Land Use Change and Wildlife Conservation—Case Analysis of LULC Change of Pench-Satpuda Wildlife Corridor in Madhya Pradesh, India
Abstract
:1. Introduction
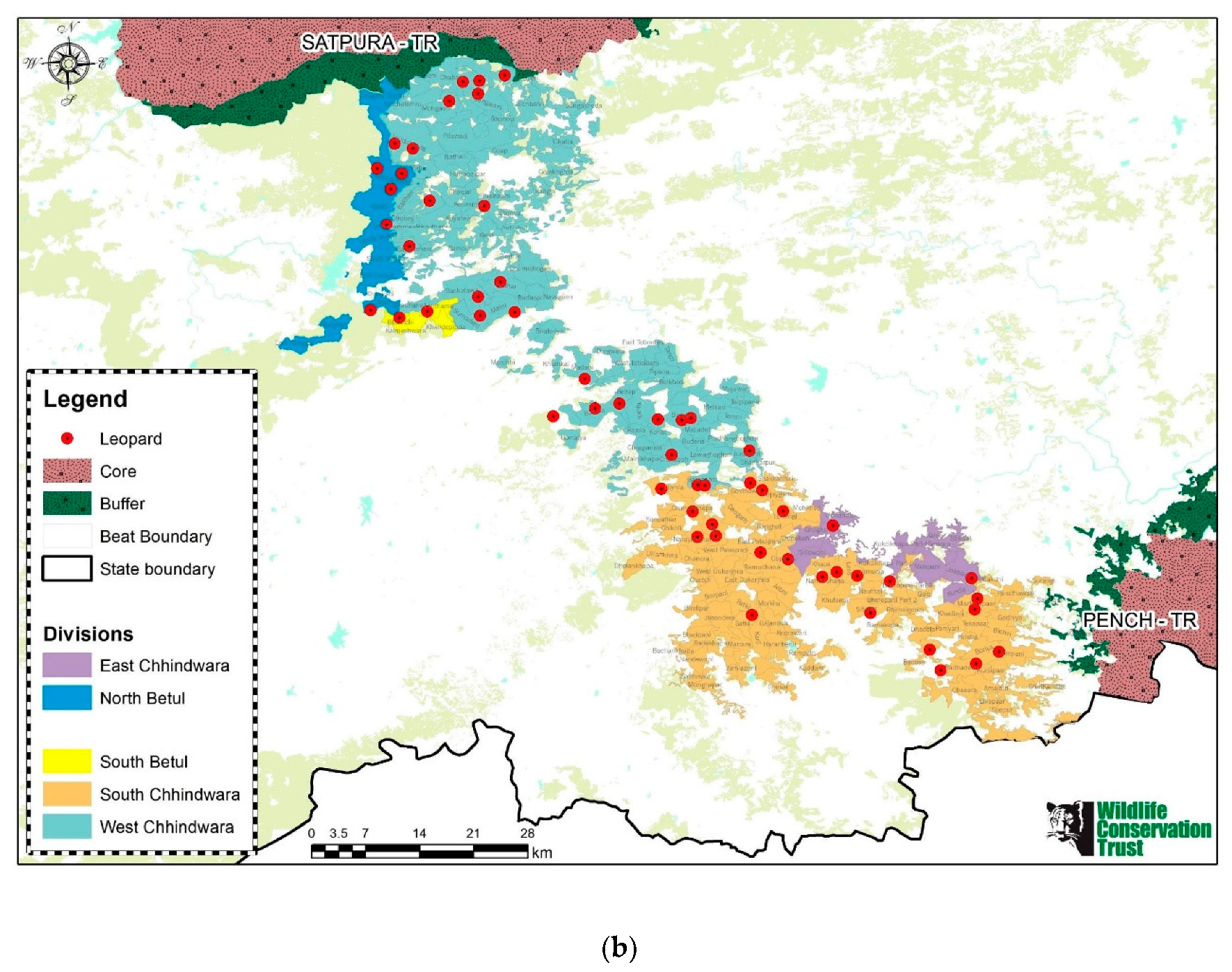
1.1. Study Area
1.2. Viability of the Corridor for Wildlife Movement
2. Material And Methods
2.1. Data Sources
2.2. LULC Classification
2.3. Image Processing
2.3.1. Classification of Images
2.3.2. Post Classification Change Detection
2.4. Accuracy Assessment
2.5. Magnitude of Change Determination
- K = magnitude of change
- A = percentage of change
- F = first date (2002)
- I = reference date (2019).
