Soil Health and Sustainable Agriculture
Abstract
:1. Introduction
2. Soil Biodiversity and Sustainability
3. Soil Health Components for Sustainable Agriculture
3.1. Distribution of Soil Microorganisms
3.1.1. Mycorrhizal Associations
3.1.2. Cyanobacteria
3.1.3. Nematodes
3.1.4. Soil Borne Pathogens
3.2. Farming Practices to Improve Soil Health Component
3.2.1. Organic Farming
3.2.2. Tillage Practices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchere, V.; Alberola, C.; Menassieu, J. Agronomy for sustainable agriculture. A review. Agron. Sustain. Dev. 2009, 29, 1–6. [Google Scholar] [CrossRef]
- Doran, J.W. Soil health and global sustainability: Translating science into practice. Agric. Ecosyst. Environ. 2002, 88, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Timsina, J. Can organic sources of nutrients increase crop yields to meet global food demand? Agronomy 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Devarinti, S.R. Natural Farming: Eco-Friendly and Sustainable? Agrotechnology 2016, 5, 147. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Lal, R. Soils and sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 57–64. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Sahu, P.; Singh, D.; Prabha, R.; Meena, K.; Abhilash, P. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y.; Dong, X. Strip tillage improves soil biological activity, fruit yield and sugar content of triploid watermelon. Soil Tillage Res. 2016, 163, 266–273. [Google Scholar] [CrossRef]
- Meena, R.; Bohra, J.; Singh, S.; Meena, V.; Verma, J.; Verma, S.; Sihag, S. Towards the prime response of manure to enhance nutrient use efficiency and soil sustainability a current need: A book Review. J. Clean. Prod. 2016, 1258–1260. [Google Scholar] [CrossRef]
- Dotaniya, M.; Meena, V.; Basak, B.; Meena, R. Potassium uptake by crops as well as microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 267–280. [Google Scholar]
- Van der Heijden, M.; Bardgett, R.; van Straalen, N. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S. Plant–Microbe Interactions: A Viable Tool for Agricultural Sustainability Plant Microbes Symbiosis: Applied Facets; Arora, N.K., Ed.; Springer: New Delhi, India; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015; p. 384. [Google Scholar]
- Al-Karaki, G.; Othman, Y.; Al-Ajmi, A. Effects of mycorrhizal fungi inoculation on landscape turf establishment under Arabian Gulf region conditions. Arab Gulf J. Sci. Res. 2007, 25, 147–152. [Google Scholar]
- Leskovar, D.; Othman, Y. Organic and conventional farming differentially influenced soil respiration, physiology, growth, and head quality of artichoke cultivars. J. Soil Sci. Plant Nutr. 2018, 18, 865–880. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Patel, D.P.; Kumar, M.; Ramkrushna, G.I.; Mukherjee, A.; Layek, J.; Ngachan, S.V.; Buragohain, J. Impact of seven years of organic farming on soil and produce quality and crop yields in eastern Himalayas, India. Agric. Ecosyst. Environ. 2017, 236, 142–153. [Google Scholar] [CrossRef]
- Crittenden, S.; Eswaramurthy, T.; de Goede, R.; Brussaard, L.; Pulleman, M. Effect of tillage on earthworms over short- and medium-term in conventional and organic farming. Appl. Soil Ecol. 2014, 83, 140–148. [Google Scholar] [CrossRef]
- Turbé, A.; De Toni, A.; Benito, P.; Lavelle, P.; Lavelle, P.; Ruiz, N.; Van der Putten, W.; Labouze, E.; Mudgal, S. Soil Biodiversity: Functions, Threats and Tools for Policy Makers. Report for European Commission, DG Environment. 2010. Available online: https://ec.europa.eu/environment/archives/soil/pdf/biodiversity_report.pdf (accessed on 8 June 2020).
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Wagg, C.; Veresoglou, S.; Hempel, S.; Rillig, M. How soil biota drive ecosystem stability. Trends Plant Sci. 2018, 23, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.M.; Wall, D.H. Trends in Global Biodiversity: Soil Biota and Processes. In The Encyclopedia of the Anthropocene; DellaSala Dominick, A., Goldstein Michael, I., Eds.; Elsevier: Oxford, UK, 2018; Volume 3, pp. 125–130. [Google Scholar] [CrossRef]
- Bardgett, R.; van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; Van Bodeom, P.M. Global drivers and patterns of microbial abundance in soil. Global Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I.; Chotte, J.L.; De Deyn, G.B.; Eggleton, P.; Fierer, N.; Fraser, T.; et al. Global Soil Biodiversity Atlas; European Commission, Publications Office of the European Union: Luxembourg, 2016; p. 176. Available online: https://esdac.jrc.ec.europa.eu/content/global-soil-biodiversity-maps-0 (accessed on 11 June 2020).
