cm2) for 6–21 min | 10 | 24 | 1.04 | 1.98 | 21 | 34 | [97] | ||
| Yeast | Mutagen | Survival Rate (%) | Screening Method | Cultivation Time (days) | DCW (g/L) | Lipid Yield (g/L) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| WT | M | WT | M | ||||||
| Cryptococcus curvatus | UV (2 W/m2) for 200 s | 10 | Cerulenin | 4 | n.r. | n.r. | 0.501 | 0.529 | [116] |
| Rhodospridium toruloides | n.r. | n.r. | 0.816 | 1.86 | |||||
| 12 | 11 | 0.94 | 1.25 | [117] | |||||
| UV (15 W/m2) for 8 min | 5 | Ethanol-H2O2 and LiCl | 7 | n.r. | n.r. | 1.5 | 2.24 | [115] | |
| ARTP | 1–5 | n.r. | 8 | n.g. | 7.2 | n.r. | 4.2 | [113] | |
| n.g. | 15.4 | n.r. | 7.4 | [114] | |||||
| ARTP + NTG (0.5 mg/L) for 45 min | 6 | 8.9 | 7.2 | 2.7 | 2.99 | [40] | |||
| Rhodotrorula glutinis | Heavy ion (40 Gy, 55 Gy) | 8–19 | Cerulenin | 4 | 2.03 | 2.21 | 0.34 | 0.65 | [118] |
| Rhodotrorula mucilaginosa | EMS (75 mM) for 60 min | 11.58 | n.r. | 2.08 | 2.72 | 0.292 | 0.52 | [119] | |
| Yarrowia lipolytica | 11.22 | 1.92 | 2.44 | 0.36 | 0.52 | ||||
| Trichosporon asatii | 7.7 | 1 | 1.16 | 0.148 | 0.236 | ||||
| Debaryomyces hansenii | EMS (75 mM) for 45 min | 3.16 | 0.64 | 0.76 | 0.072 | 0.076 | |||
| Candida tenuis | EMS (75 mM) for 30 min | 6.11 | 0.68 | 0.84 | 0.088 | 0.096 | |||
| Lipomyces starkeyi | UV (15 W/m2) for 40 min | 5 | Cerulenin | 7 | 12.31 | 13.74 | 4.41 | 5.44 | [63] |
| EMS (340 µL) for 30 min | 6 | Percoll density gradient | 11 | 14 | 4.3 | 6.3 | [55] | ||
| Microorganism | Stress Condition | Generations | Time for ALE (Days) | Cultivation Time (Days) | DCW (g/L) | Lipid Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Microalga | |||||||
| Aurantiochytrium sp. | Sugarcane bagasse hydrolysate | 10 | n.r. | 5 | 25 | 30 | [127] |
| Crypthecodinium cohnii | Glucose tolerant | 260 | 650 | 4 | n.r. | 35 | [99] |
| Sethoxydoxin + Seasamol | 100 | 300 | 5 | 6 | 60 | [128] | |
| Chlorella sp. | Flue gas | 110 | 138 | 7 | 3.4 | n.r. | [49] |
| CO2 tolerance | 31 | 97 | 11 | 3.68 | 20 | [129] | |
| Phenol degradation | 31 | 95 | 8 | 3.40 | 26 | [125] | |
| Salinity | 46 | 138 | 7 | 2.7 | 18.14 | [47] | |
| Schizochytrium sp. | n.r. | 150 | 3 | 52.3 | 22.7 | [130] | |
| Salinity + low temperature | 40 | n.r. | 47.23 | 37.60 | [131] | ||
| Glucose tolerant | 40 | n.r. | 62.15 | 49.78 | [132] | ||
| Chlamydomonas reinhardtii | n.r. | 28 | 84 | 9 | 0.48 | 40 | [122] |
| High salt | 1255 | 510 | n.r. | n.r. | n.r. | [133] | |
| Non-intentional | n.r. | 4 years | 8 | 1.71 | 8.18 | [121] | |
| Chlorella vulgaris | LED-Red light (660 nm) | 38 | 114 | 3 | 5.2 | n.r. | [134] |
| Yeast | |||||||
| Yarrowia lipolytica | Ionic liquids (18%; v/v) | 200 | n.r. | 4 | n.r. | n.r. | [86] |
| Nitrogen and Magnesium limited | 77 | n.r. | 7 | 8.8 | 44 | [135] | |
| Rhodospridium toruloides | Non-detoxified hydrolysate (75%) | 8 | 4 months | 4 | 6.6 | 55 | [136] |
| Metshnikowia pulcherrima | Inhibitors | 22 | n.r. | 7 | 14.5 | 33.3 | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, N.; Yen, H.-W.; Philippidis, G.P. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 2020, 12, 5125. https://doi.org/10.3390/su12125125
Arora N, Yen H-W, Philippidis GP. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability. 2020; 12(12):5125. https://doi.org/10.3390/su12125125
Chicago/Turabian StyleArora, Neha, Hong-Wei Yen, and George P. Philippidis. 2020. "Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts" Sustainability 12, no. 12: 5125. https://doi.org/10.3390/su12125125
APA StyleArora, N., Yen, H.-W., & Philippidis, G. P. (2020). Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability, 12(12), 5125. https://doi.org/10.3390/su12125125