3. Results
3.1. Evaluation of LULC Cover Maps
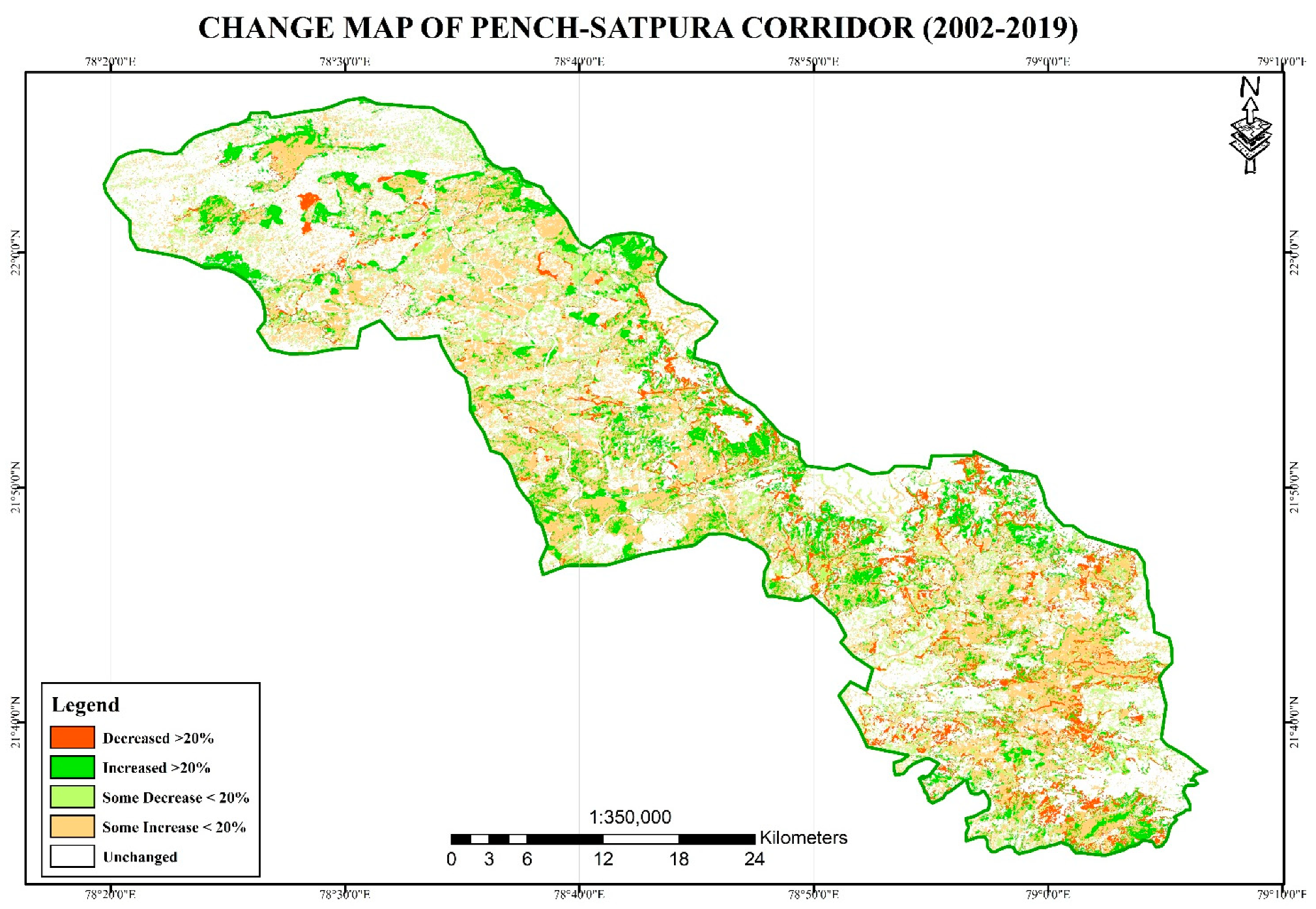
3.2. Land Use Land Cover Change Analysis
4. Discussion
5. Conclusions
- Detailed survey and demarcation exercise to identify the boundaries of forests and eviction of illegal encroachers undertaking cultivation on forest lands.