- Ritz, K.; Black, H.I.J.; Campbell, C.D.; Harris, J.A.; Wood, C. Selecting the biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecol. Indic. 2009, 9, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whalen, J. Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Adv. Agric. 2014, 1–13. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.; Andrews, S.S. Soil quality: Why and how? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Sahu, N.; Vasu, D.; Sahu, A.; Lal, N.; Singh, S.K. Strength of Microbes in Nutrient Cycling: A Key to Soil Health. In Agriculturally Important Microbes for Sustainable Agriculture; Meena, V., Mishra, P., Bisht, J., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 69–86. [Google Scholar] [CrossRef]
- Karlen, D.; Mausbach, M.; Doan, J.; Cline, R.; Harris, R.; Schuman, G. Soil quality: A concept definition and framework or evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bouma, J.; van Ittersum, M.; Stoorvogel, J.; Batjes, N.; Droogers, P.; Pulleman, M. Soil capability: Exploring the functional potentials o soil. In Global Soil Security; Springer: Cham, Switzerland, 2017; pp. 27–44. [Google Scholar]
- Tóth, G. Agri-Environmental Soil Quality Indicator in the European Perspective; European Commission Joint Research Centre, OECD: Ispra, Italy, 2008; p. 12. [Google Scholar]
- Chaussod, R. La qualité biologique des sols: Des concepts aux applications. Comptes Rendus de l’Académie d’Agriculture de France 2002, 88, 61–68. [Google Scholar]
- Acton, D.; Gregorich, L. Understanding soil health. In The Health of Our Soil; Acton, D.F., Gregorich, L.J., Eds.; Towards sustainable agriculture in Canada; Centre or Land and Biological Resources Research Branch, Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1995; p. 138. [Google Scholar]
- More, S.D. Soil quality indicators or sustainable crop productivity. J. Indian Soc. Soil Sci. 2010, 58, 5–11. [Google Scholar]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Harris, J. Soil microbial communities and restoration ecology: Facilitators or followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Soil fertility management and insect pests: Harmonizing soil and plant health in agroecosystems. Soil Tillage Res. 2003, 72, 203–211. [Google Scholar] [CrossRef]
- Giller, K.; Bignell, D.; Lavelle, P.; Swift, M.; Barrios, E.; Moreia, F.; van Noordwijk, M.; Barois, I.; Karanja, N.; Huising, J. Soil Biodiversity in Rapidly Changing Tropical Landscapes: Scaling down and Scaling up. In Biological Diversity and Function in Soils (Ecological Reviews); Bardgett, R., Usher, M., Hopkins, D., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 295–318. [Google Scholar] [CrossRef] [Green Version]
- Drażkiewicz, M. Distribution of microorganisms in soil aggregates: Effect of aggregate size. Folia Microbiol. 1994, 39, 276–282. [Google Scholar] [CrossRef]
- Tsiknia, M.; Paranychianakis, N.V.; Varouchakis, E.A.; Moraetis, D.; Nikolaidis, N.P. Environmental divers of soil microbial community distribution at the Koiliaris Critical Zone Observatory. FEMS Microbiol. Ecol. 2014, 90, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Dong, X.; Jifon, J.; Leskovar, D. Rhizosphere microbial biomass is affected by soil type, organic and water inputs in a bell pepper system. Appl. Soil Ecol. 2019, 138, 80–87. [Google Scholar] [CrossRef]
- Bowen, G.; Rovira, A. The rhizosphere and its management to improve plant growth. Adv. Agron. 1999, 66, 1–102. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Rhodes, L.; Gerdeman, J. Phosphate uptake zones ofmycorrhizal and non-mycorrhizal onions. New Phytol. 1975, 75, 555–561. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moenne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Dessaux, Y.; Grandcle’ment, C.; Faure, D. Engineering the rhizosphere. Trends Plant Sci. 2016, 21, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ruyter-Spira, C.; Bouwmeester, H.J. Engineering the plant rhizosphere. Curr. Opin. Biotechnol. 2015, 32, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Klironomos, J.N.; HilleRisLambers, J.; Kinkel, L.L.; Reich, P.B.; Xiao, K.; Rillig, M.C.; Sikes, B.A.; Callaway, R.M.; Mangan, S.A.; et al. Soil microbes drive the classic plant diversity-productivity pattern. Ecology 2011, 92, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Jansa, J.; Schmid, B.; van der Heijden, M.G.A. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 2011, 14, 1001–1009. [Google Scholar] [CrossRef]
