Environmental Fate of Multistressors on Carpet Shell Clam Ruditapes decussatus: Carbon Nanoparticles and Temperature Variation
Abstract
:1. Introduction
2. Material and Methods
2.1. Contaminants
2.1.1. Description
2.1.2. Characterization
2.2. Sampling and Laboratory Conditions
2.3. Biologic Analyses
2.4. Data Analyses
2.4.1. Principal Coordinates Analysis
2.4.2. Statistical Analysis
3. Results and Discussion
3.1. Characterization Analysis
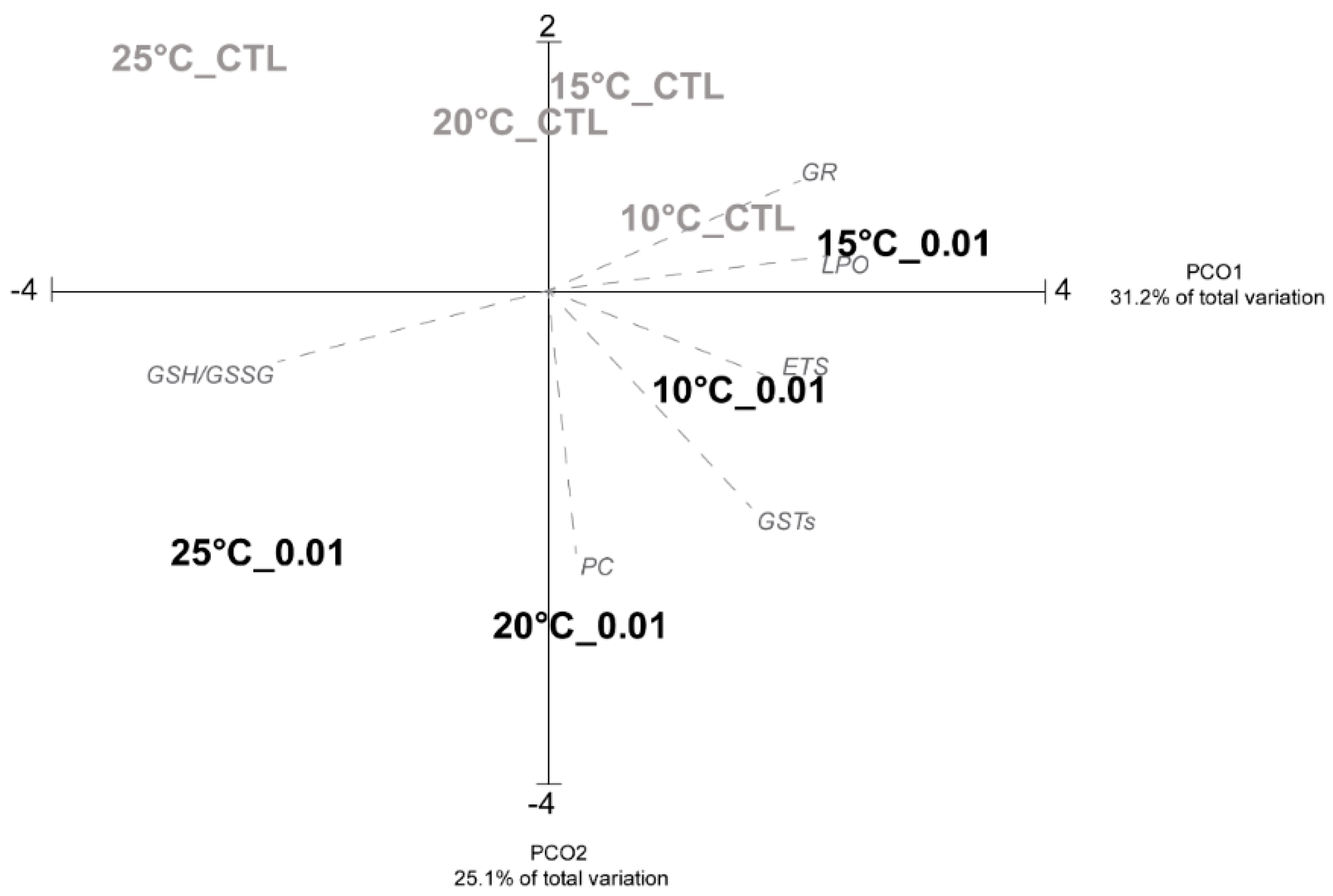
3.2. Biological Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- De Montaudouin, X.; Bachelet, G. Experimental evidence of complex interactions between biotic and abiotic factors in the dynamics of an intertidal population of the bivalve Cerastoderma edule. Oceanol. Acta 1996, 19, 449–463. [Google Scholar]
- Gerwing, T.G.; Drolet, D.; Hamilton, D.J.; Barbeau, M.A. Relative Importance of Biotic and Abiotic Forces on the Composition and Dynamics of a Soft-Sediment Intertidal Community. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grilo, T.F.; Cardoso, P.G.; Dolbeth, M.; Bordalo, M.D.; Pardal, M.A. Effects of extreme climate events on the macrobenthic communities’ structure and functioning of a temperate estuary. Mar. Pollut. Bull. 2011, 62, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Jenewein, B.T. Determining the extent to which weather-related abiotic factors influence daily variation in early benthic phase mortality of intertidal marine invertebrates. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, June 2009. [Google Scholar]
- Woodin, S.A.; Wethey, D.S.; Dubois, S.F. Population structure and spread of the polychaete Diopatra biscayensis along the French Atlantic coast: Human-assisted transport by-passes larval dispersal. Mar. Environ. Res. 2014, 102, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Bindoff, N.L.; Stott, P.A.; AchutaRao, K.M.; Allen, M.R.; Gillett, N.; Gutzler, D.; Hansingo, K.; Hegerl, G.; Hu, Y.; Jain, S.; et al. Chapter 10–Detection and attribution of climate change: From global to regional. In Climate Change 2013: The Physical Science Basis. IPCC Working Group I Contribution to AR5; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Weber, K.; Sturmer, L.; Hoover, E.; Baker, S. The Role of Salinity in Hard Clam Aquaculture; University of Florida IFAS Extension: Gainesville, FL, USA, 2013; pp. 1–10. [Google Scholar]
- Andrade, M.; De Marchi, L.; Pretti, C.; Chiellini, F.; Morelli, A.; Figueira, E.; Rocha, R.J.M.; Soares, A.M.V.M.; Freitas, R. The impacts of warming on the toxicity of carbon nanotubes in mussels. Mar. Environ. Res. 2019, 145, 11–21. [Google Scholar] [CrossRef]
- Attig, H.; Kamel, N.; Sforzini, S.; Dagnino, A.; Jamel, J.; Boussetta, H.; Viarengo, A.; Banni, M. Effects of thermal stress and nickel exposure on biomarkers responses in Mytilus galloprovincialis (Lam). Mar. Environ. Res. 2014, 94, 65–71. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Taylor, C.; Sokolova, I.M. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat. Toxicol. 2009, 91, 245–254. [Google Scholar] [CrossRef]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.I. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. Mol. Integr. Physiol. 