- Enrichment of the forests through Assisted Natural Regeneration (ANR) or artificial regeneration (gap planting). This will improve the water regime of the area to reduce human-wildlife conflict.
- Escalating the level of protection of the forests to prevent illegal felling of trees in the wildlife corridor and taking stringent action under the law against those involved in the illicit felling of trees.
- Scientific management of grasslands for supporting the herbivore population. This will reduce cattle depredation by carnivores.
- Declaring the Pench-Satpuda wildlife corridor as an absolute “prohibited area” for coal mining, and even underground coal mining should not be allowed under any circumstance.
- Scientific monitoring of wildlife through systematic studies and other techniques such as camera trapping, presence-absence surveys, and maintaining wildlife sighting registers by the field staff.
- Necessity of elaboration of alternatives for local communities to prevent illegal encroachment of forests for cultivation in the future. Such plans should be elaborated jointly by the multiple stakeholders including the administration, local people, and scientists.
- There is a pressing need to introduce a wide information campaign among local communities to reduce human-wildlife conflict, and for reducing the biotic pressures on the forests.
Author Contributions
Funding
Conflicts of Interest
References and Note
- Pearce, D.W. The Economic Value of Forest Ecosystems. Ecosyst. Health 2001, 7, 284–296. [Google Scholar] [CrossRef]
- Lange, G.M. Manual for Environmental and Economic Accounts for Forestry: A Tool for Cross-Sectoral Policy Analysis; Working Paper; FAO Forestry Department: Rome, Italy, 2004. [Google Scholar]
- Costanza, R.; d’Arget, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The Value of The World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- FAO. Global Forest Resource Assessment. How are the World’s Forests Changing? 2nd ed.; FAO: Rome, Italy, 2015. [Google Scholar]
- UNEP. Global Environmental Outlook 3: Past, Present and Future Perspectives; Earthscan: London, UK, 2002. [Google Scholar]
- Smeraldo, S.; Bosso, L.; Fraissinet, M.; Bordignon, L.; Brunelli, M.; Ancillotto, L.; Russo, D. Modelling Risks Posed by Wind Turbines and Power Lines to Soaring Birds: The Black Stork (Ciconia nigra) in Italy as a Case Study. Biodivers. Conserv. 2020, 29, 1959–1976. [Google Scholar] [CrossRef]
- Jensen, A.M.; Oneil, N.P.; Iwaniuk, A.N.; Burg, T.M. Landscape Effects on the Contemporary Genetic Structure of Ruffed Grouse (Bonasa umbellus) Populations. Ecol. Evol. 2019, 9, 5572–5592. [Google Scholar] [CrossRef] [Green Version]
- Bennett, A.F.; Saunders, D.A. Habitat Fragmentation and Landscape Change. Conserv. Biol. All 2010, 93, 1544–1550. [Google Scholar]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Beier, P.; Noss, R.F. Do Habitat Corridors Provide Connectivity? Conserv. Biol. 1998, 12, 1241–1252. [Google Scholar] [CrossRef]
- Bennett, A.F. Linkages in the Landscape: The Role of Corridors and Connectivity in Wildlife Conservation; IUCN: Gland, Switzerland; Cambridge, UK, 2003. [Google Scholar]
- Beier, P.; Loe, S. A Checklist for Evaluating Impacts to Wildlife Movement Corridors. Biol. Conserv. 1994, 69, 434–440. [Google Scholar]
- Salinas-Ramos, V.B.; Ancillotto, L.; Bosso, L.; Sánchez-Cordero, V.; Russo, D. Interspecific Competition in Bats: State of Knowledge and Research Challenges. Mammal Rev. 2019, 50, 68–81. [Google Scholar] [CrossRef]
- Wauters, L.A.; Mazzamuto, M.V.; Santicchia, F.; Dongen, S.V.; Preatoni, D.G.; Martinoli, A. Interspecific Competition Affects the Expression of Personality-Traits in Natural Populations. Sci. Rep. 2019, 9, 11189. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, R. Asian Elephant (Elephas Maximus) and Riparian Wildlife Corridor: A Case Study from Lesser Himalayan Zone of Uttarakhand. J. Am. Sci. 2008, 4, 63–75. [Google Scholar]
- Brooks, T.M.; Pimm, S.L.; Oyugi, J.O. Time Lag between Deforestation and Bird Extinction in Tropical Forest Fragments. Conserv. Biol. 1999, 13, 1140–1150. [Google Scholar] [CrossRef]
- Osborn, F.V.; Parker, G.E. Linking Two Elephant Refuges with a Corridor in the Communal Lands of Zimbabwe. Afr. J. Ecol. 2003, 41, 68–74. [Google Scholar] [CrossRef]
- Government of India. State of Environment Report; Ministry of Environment & Forests: New Delhi, India, 2009.