- Buckling, A.; Harrison, F.; Vos, M.; Brockhurst, M.A.; Gardner, A.; West, S.A.; Griffin, A. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol. Ecol. 2007, 62, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.J.; Thomashow, L.S.; Weller, D.M.; Fujimoto, D.; Mazzola, M.; Bangera, G.; Kim, D.S. Molecular mechanisms of defense by Rhizobacteria against root disease. Proc. Natl. Acad. Sci. USA 1995, 92, 4197–4201. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and pathogenic soil bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Schenk, S.T.; Stein, E.; Kogel, K.H.; Schikora, A. Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal. Behav. 2012, 7, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Mela, F.; Fritsche, K.; De Boer, W.; Van Veen, J.A.; De Graaff, L.H.; Van Den Berg, M.; Leveau, J.H. Dual transcriptional profiling of a bacterial/fungal confrontation: Collimonas fungivorans versus Aspergillus niger. ISME J. 2011, 5, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Kent, A.D.; Triplett, E.W. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu. Rev. Microbiol. 2002, 56, 211–236. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihajlović, M.; Emil, R.; Jovana, H.; Mila, G.; Brankica, T. Methods for management of soil borne plant pathogens. Pesticidi I fitomedicina 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cassán, F.; Perrig, D.; Sgroy, V.; Masciarelli, O.; Penna, C.; Luna, V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur. J. Soil Biol. 2009, 45, 28–35. [Google Scholar] [CrossRef]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere engineering and management for sustainable agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar] [CrossRef]
- Loper, J.E.; Gross, H. Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. Eur. J. Plant Pathol. 2007, 119, 265–278. [Google Scholar] [CrossRef]
- Schrey, S.D.; Schellhammer, M.; Ecke, M.; Hampp, R.; Tarkka, M.T. Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol. 2005, 168, 205–216. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef] [Green Version]
- Long, S.R. Genes and signals in the rhizobium legume symbiosis. Plant Physiol. 2001, 125, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.A.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species- opportunistic, a virulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization-a global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, D.; Priyadarshani, I.; Rath, B. Cyanobacteria-as potential biofertilizer. CIBTech. J. Microbiol. 2012, 1, 20–26. [Google Scholar]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef]
- Shodo, M. Bacterial control of plant diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant diseases. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Van Loon, L. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Endomycorrhizal and rhizobial symbiosis: How much do they share? J. Plant Interact. 2007, 2, 79–88. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology and Biochemistry, 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; p. 598. [Google Scholar] [CrossRef]
- Linderman, R.G. Mycorrhizal interactions in the rhizosphere. In The Rhizosphere and Plant Growth. Beltsville Symposia in Agricultural Research; Keister, D.L., Cregan, P.B., Eds.; Springer: Dordrecht, The Netherlands, 1991; Volume 14, pp. 343–348. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Johansen, A.; George, E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 2000, 226, 275–285. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. In Management of Mycorrhizas in Agriculture, Horticulture and Forestry; Robson, A.D., Abott, L.K., Malaccjuk, N., Eds.; Plant Soil; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1994; Volume 159, pp. 89–102. [Google Scholar] [CrossRef]
- Morrison, E.N.; Emery, R.J.N.; Saville, B.J. Phytohormone Involvement in the Ustilago maydis–Zea mays Pathosystem: Relationships between abscisic acid and cytokinin levels and strain virulence in infected cob tissue. PLoS ONE 2015, 10, e0130945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahat, M.M.; Kamaruzaman, S.; Othman, R. Mycorrhizal fungi as a biocontrol agent. Plant Pathol. J. 2010, 9, 198–207. [Google Scholar] [CrossRef]
- Panwar, J.; Yadav, R.S.; Yadav, B.K.; Tarafdar, J.C. Arbuscular Mycorrhizae: A dynamic micro-symbiont for sustainable agriculture: In Mycorrhizae: Sustainable Agriculture and Forestry; Springer: Dordrecht, The Netherlands, 2008; Volume 15, pp. 9–176. [Google Scholar] [CrossRef]
- Heidari, M.; Karami, V. Effects of different mycorrhiza species on grain yield, nutrient uptake and oil content of sunflower under water stress. J. Saudi Soc. Agric. Sci. 2014, 13, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Mobasser, H.R.; Moradgholi, A.; Mehraban, A.; Koohkan, S. Investigation of mycorrhizal effect on agronomic traits and protein percent of corn varieties in Sistan. Int. J. Agric. Sci. 2012, 2, 108–119. [Google Scholar]
- Prasanna, R.; Jaiswal, P.; Nayak, S.; Sood, A.; Kaushik, B.D. Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J. Microbiol. 2009, 49, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Zulpa, G.; Zaccaro, M.C.; Boccazzi, F.; Parada, J.L.; Storni, M. Bioactivity of intra and extracellular substances from cyanobacteria and lactic acid bacteria on “wood blue stain” fungi. Biol. Control 2003, 27, 345–348. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Craig, L.M.; Kerr, E.D.; Steadman, J.R. Influences of antagonist population levels, blossom development stage and canopy temperature on the inhibition of the Sclerotinia sclerotiorum on dry edible bean by Erwinia herbicola. Phytopathology 1994, 84, 495–501. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shouny, W.A.; Osman, M.E.; El-gammal, E.W. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- El-Enany, A.E.; Issa, A.A. Cyanobacteria as a biosorbent of heavy metals in sewage water. Environ. Toxicol. Pharmacol. 2000, 8, 95–101. [Google Scholar] [CrossRef]