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Meijide, F.J.; Da Cuña, R.H.; Prieto, J.P.; Dorelle, L.S.; Babay, P.A.; Lo Nostro, F.L. Effects of waterborne exposure to the antidepressant fluoxetine on swimming, shoaling and anxiety behaviours of the mosquitofish Gambusia holbrooki. Ecotoxicol. Environ. Saf. 2018, 163, 646–655. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centi, G.; Perathoner, S. Carbon Nanotubes for Sustainable Energy Applications. ChemSusChem 2011, 4, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.N.; Wang, N.; Ingersoll, C.G.; Hardesty, D.K.; Brunson, E.L.; Li, H.; Deng, B. Toxicity of carbon nanotubes to freshwater aquatic invertebrates. Environ. Toxicol. Chem. 2012, 31, 1823–1830. [Google Scholar] [CrossRef]
- Yeung, J.W.Y.; Zhou, G.J.; Leung, K.M.Y. Sub-lethal effects of cadmium and copper on RNA/DNA ratio and energy reserves in the green-lipped mussel Perna viridis. Ecotoxicol. Environ. Saf. 2016, 132, 59–67. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Freitas, R. Toxic effects of multi-walled carbon nanotubes on bivalves: Comparison between functionalized and nonfunctionalized nanoparticles. Sci. Total Environ. 2018, 622–623, 1532–1542. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Freitas, R. Effects of multi-walled carbon nanotube materials on Ruditapes philippinarum under climate change: The case of salinity shifts. Aquat. Toxicol. 2018, 199, 199–211. [Google Scholar] [CrossRef]
- Kádár, E.; Lowe, D.M.; Solé, M.; Fisher, A.S.; Jha, A.N.; Readman, J.W.; Hutchinson, T.H. Uptake and biological responses to nano-Fe versus soluble FeCl3 in excised mussel gills. Anal. Bioanal. Chem. 2010, 396, 657–666. [Google Scholar] [CrossRef]
- Rocha, T.L.; Gomes, T.; Sousa, V.S.; Mestre, N.C.; Bebianno, M.J. Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: An overview. Mar. Environ. Res. 2015, 111, 74–88. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, R.; Gallo, G.; Vallotto, D.; Marcomini, A.; Pojana, G. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat. Toxicol. 2010, 100, 168–177. [Google Scholar] [CrossRef]
- Moore, M.N.; Readman, J.A.J.; Readman, J.W.; Lowe, D.M.; Frickers, P.E.; Beesley, A. Lysosomal cytotoxicity of carbon nanoparticles in cells of the molluscan immune system: An in vitro study. Nanotoxicology 2009, 3, 40–45. [Google Scholar] [CrossRef]
- Parache, A. La palourde. La Pêche Marit. 1982, 61, 496–506. [Google Scholar]
- Bebianno, M.J.; Géret, F.; Hoarau, P.; Serafim, M.A.; Coelho, M.R.; Gnassia-Barelli, M.; Roméo, M. Biomarkers in Ruditapes decussatus: A potential bioindicator species. Biomarkers 2004, 9, 305–330. [Google Scholar] [CrossRef] [PubMed]
- FAO. Aquaculture Production by Species and Country or Area. 2020. Available online: http://www.fao.org/fishery/static/Yearbook/YB2017_USBcard/root/aquaculture/b56.pdf (accessed on 13 June 2020).
- IPMA. Lista de Espécies. Available online: https://www.ipma.pt/pt/bivalves/docs/files/Lista_de_espxcies_em_14_04_2020.pdf (accessed on 9 June 2020).