- Government of India. State of Forest Report 2017; Forest Survey of India: Dehradun, India, 2017.
- Lambin, E.F.; Geist, H.J. Causes of Land Use and Land Cover Change; Encyclopedia of Earth, Environmental Information Coalition: Washington, DC, USA, 2007. [Google Scholar]
- Twisa, S.; Buchroithner, M.F. Land-Use and Land-Cover (LULC) Change Detection in Wami River Basin, Tanzania. Land 2019, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Nukala, R.B.; Mutz, D. Strategic Approach for Sustainable Land Use in an Emerging Country—Case of India. In Proceedings of the World Bank Conference on Land and Poverty 2015, Washington, DC, USA, 23–27 March 2015. [Google Scholar]
- Jhala, Y.V.; Qureshi, Q.; Gopal, R.; Sinha, P.R. (Eds.) Status of the Tigers, Co-predators, and Prey in India. In National Tiger Conservation Authority; TR 2011/003; Government of India, New Delhi & Wildlife Institute of India: Dehradun, India, 2011; p. 302. [Google Scholar]
- Joshi, A.; Pariwakam, M. Status of Large Carnivores in the Satpura-Pench Corridor, Madhya Pradesh; Technical Report; Wildlife Conservation Trust: Mumbai, India, 2017.
- Attack on Humans by Wildlife Resulting in Death or Injury, Livestock Depredation by Wild Animals; Unpublished Official Records; Forest Department: Madhya Pradesh, India, 2012–2019.
- CMPDI. Land Use/Vegetation Cover Mapping of Pench-Kanhan-Tawa-Satpura Valley Coalfield based on Satellite Data for the Year- 2017; Central Mine Planning and Design Institute: Ranchi, India, 2018. [Google Scholar]
- WCL. Annual Reports and Accounts; Western Coalfields Limited, Government of India: Nagpur, India, 2019.
- WCL. Presentation by Western Coalfields Limited in Workshop on ‘The Technology Development & Mechanisation of Mines for Enhancing Coal Production & Productivity’; Western Coalfields Limited, Government of India: Nagpur, India, 2015.
- Anderson, J.R.; Hardy, E.E.; Roach, J.T.; Witmer, R.E. A Land Use and Land Cover Classification System for Use with Remote Sensor Data; Geological Survey Professional Paper 964; United States Department of the Interior: Alexandria, VA, USA, 1976.
- Lizarazo, I. Accuracy Assessment of Object-Based Image Classification: Another STEP. Int. J. Remote Sens. 2014, 35, 6135–6156. [Google Scholar] [CrossRef]
- Congalton, R.G. A Review of Assessing the Accuracy of Classifications of Remotely Sensed Data. Remote Sens. Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Congalton, R.; Green, K. Assessing the Accuracy of Remotely Sensed Data; Mapping Science: Boca Raton, FL, USA, 1998. [Google Scholar]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest Biodiversity, Ecosystem Functioning and the Provision of Ecosystem Services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef] [Green Version]
- Symes, W.S.; Edwards, D.P.; Miettinen, J.; Rheindt, F.E.; Carrasco, L.R. Combined Impacts of Deforestation and Wildlife Trade on Tropical Biodiversity Are Severely Underestimated. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- de Araujo Lima Constantino, P. Deforestation and Hunting Effects on Wildlife across Amazonian Indigenous Lands. Ecol. Soc. 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Newhouse, M.C. Wildlife Habitat Analysis for Site Planning. Master’s Thesis, Iowa State University, Ames, IA, USA, 1984. Available online: https://lib.dr.iastate.edu/rtd/17290 (accessed on 29 March 2020).