- Dominic, T.; Madhusoodanan, P. Cyanobacteria from extreme acidic environments. Curr. Sci. 1999, 77, 1021–1023. [Google Scholar]
- Belnap, J.; Lange, O. Biological soil crusts: Structure, function, and management. Ecol. Stud. 2001, 150. [Google Scholar] [CrossRef]
- Perez, R.; Forchhammer, K.; Salerno, G.; Maldener, I. Clear differences in metabolic and morphological adaptations of akinetes of two Nostocales living in different habitats. Microbiology 2016, 162, 214–223. [Google Scholar] [CrossRef]
- Kultschar, B.; Llewellyn, C. Secondary metabolites in cyanobacteria. In Secondary Metabolites-Sources and Applications; InTech: London, UK, 2018; Volume 2, pp. 23–36. [Google Scholar]
- Nisha, R.; Kaushik, A.; Kaushik, C.P. Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma 2007, 138, 49–56. [Google Scholar] [CrossRef]
- Kaushik, S.; Sahu, B.K.; Lawania, R.K.; Tiwari, R.K. Occurrence of heavy metals in lentic water of Gwalior region. Pollut. Res. 1999, 18, 137–140. [Google Scholar]
- Singh, J.S. Cyanobacteria: A vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Clim. Chang. Environ. Sustain. 2014, 2, 133–137. [Google Scholar]
- Acea, M.J.; Prieto Fernandez, A.; Diz Cid, N. Cyanobacterial inoculation of heated soils: Effect on microorganisms of C and N cycles and on chemical composition in soil surface. Soil Biol. Biochem. 2003, 35, 513–524. [Google Scholar] [CrossRef]
- DeCaire, G.Z.; DeCano, M.S.; DeMule, M.C.; Palma, R.M.; Colombo, K. Exopolysaccharide of Nostoc muscorum (cyanobacteria) in the aggregation of soil particles. J. Appl. Phycol. 1997, 4, 249–253. [Google Scholar] [CrossRef]
- Tassara, C.; Zaccaro, M.C.; Storni, M.M.; Palma, M.; Zulpa, G. Biological control of lettuce white mold with cyanobacteria. Int. J. Agric. Biol. 2008, 10, 487–492. [Google Scholar]
- Moura, G.S.; Franzener, G. Biodiversity of nematodes biological indicators of soil quality in the agroecosystems. Arq. Inst. Biol. 2017, 84, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ritz, K.; Trudgill, D. Utility of nematode community analysis as an integrated measure of the functional state of soils: Perspectives and challenges. Plant Soil 1999, 212, 1–11. [Google Scholar] [CrossRef]
- Stone, D.; Costa, D.; Daniell, T.; Mitchell, S.; Topp, C.; Griffiths, B. Using nematode communities to test a European scale soil biological monitoring programme for policy development. Appl. Soil Ecol. 2016, 97, 78–85. [Google Scholar] [CrossRef]
- Lambert, K.; Bekal, S. Introduction to plant-parasitic nematodes. Plant Health Instr. 2002, 10, 1094–1218. [Google Scholar] [CrossRef]
- Mekonen, S.; Petros, I.; Hailemariam, M. The role of nematodes in the processes of soil ecology and their use as bio-indicators. Agric. Biol. J. N. Am. 2017, 8, 132–140. [Google Scholar]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Moreno, S.; Nicola, N.L.; Ferris, H.; Zalom, F.G. Effects of agricultural management on nematode-mite assemblages: Soil food web indices as predictors of mite community composition. Appl. Soil Ecol. 2009, 41, 107–117. [Google Scholar] [CrossRef]
- Johan, H.; Leveau, J.; Gail, M.P. Bacterial mycophagy: Definition and diagnosis of a unique bacterial–fungal interaction. New Phytol. 2008, 177, 859–876. [Google Scholar] [CrossRef]
- Khan, Z.; Kim, Y.H. A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl. Soil Ecol. 2007, 35, 370–379. [Google Scholar] [CrossRef]
- Chen, J.; Ferris, H. The effects of nematode grazing on nitrogen mineralization during fungal decomposition of organic matter. Soil Biol. Biochem. 1999, 3, 1265–1279. [Google Scholar] [CrossRef]
- Quist, C.W.; Gort, G.; Mulder, C.; Wilbers, R.H.P.; Termorshuizen, A.J.; Bakker, J.; Helder, J. Feeding preference as a main determinant of microscale patchiness among terrestrial nematodes. Mol. Ecol. Resour. 2017, 17, 1257–1270. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar] [PubMed]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar]
- Susilo, F.X.; Neutel, A.M.; van Noordwijk, M.; Hairiah, K.; Brown, G.; Swift, M.J. Soil biodiversity and food webs. In Below-Ground Interactions in Tropical Agroecosystems: Concepts and Models with Multiple Plant Components; Van Noordwijk, M., Cadisch, G., Ong, C.K., Eds.; CABI, International: Wallingford, UK, 2004; pp. 285–307. [Google Scholar] [CrossRef]
- Koike, S.; Subbarao, K.; Davis, R.M.; Turini, A.T. Vegetable Diseases Caused by Soilborne Pathogens; UCANR Publications University of California: Oakland, CA, USA, 2003; Available online: https://anrcatalog.ucanr.edu/pdf/8099.pdf (accessed on 7 July 2019).