- Matias, D.; Joaquim, S.; Matias, A.M.; Moura, P.; de Sousa, J.T.; Sobral, P.; Leitão, A. The reproductive cycle of the European clam Ruditapes decussatus (L. 1758) in two Portuguese populations: Implications for management and aquaculture programs. Aquaculture 2013, 406–407, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Chalghmi, H.; Zrafi, I.; Gourves, P.Y.; Bourdineaud, J.P.; Saidane-Mosbahi, D. Combined effects of metal contamination and abiotic parameters on biomarker responses in clam: Ruditapes decussatus gills: An integrated approach in biomonitoring of Tunis lagoon. Environ. Sci. Process. Impacts 2016, 18, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Cravo, A.; Pereira, C.; Gomes, T.; Cardoso, C.; Serafim, A.; Almeida, C.; Rocha, T.; Lopes, B.; Company, R.; Medeiros, A.; et al. A multibiomarker approach in the clam Ruditapes decussatus to assess the impact of pollution in the Ria Formosa lagoon, South Coast of Portugal. Mar. Environ. Res. 2012, 75, 23–34. [Google Scholar] [CrossRef]
- El-Shenawy, N.S. Heavy-metal and microbial depuration of the clam Ruditapes decussatus and its effect on bivalve behavior and physiology. Environ. Toxicol. 2004, 19, 143–153. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A. Usefulness of Bioindicators and Biomarkers in Pollution Biomonitoring. Int. J. Biotechnol. Wellness Ind. 2014, 3, 19–26. [Google Scholar] [CrossRef]
- Caro, A.; Chereau, G.; Briant, N.; Roques, C.; Freydier, R.; Delpoux, S.; Escalas, A.; Elbaz-Poulichet, F. Contrasted responses of Ruditapes decussatus (filter and deposit feeding) and Loripes lacteus (symbiotic) exposed to polymetallic contamination (Port-Camargue, France). Sci. Total Environ. 2015, 505, 526–534. [Google Scholar] [CrossRef]
- Chalghmi, H.; Bourdineaud, J.P.; Haouas, Z.; Gourves, P.Y.; Zrafi, I.; Saidane-Mosbahi, D. Transcriptomic, Biochemical, and Histopathological Responses of the Clam Ruditapes decussatus from a Metal-Contaminated Tunis Lagoon. Arch. Environ. Contam. Toxicol. 2016, 70, 241–256. [Google Scholar] [CrossRef]
- Fathallah, S.; Medhioub, M.N.; Medhioub, A.; Kraiem, M.M. Toxicity of Hg, Cu and Zn on early developmental stages of the European clam (Ruditapes decussatus) with potential application in marine water quality assessment. Environ. Monit. Assess. 2010, 171, 661–669. [Google Scholar] [CrossRef]
- Figueira, E.; Cardoso, P.; Freitas, R. Ruditapes decussatus and Ruditapes philippinarum exposed to cadmium: Toxicological effects and bioaccumulation patterns. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 156, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Roméo, M.; Gnassia-Barelli, M. Metal distribution in different tissues and in subcellular fractions of the Mediterranean clam Ruditapes decussatus treated with cadmium, copper, or zinc. Comp. Biochem. Physiol. Part C Comp. 1995, 111, 457–463. [Google Scholar] [CrossRef]
- Serafim, A.; Bebianno, M.J. Effect of a polymetallic mixture on metal accumulation and metallothionein response in the clam Ruditapes decussatus. Aquat. Toxicol. 2010, 99, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Velez, C.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. The impacts of As accumulation under different pH levels: Comparing Ruditapes decussatus and Ruditapes philippinarum biochemical performance. Environ. Res. 2016, 151, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Abdelhafidh, K.; Ali, M.; Hassen, K.; Badreddine, S.; Jaume, A.; Sandra, P.; Ethel, E.; Damià, B.; Hamouda, B.; Ezzeddine, M. Uptake and metabolism of carbamazepine (CBZ) by clam Ruditapes decussatus and its effects in biochemical responses. Xenobiotica 2018, 48, 727–733. [Google Scholar] [CrossRef]