- Chakravarty, S.; Ghosh, S.K.; Suresh, C.P.; Dey, A.N.; Shukla, G. Deforestation: Causes, Effects and Control Strategies. Glob. Perspect. Sustain. For. Manag. 2012, 1, 3–28. [Google Scholar] [CrossRef] [Green Version]
- Qazi, N.Q.; Bruijnzeel, L.A.; Rai, S.P.; Ghimire, C.P. Impact of Forest Degradation on Streamflow Regime and Runoff Response to Rainfall in the Garhwal Himalaya, Northwest India. Hydrol. Sci. J. 2017, 62, 1114–1130. [Google Scholar] [CrossRef]
- Agarwal, S.; Nagendra, H.; Ghate, R. The Influence of Forest Management Regimes on Deforestation in a Central Indian Dry Deciduous Forest Landscape. Land 2016, 5, 27. [Google Scholar] [CrossRef]
- Agarwala, M.; Defries, R.S.; Qureshi, Q.; Jhala, Y.V. Changes in the Dry Tropical Forests in Central India with Human Use. Reg. Environ. Chang. 2016, 16, 5–15. [Google Scholar] [CrossRef]
- Ranjan, R. Assessing the Impact of Mining on Deforestation in India. Resour. Policy 2019, 60, 23–35. [Google Scholar] [CrossRef]
- Darmody, R.; Hetzler, R.; Simmons, F. Coal Mine Subsidence: Effects of Mitigation on Crop Yields. Int. J. Surf. Min. Reclam. Environ. 1992, 6, 187–190. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S. Underground Coal Mine Subsidence Impacts Forest Ecosystem; Central Institute of Mining and Fuel Research: Dhanbad, India, 2010. [Google Scholar]
- Berger, J. The Last Mile: How to Sustain Long-Distance Migration in Mammals. Conserv. Biol. 2004, 18, 320–331. [Google Scholar] [CrossRef]
- Jones, T.; Bamford, A.J.; Ferrol-Schulte, D.; Hieronimo, P.; Mcwilliam, N.; Rovero, F. Vanishing Wildlife Corridors and Options for Restoration: A Case Study from Tanzania. Trop. Conserv. Sci. 2012, 5, 463–474. [Google Scholar] [CrossRef]
- Srivastava, R.; Tyagi, R. Wildlife Corridors in India. Environ. Law Rev. 2016, 18, 205–223. [Google Scholar] [CrossRef] [Green Version]





| Path and Row | Year 2002 Source: Landsat 7 ETM + Level-1 | Year 2019 Source: Landsat 8 OLI/TIRS Level-1 |
|---|---|---|
| 144, 45 | 23 April | 22 April |
| 145, 45 | 29 March | 14 April |
| Class Name | Reference Totals | Classified Totals | Number Correct | Producer’s Accuracy | User’s Accuracy |
|---|---|---|---|---|---|
| 2002 | |||||
| Dense Forest | 51 | 54 | 42 | 82.35% | 77.78% |
| Open Forest | 33 | 30 | 27 | 81.82% | 90.00% |
| Scrub Forest | 9 | 18 | 9 | 100.00% | 50.00% |
| Uncultivated Land | 21 | 15 | 15 | 71.43% | 100.00% |
| Agriculture | 60 | 57 | 54 | 90.00% | 94.74% |
| Water Bodies | 6 | 6 | 6 | 100.00% | 100.00% |
| TOTAL | 180 | 180 | 153 | ||
| 2019 | |||||
| Dense Forest | 21 | 24 | 15 | 71.43% | 62.50% |
| Open Forest | 39 | 33 | 27 | 69.23% | 81.82% |
| Scrub Forest | 39 | 42 | 36 | 92.31% | 85.71% |
| Uncultivated Land | 12 | 12 | 12 | 100.00% | 100.00% |
| Agriculture | 63 | 63 | 60 | 95.24% | 95.24% |
| Water Bodies | 6 | 6 | 6 | 100.00% | 100.00% |
| TOTAL | 180 | 180 | 156 | ||
| Class Name | 2002 | 2019 |
|---|---|---|
| Dense Forest | 0.69 | 0.58 |
| Open Forest | 0.88 | 0.77 |
| Scrub Forest | 0.47 | 0.82 |
| Uncultivated Land | 1.00 | 1.00 |
| Agriculture | 0.92 | 0.93 |
| Water | 1.00 | 1.00 |
| Overall Kappa | 0.80 | 0.83 |
| Class | AREA CHANGE | |||||
|---|---|---|---|---|---|---|