- Astrom, B.; Gerhardson, B. Differential reactions of wheat and pea genotypes to root inoculation with growth-affecting rhizosphere bacteria. Plant Soil 1998, 109, 263–269. [Google Scholar] [CrossRef]
- Stirling, G.; Hayden, H.; Pattison, T.; Stirling, M. Soil health, soil biology, soilborne diseases and sustainable agriculture: A guide. Australas. Plant Pathol. 2017, 46, 387. [Google Scholar] [CrossRef] [Green Version]
- Ellouze, W.; Esmaeili Taheri, A.; Bainard, L.D.; Yang, C.; Bazghaleh, N.; Navarro-Borrell, A.; Hanson, K.; Hamel, C. Soil fungal resources in annual cropping systems and their potential for management. Biomed. Res. Int. 2014, 53, 18–24. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Olanya, O.M.; Halloran, J.M.; He, Z. Impacts of crop rotation and irrigation on soilborne diseases and soil microbial communities. In Sustainable Potato Production: Global Case Studies; He, Z., Larkin, R., Honeycutt, W., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Goreta Ban, S.; Zanic, K.; Dumiciv, G.; Raspudic, E.; Selak, G.; Ban, D. Growth and yield of grafted cucumbers in soil infested with root-knot nematodes. Chil. J. Agric. Res. 2014, 74, 29–34. [Google Scholar] [CrossRef]
- Govaerts, B.; Fuentes, M.; Mezzalama, M.; Nicol, J.M.; Deckers, J.; Etchevers, J.D.; Figueroa-Sandoval, B.; Sayre, K.D. Infiltration, soil moisture, root rot and nematode populations after 12 years of different tillage, residue and crop rotation managements. Soil Tillage Res. 2007, 94, 209–219. [Google Scholar] [CrossRef]
- Dong, K.; Dong, Y.; Zheng, L.; Tang, Z.; Yang, X. Faba bean fusarium wilt (Fusarium oxysporum) control and its mechanism in different wheat varieties and faba bean intercropping system. Chin. J. Appl. Ecol. 2014, 25, 1979–1987. [Google Scholar]
- Gutierrez, W.A.; Shew, H.D.; Melton, T.A. Sources of inoculum and management for Rhizoctonia solani damping-off on tobacco transplants under greenhouse conditions. Plant Dis. 1997, 81, 604–606. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.L.; Subbarao, K.V.; Schulback, K.F.; Koike, S.T. Effects of crop rotation and irrigation on Verticillium dahliae microsclerotia in soil and wilt in cauliflower. Phytopathology 1998, 88, 1046–1055. [Google Scholar] [CrossRef] [Green Version]
- Ristaino, J.B.; Perry, K.B.; Lumsden, R.D. Effect of solarization and Gilocladium virens on sclerotia of Sclerotium folfsii, soil microbiota and the incidence of southern blight of tomato. Phytopathology 1991, 81, 1117–1124. [Google Scholar] [CrossRef]
- Sid, A.; Pérez, S.C.; Egea, G.C.; Candela, M.E. Evaluation of the capacity of Trichoderma harzianum in controlling rot caused by Phytophthora capsici in pepper plants. Plant Pathol. 1999, 48, 58–65. [Google Scholar] [CrossRef]
- Tanović, B.; Potočnik, I.; Delibašic, G.; Ristić, M.; Kostić, M.; Marković, M. In vitro effect of essential oils from aromatic and medicinal plants on mushroom pathogens: Verticillium fungicola var. fungicola, Mycogone perniciosa, and Cladobotryum sp. Arch. Biol. Sci. 2009, 61, 231–237. [Google Scholar]
- Taiwo, L.B.; Adebayo, D.T.; Adebayo, O.S.; Adediran, J.A. Compost and Glomus mosseae for management of bacterial and Fusarium wilts of tomato. Int. J. Veg. Sci. 2007, 13, 49–61. [Google Scholar] [CrossRef]
- Norman, D.J.; Chen, J.; Yuen, J.M.F.; Mangravita-Novo, A.; Byrne, D.; Walsh, L. Control of bacterial wilt of geranium with phosphorous acid. Plant Dis. 2006, 90, 798–802. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; de Freitas, V.; Delerue-Matos, C.; Mateus, N. Strawberries from integrated pest management and organic farming: Phenolic composition and antioxidant properties. Food Chem. 2012, 134, 1926–1931. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term application of chemical and organic fertilizers on plant-available nitrogen pools and nitrogen management index. Biol. Fertil. Soils 2011, 47, 767–775. [Google Scholar] [CrossRef]