- Costa, S.; Coppola, F.; Pretti, C.; Intorre, L.; Meucci, V.; Soares, A.M.V.M.; Freitas, R.; Solé, M. The influence of pre-exposure to climate change related factors on the response of two clam’s species to Diclofenac. Ecotoxicol. Environ. Saf. 2020, 189, 109899. [Google Scholar] [CrossRef]
- Sellami, B.; Aouani, I.; Maalaoui, A.; Dellali, M.; Aïssa, P.; Touil, S.; Sheehan, D.; Mahmoudi, E.; Hamouda, B. Effects of 2-(4-Methoxyphenyl)-5, 6-trimethylene-4H-1, 3, 2-oxathiaphosphorine-2-sulfide on biomarkers of Mediterranean clams Ruditapes decussatus. Ecotoxicol. Environ. Saf. 2015, 120, 263–269. [Google Scholar] [CrossRef]
- Campillo, J.A.; Sevilla, A.; Albentosa, M.; Bernal, C.; Lozano, A.B.; Cánovas, M.; León, V.M. Metabolomic responses in caged clams, Ruditapes decussatus, exposed to agricultural and urban inputs in a Mediterranean coastal lagoon (Mar Menor, SE Spain). Sci. Total Environ. 2015, 524–525, 136–147. [Google Scholar] [CrossRef]
- Kamel, N.; Jebali, J.; Banni, M.; Ben Khedher, S.; Chouba, L.; Boussetta, H. Biochemical responses and metals levels in Ruditapes decussatus after exposure to treated municipal effluents. Ecotoxicol. Environ. Saf. 2012, 82, 40–46. [Google Scholar] [CrossRef]
- Saidani, W.; Sellami, B.; Khazri, A.; Mezni, A.; Dellali, M.; Joubert, O.; Sheehan, D.; Beyrem, H. Metal accumulation, biochemical and behavioral responses on the Mediterranean clams Ruditapes decussatus exposed to two photocatalyst nanocomposites (TiO2 NPs and AuTiO2NPs). Aquat. Toxicol. 2019, 208, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sellami, B.; Bouzidi, I.; Saidani, W.; Mezni, A.; Sheehan, D.; Beyrem, H. Effects of Gold Nanoparticles on the Mediterranean Clams Ruditapes decussatus: Chemical and Biochemical Investigations. In Proceedings of the Euro-Mediterranean Conference for Environmental Integration, Sousse, Tunisia, 20–25 November 2017; Springer: Cham, Switzerland, 2018; pp. 577–580. [Google Scholar]
- Peng, X.; Jia, J.; Gong, X.; Luan, Z.; Fan, B. Aqueous stability of oxidized carbon nanotubes and the precipitation by salts. J. Hazard. Mater. 2009, 165, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- OECD. List of Manufactured Nanomaterials and List of Endpoints for Phase One of the Sponsorship Programme for the Testing of Manufactured Nanomaterials: Revision. In OECD Environment, Health and Safety Publications; Series on the Safety of Manufactured Nanomaterials; Organization for Economic Cooperation and Development: Paris, France, 2010. [Google Scholar]
- Zhang, X.; Zhou, Q.; Zou, W.; Hu, X. Molecular Mechanisms of Developmental Toxicity Induced by Graphene Oxide at Predicted Environmental Concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef]