| 2002 | 2019 | CHANGE | ||||
| Ha | % Age | Ha | % Age | Ha(4 − 2) | % Age(6/2) * 100 | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Dense Forest | 46,074.48 | 30.31 | 20,767.05 | 13.66 | −25,307.43 | −54.93 |
| Open Forest | 23,931.29 | 15.74 | 27,709.03 | 18.23 | 3777.74 | 15.79 |
| Scrub Forest | 13,815.12 | 9.09 | 34,667.88 | 22.81 | 20,852.75 | 150.94 |
| Uncultivated Land | 13,229.27 | 8.70 | 10,489.25 | 6.90 | −2740.02 | −20.71 |
| Agriculture | 48,947.70 | 32.20 | 53,893.42 | 35.45 | 4945.72 | 10.10 |
| Water | 6017.29 | 3.96 | 4488.53 | 2.95 | −1528.75 | −25.41 |
| Total | 152015.15 | 100.00 | 152015.15 | 100.00 | 0.00 | |
| 2002 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LULC Classes | DF | OF | SF | UL | Ag | W | Total (2019) | % Change 2002–2019 | |
| 2019 | DF | 13,236.60 | 4733.15 | 1859.96 | 104.92 | 442.95 | 389.47 | 20,767.05 | −121.86 |
| OF | 17,286.60 | 5673.79 | 2533.59 | 206.62 | 1431.53 | 576.90 | 27,709.03 | 13.63 | |
| SF | 12,236.70 | 9939.83 | 7884.06 | 822.00 | 2664.13 | 1121.16 | 34,667.88 | 60.15 | |
| UL | 286.79 | 221.43 | 177.39 | 4247.43 | 5294.55 | 261.66 | 10,489.25 | −26.12 | |
| Ag | 2309.93 | 3182.97 | 1100.31 | 7658.06 | 38,332.00 | 1310.15 | 53,893.42 | 9.18 | |
| W | 717.86 | 180.12 | 259.81 | 190.25 | 782.54 | 2357.95 | 4488.53 | −34.06 | |
| Total (2002) | 46,074.48 | 23,931.29 | 13,815.12 | 13,229.27 | 48,947.70 | 6017.29 | 152,015.15 | ||
| 2002 | |||||||
|---|---|---|---|---|---|---|---|
| LULC Classes | Dense Forests | Open Forests | Scrub Forests | Uncultivated Land | Agriculture | Water | |
| 2019 | Dense Forest | 0.00 | 12,553.45 | 10376.74 | 181.88 | 1866.98 | 328.39 |
| Open Forest | −12,553.45 | 0.00 | 7406.24 | 14.81 | 1751.44 | −396.78 | |
| Scrub Forest | −10376.74 | −7406.24 | 0.00 | −644.60 | −1563.82 | −861.35 | |
| Uncultivated Land | −181.88 | −14.81 | 644.60 | 0.00 | 2363.51 | −71.41 | |
| Agriculture | −1866.98 | −1751.44 | 1563.82 | −2363.51 | 0.00 | −527.61 | |
| Water | −328.39 | 396.78 | 861.35 | 71.41 | 527.61 | 0.00 | |
| Area Change 2002–2019 | −25,307.43 | 3777.74 | 20852.75 | −2740.02 | 4945.72 | −1528.75 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Kauranne, T.; Mikkila, M. Land Use Change and Wildlife Conservation—Case Analysis of LULC Change of Pench-Satpuda Wildlife Corridor in Madhya Pradesh, India. Sustainability 2020, 12, 4902. https://doi.org/10.3390/su12124902
Banerjee S, Kauranne T, Mikkila M. Land Use Change and Wildlife Conservation—Case Analysis of LULC Change of Pench-Satpuda Wildlife Corridor in Madhya Pradesh, India. Sustainability. 2020; 12(12):4902. https://doi.org/10.3390/su12124902
Chicago/Turabian StyleBanerjee, Sujoy, Tuomo Kauranne, and Mirja Mikkila. 2020. "Land Use Change and Wildlife Conservation—Case Analysis of LULC Change of Pench-Satpuda Wildlife Corridor in Madhya Pradesh, India" Sustainability 12, no. 12: 4902. https://doi.org/10.3390/su12124902
APA StyleBanerjee, S., Kauranne, T., & Mikkila, M. (2020). Land Use Change and Wildlife Conservation—Case Analysis of LULC Change of Pench-Satpuda Wildlife Corridor in Madhya Pradesh, India. Sustainability, 12(12), 4902. https://doi.org/10.3390/su12124902