- Mäder, P.; Fließbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Schrama, M.; de Haan, J.; Kroonen, M.; Verstegen, H.; Van der Putten, W. Crop yield gap and stability in organic and conventional farming systems. Agric. Ecosyst. Environ. 2018, 256, 123–130. [Google Scholar] [CrossRef]
- Organic Production/Organic Food: Information Access Tools. USDA. Available online: https://www.nal.usda.gov/afsic/organic-productionorganic-food-information-access-tools (accessed on 8 June 2020).
- Matoh, T.; Saraswati, R.; Phupaibul, P.; Sekiya, J. Growth characteristics of Sesbania species under adverse edaphic conditions in relation to use as green manure in Japan. J. Soil Sci. Plant Nutr. 1992, 38, 741–747. [Google Scholar] [CrossRef]
- Chang, E.; Wang, C.; Chen, C.; Chung, R. Effects of long-term treatments of different organic fertilizers complemented with chemical N fertilizer on the chemical and biological properties of soils. J. Soil Sci. Plant Nutr. 2014, 60, 499–511. [Google Scholar] [CrossRef]
- Chou, Y.; Shen, F.; Chiang, S.; Chang, C. Functional diversity and dominant populations of bacteria in banana plantation soils as influenced by long-term organic and conventional farming. Appl. Soil Ecol. 2017, 110, 21–33. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, R.; Xue, C.; Zhang, S.; Li, S.; Zhang, N.; Shen, Q. Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol. Fertil. Soils 2012, 48, 807–816. [Google Scholar] [CrossRef]
- Atandi, J.G.; Haukeland, S.; Kariuki, G.M.; Coyne, D.L.; Karanja, E.N.; Musyoka, M.W.; Fiaboe, K.K.; Bautze, D.; Adamtey, N. Organic farming provides improved management of plant parasitic nematodes in maize and bean cropping systems. Agric. Ecosyst. Environ. 2017, 247, 265–272. [Google Scholar] [CrossRef]
- Chang, E.; Chung, R.; Wang, F. Effect of different types of organic fertilizers on the chemical properties and enzymatic activities of an Oxisol under intensive cultivation of vegetables for 4 years. J. Soil Sci. Plant Nutr. 2008, 54, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Othman, Y.; Leskovar, D. Organic soil amendments influence soil health, yield, and phytochemicals of globe artichoke heads. Biol. Agric. Hortic. 2018, 34, 258–267. [Google Scholar] [CrossRef]
- Mangalasserya, S.; Kalaivananb, D.; Philipc, P. Effect of inorganic fertilisers and organic amendments on soil aggregation and biochemical characteristics in a weathered tropical soil. Soil Tillage Res. 2019, 187, 144–151. [Google Scholar] [CrossRef]
- Fischer, R.A. Definitions and determination of crop yield, yield gaps, and of rates of change. Field Crops Res. 2015, 182, 9–18. [Google Scholar] [CrossRef]
- De Ponti, T.; Rijk, B.; van Ittersum, M. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Bassouny, M.; Chen, J. Effect of long-term organic and mineral fertilizer on physical properties in root zone of a clayey Ultisol. Arch. Agron. Soil Sci. 2016, 62, 819–828. [Google Scholar] [CrossRef]
- Escanhoela, A.S.; Pitombo, L.M.; Brandani, C.B.; Navarrete, A.A.; Bento, C.B.; Carmo, J.B. Organic management increases soil nitrogen but not carbon content in a tropical citrus orchard with pronounced N2O emissions. J. Environ. Manag. 2019, 234, 326–335. [Google Scholar] [CrossRef]
- Suja, G.; Byju, G.; Jyothi, A.N.; Veena, S.S.; Sreekumar, J. Yield, quality and soil health under organic vs conventional farming in taro. Sci. Hortic. 2017, 218, 334–343. [Google Scholar] [CrossRef]
- Maqueda, C.; Herencia, J.F.; Ruiz, J.C.; Hidalgo, M.F. Organic and inorganic fertilization effects on DTPA-extractable Fe, Cu, Mn and Zn, and their concentration in the edible portion of crops. J. Agric. Sci. 2011, 149, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Gaskell, M.; Smith, R. Nitrogen sources for organic vegetable crops. HortTechnology 2007, 17, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, R.; Hartz, T. Nitrogen sources for organic crop production. Better Crops 2008, 92, 16–19. [Google Scholar]