- Cravo, A.; Rosa, A.; Jacob, J.; Correia, C. Dissolved oxygen dynamics in Ria Formosa Lagoon (South Portugal)—A real time monitoring station observatory. Mar. Chem. 2020, 223, 103806. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Wang, Q.; Zeng, Y.; Ding, L.; Jiang, W. Effects of ionic strength and temperature on the aggregation and deposition of multi-walled carbon nanotubes. J. Environ. Sci. China 2017, 51, 248–255. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Smolders, R.; Bervoets, L.; De Coen, W.; Blust, R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004, 129, 99–112. [Google Scholar] [CrossRef]
- Reid, G.K.; Gurney-Smith, H.J.; Marcogliese, D.J.; Knowler, D.; Benfey, T.; Garber, A.F.; Forster, I.; Chopin, T.; Brewer-Dalton, K.; Moccia, R.D.; et al. Climate change and aquaculture: Considering biological response and resources. Aquac. Environ. Interact. 2019, 11, 569–602. [Google Scholar] [CrossRef] [Green Version]
- Talmage, S.C.; Gobler, C.J. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Velez, C.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar. Environ. Res. 2017, 123, 62–70. [Google Scholar] [CrossRef]
- Freitas, R.; Coppola, F.; Henriques, B.; Wrona, F.; Figueira, E.; Pereira, E.; Soares, A.M.V.M. Does pre-exposure to warming conditions increase Mytilus galloprovincialis tolerance to Hg contamination? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Leite, C.; Pinto, J.; Costa, M.; Monteiro, R.; Henriques, B.; Di Martino, F.; Coppola, F.; Soares, A.M.V.M.; Solé, M.; et al. The influence of temperature and salinity on the impacts of lead in Mytilus galloprovincialis. Chemosphere 2019, 235, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anacleto, P.; Maulvault, A.L.; Bandarra, N.M.; Repolho, T.; Nunes, M.L.; Rosa, R.; Marques, A. Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res. Int. 2014, 64, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Q. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Maria, V.L.; Bebianno, M.J. Antioxidant and lipid peroxidation responses in Mytilus galloprovincialis exposed to mixtures of benzo(a)pyrene and copper. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 154, 56–63. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Gagné, F.; Chemello, G.; Riedl, V.; Finos, L.; Pastore, P.; Badocco, D.; Marin, M.G. Does exposure to reduced pH and diclofenac induce oxidative stress in marine bivalves? A comparative study with the mussel Mytilus galloprovincialis and the clam Ruditapes philippinarum. Environ. Pollut. 2018, 240, 925–937. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Lannig, G.; Flores, J.F.; Sokolova, I.M. Temperature-dependent stress response in oysters, Crassostrea virginica: Pollution reduces temperature tolerance in oysters. Aquat. Toxicol. 2006, 79, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Felton, G.W. Oxidative Stress of Vertebrates and Invertebrates. In Oxidative Stress and Antioxidant Defenses in Biology; Springer: New York, NY, USA, 1995; pp. 356–434. [Google Scholar]
- Franco, J.L.; Trivella, D.B.B.; Trevisan, R.; Dinslaken, D.F.; Marques, M.R.F.; Bainy, A.C.D.; Dafre, A.L. Antioxidant status and stress proteins in the gills of the brown mussel Perna perna exposed to zinc. Chem. Biol. Interact. 2006, 160, 232–240. [Google Scholar] [CrossRef]