- Zhao, W.; Yang, X.; Yu, H.; Jiang, W.; Sun, N.; Liu, X.; Liu, X.; Zhang, X.; Wang, Y.; Gu, X. RNA-Seq-based transcriptome profiling of early nitrogen deficiency response in cucumber seedlings provides new insight into the putative nitrogen regulatory network. Plant Cell Physiol. 2015, 56, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonious, G.; Turley, E.; Snyder, J. Chicken manure enhanced yield and quality of field-grown kale and collard greens. J. Environ. Sci. Health B 2014, 49, 299–304. [Google Scholar] [CrossRef]
- Woese, K.; Lange, D.; Boess, C.; Bögl, K. A comparison of organically and conventionally grown foods-results of a review of the relevant literature. J. Sci. Food Agric. 1997, 74, 281–293. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional quality of organic versus conventional fruits, vegetables, and grains. J. Altern. Complement Med. 2001, 7, 161–173. [Google Scholar] [CrossRef]
- Fernandez-Salvador, J.; Strik, B. Liquid corn and fish fertilizers are good options for fertigation in blackberry cultivars grown in an organic production system. Hortic. Sci. 2015, 50, 225–233. [Google Scholar] [CrossRef]
- Vyn, T.; Faber, J.; Janovicek, K.; Beauchamp, E. Cover crop effects on nitrogen availability to corn following wheat. Agron. J. 2000, 92, 915–924. [Google Scholar] [CrossRef]
- Fauci, M.F.; Dick, R.P. Plant response to organic amendments and decreasing inorganic nitrogen rates in soils from long-term experiment. Soil Sci. Soc. Am. J. 1994, 58, 134–138. [Google Scholar] [CrossRef]
- Fageria, N. Green manuring in crop production. J. Plant Nutr. 2007, 30, 691–719. [Google Scholar] [CrossRef]
- Gathala, M.K.; Timsina, J.; Islam, M.S.; Rahman, M.M.; Hossain, M.I.; Harun-Ar-Rashid, M.; Ghosh, A.K.; Krupnik, T.J.; Tiwari, T.P.; McDonald, A. Conservation agriculture based tillage and crop establishment options can maintain farmers’ yields and increase profits in South Asia’s rice–maize systems: Evidence from Bangladesh. Field Crops Res. 2015, 172, 85–98. [Google Scholar] [CrossRef]
- Jabro, J.D.; Stevens, W.B.; Evans, R.G.; Iversen, W.M. Tillage effects on physical properties in two soils of the Northern Great Plains. Appl. Eng. Agric. 2009, 25, 377–382. [Google Scholar] [CrossRef]
- Gozubuyuk, Z.; Sahin, U.; Ozturk, I.; Celik, A.; Adiguzel, M.C. Tillage effects on certain physical and hydraulic properties of a loamy soil under a crop rotation in a semi-arid region with a cool climate. CATENA 2014, 118, 195–205. [Google Scholar] [CrossRef]
- Cannell, R.; Hawes, J. Trends in tillage practices in relation to sustainable crop production with special reference to temperate climates. Field Crops Res. 1994, 30, 245–282. [Google Scholar] [CrossRef]
- Jat, M.L.; Gathala, M.K.; Saharawat, Y.S.; Tetarwal, J.P.; Gupta, R. Double no-till and permanent raised beds in maize–wheat rotation of North-Western Indo-Gangetic Plains of India: Effects on crop yields, water productivity, profitability and soil physical properties. Field Crops Res. 2013, 149, 291–299. [Google Scholar] [CrossRef]
- Al-Kaisi, M.; Douelle, A.; Kwaw-Mensah, D. Soil microaggregate and macroaggregate decay over time and soil carbon change as influenced by different tillage systems. J. Soil Water Conserv. 2014, 69, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Xie, Y.; Wang, C.; Yue, J.; Yao, Y.; Liu, W. Effects of different soil conservation tillage approaches on soil nutrients, water use and wheat-maize yield in rainfed dry-land regions of North China. Eur. J. Agron. 2016, 81, 37–45. [Google Scholar] [CrossRef]
- Celik, A.; Altikat, S.; Way, T. Strip tillage width effects on sunflower seed emergence and yield. Soil Tillage Res. 2013, 131, 20–27. [Google Scholar] [CrossRef]