- Everatt, M.J.; Convey, P.; Bale, J.S.; Worland, M.R.; Hayward, S.A.L. Responses of invertebrates to temperature and water stress: A polar perspective. J. Therm. Biol. 2015, 54, 118–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handy, R.D.; Von Der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, C.; Ueda, K.; Haniu, H.; Ishida, H.; Okano, S.; Takizawa, T.; Sobajima, A.; Kamanaka, T.; Yoshida, K.; Okamoto, M.; et al. Different aggregation and shape characteristics of carbon materials affect biological responses in RAW264 cells. Int. J. Nanomed. 2018, 13, 6079–6088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielen, A.; Bošnjak, I.; Sepčić, K.; Jaklič, M.; Cvitanić, M.; Lušić, J.; Lajtner, J.; Simčič, T.; Hudina, S. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci. Total Environ. 2016, 543, 449–459. [Google Scholar] [CrossRef]



| Technical Data | ||||||
|---|---|---|---|---|---|---|
| MWCNTs–COOH | Diameter (nm) | Length (um) | Carbon Purity (%) | Surface Area (m2/g) | Amorphous Carbon (mol%) | -COOH (wt%) |
| 2–5 | 10–30 | 98 | 400 | 8–10 | 3.86 | |
| Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | ||||||
| T0 | T96 | T0 | T96 | T0 | T96 | T0 | T96 | ||
| MWCNTs–COOH | Particle size (nm) | 2307.1 | 2289.1 | 2564.3 | 2500.1 | 2601.2 | 2790.8 | 3989.1 | 4001.2 |
| PDI | 0.98 | 0.97 | 1.01 | 0.91 | 1.13 | 1.16 | 0.26 | 0.33 | |
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | |||
| Energy reserves content | PROT (mg/g) | CTL | 80.99 ± 19.10 A | 93.68 ± 17.53 A | 91.23 ± 9.59 A | 89.12 ± 20.84 A |
| Exp | 89.24 ± 9.92 a | 102.87 ± 15.47 a | 78.46 ± 15.08 a | 81.40 ± 21.01 a | ||
| GLY (mg/g) | CTL | 1.37 ± 0.36 A | 1.63 ± 0.30 A | 1.95 ± 0.52 B | 1.58 ± 0.21 A | |
| Exp | 1.65 ± 0.41 a | 1.60 ± 0.49 a | 1.42 ± 0.50 a | 1.65 ± 0.11 a | ||
| Antioxidant defenses | CAT (U/g) | CTL | 9.25 ± 1.65 A | 10.83 ± 1.70 A | 9.92 ± 2.23 A | 11.10 ± 1.98 A |
| Exp | 8.87 ± 0.87 a | 10.58 ± 0.49 a | 10.28 ± 1.50 a | 9.93 ± 0.79 a | ||
| GPx (U/g) | CTL | 0.05 ± 0.02 A | 0.05 ± 0.02 A* | 0.10 ± 0.02 A | 0.06 ± 0.02 A | |
| Exp | 0.08 ± 0.02 a | 0.13 ± 0.01 b | 0.14 ± 0.01 b | 0.14 ± 0.02 b | ||
| Neuro status | AChE (nmol/min/g) | CTL | 0.08 ± 0.02 A | 0.09 ± 0.01 A | 0.08 ± 0.02 A | 0.18 ± 0.05 B,* |
| Exp | 0.09 ± 0.02 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.01 ± 0.00 b | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marchi, L.; Rocha, R.J.M.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Pretti, C.; Chiellini, F.; Freitas, R. Environmental Fate of Multistressors on Carpet Shell Clam Ruditapes decussatus: Carbon Nanoparticles and Temperature Variation. Sustainability 2020, 12, 4939. https://doi.org/10.3390/su12124939
De Marchi L, Rocha RJM, Rodrigues ACM, Soares AMVM, Pretti C, Chiellini F, Freitas R. Environmental Fate of Multistressors on Carpet Shell Clam Ruditapes decussatus: Carbon Nanoparticles and Temperature Variation. Sustainability. 2020; 12(12):4939. https://doi.org/10.3390/su12124939
Chicago/Turabian StyleDe Marchi, Lucia, Rui Jorge Miranda Rocha, Andreia C.M. Rodrigues, Amadeu M.V.M. Soares, Carlo Pretti, Federica Chiellini, and Rosa Freitas. 2020. "Environmental Fate of Multistressors on Carpet Shell Clam Ruditapes decussatus: Carbon Nanoparticles and Temperature Variation" Sustainability 12, no. 12: 4939. https://doi.org/10.3390/su12124939