- Mullins, G.L.; Alley, S.E.; Reeves, D.W. Tropical maize response to nitrogen and starter fertilizer under strip and conventional tillage systems in southern Alabama. Soil Tillage Res. 1998, 45, 1–15. [Google Scholar] [CrossRef]
- Jat, M.L.; Gathala, M.K.; Ladha, J.K.; Saharawat, Y.S.; Jat, A.S.; Vipin, K.; Sharma, S.K.; Kumar, V.; Gupta, R.K. Evaluation of precision land leveling and double zero-till systems in the rice–wheat rotation: Water use, productivity, profitability and soil physical properties. Soil Tillage Res. 2009, 105, 112–121. [Google Scholar] [CrossRef]
- Saharawat, Y.S.; Ladha, J.K.; Pathak, H.; Gathala, M.; Chaudhary, N.; Jat, M.L. Simulation of resource-conserving technologies on productivity, income and greenhouse gas (GHG) emission in rice–wheat system. J. Soil Sci. Environ. 2012, 3, 9–22. [Google Scholar]
- Vivak, K.; Saharawat, Y.S.; Gathala, M.K.; Jat, A.S.; Singh, S.K.; Chaudhary, N.; Jat, M.L. Effect of different tillage and seeding methods on energy use efficiency and productivity of wheat in Indo-Gangetic plains. Field Crops Res. 2013, 142, 1–8. [Google Scholar] [CrossRef]
- Bhushan, L.; Ladha, J.K.; Gupta, R.K.; Singh, S.; Tirol-Padre, A.; Saharawat, Y.S.; Gathala, M.; Pathak, H. Saving of water and labor in a rice-wheat system with no-tillage and direct seeding technologies. Agron. J. 2008, 99, 1288–1296. [Google Scholar] [CrossRef]
- Mohammad, W.; Shah, S.M.; Shehzadi, S.; Shah, S.A. Effect of tillage, rotation and crop residues on wheat crop productivity: Fertilizer nitrogen and water use efficiency and soil organic carbon status in dry area (rainfed) of north-west Pakistan. J. Soil Sci. Plant Nutr. 2012, 12, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Wang, S.; Wang, H.; Ning, F.; Zhang, Y.; Dong, Z.; Wen, P.; Wang, R.; Wang, X.; Jun, L. The effects of rotating conservation tillage with conventional tillage on soil properties and grain yields in winter wheat-spring maize rotations. Agric. For. Meteorol. 2018, 263, 107–117. [Google Scholar] [CrossRef]
- Sengupta, A.; Dick, W. Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb. Ecol. 2015, 70, 853–859. [Google Scholar] [CrossRef]
- Yang, S.; Kim, M.; Seo, Y.; Choi, K.; Lee, S.; Kwak, Y.; Lee, Y. Soil microbial community analysis of between no-till and tillage in a controlled horticultural field. World J. Microbiol. Biotechnol. 2012, 28, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, L.; Hoyt, G.; Imbriani, J. Comparing nematode and earthworm communities under combinations of conventional and conservation vegetable production practices. Soil Tillage Res. 2010, 110, 42–50. [Google Scholar] [CrossRef]
- Sharratt, B.; Zhang, M.; Sparrow, S. Twenty years of conservation tillage research in subarctic Alaska II. Impact on soil hydraulic properties. Soil Tillage Res. 2006, 91, 82–88. [Google Scholar] [CrossRef]
- Hobbs, P.R. Conservation agriculture: What is it and why is it important for future sustainable food production? J. Agric. Sci. 2007, 145, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Araki, M.; Komatsuzaki, M.; Kaneko, N.; Ohta, H. Soil nematode community structure affected by tillage systems and cover crop managements in organic soybean production. Appl. Soil Ecol. 2015, 86, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Melman, D.; Kelly, C.; Schneekloth, J.; Calderón, F.; Fonte, S. Tillage and residue management drive rapid changes in soil macrofauna communities and soil properties in a semiarid cropping system of Eastern Colorado. Appl. Soil Ecol. 2019, 143, 98–106. [Google Scholar] [CrossRef]
- Johnson-Maynard, J.L.; Umiker, K.J.; Guy, S.O. Earthworm dynamics and soil physical properties in the first three years of no-till management. Soil Tillage Res. 2007, 94, 338–345. [Google Scholar] [CrossRef]
- Van Schaik, L.; Palm, J.; Klaus, J.; Zehe, E.; Schröder, B. Potential effects of tillage and field borders on within-field spatial distribution patterns of earthworms. Agric. Ecosyst. Environ. 2016, 228, 82–90. [Google Scholar] [CrossRef]
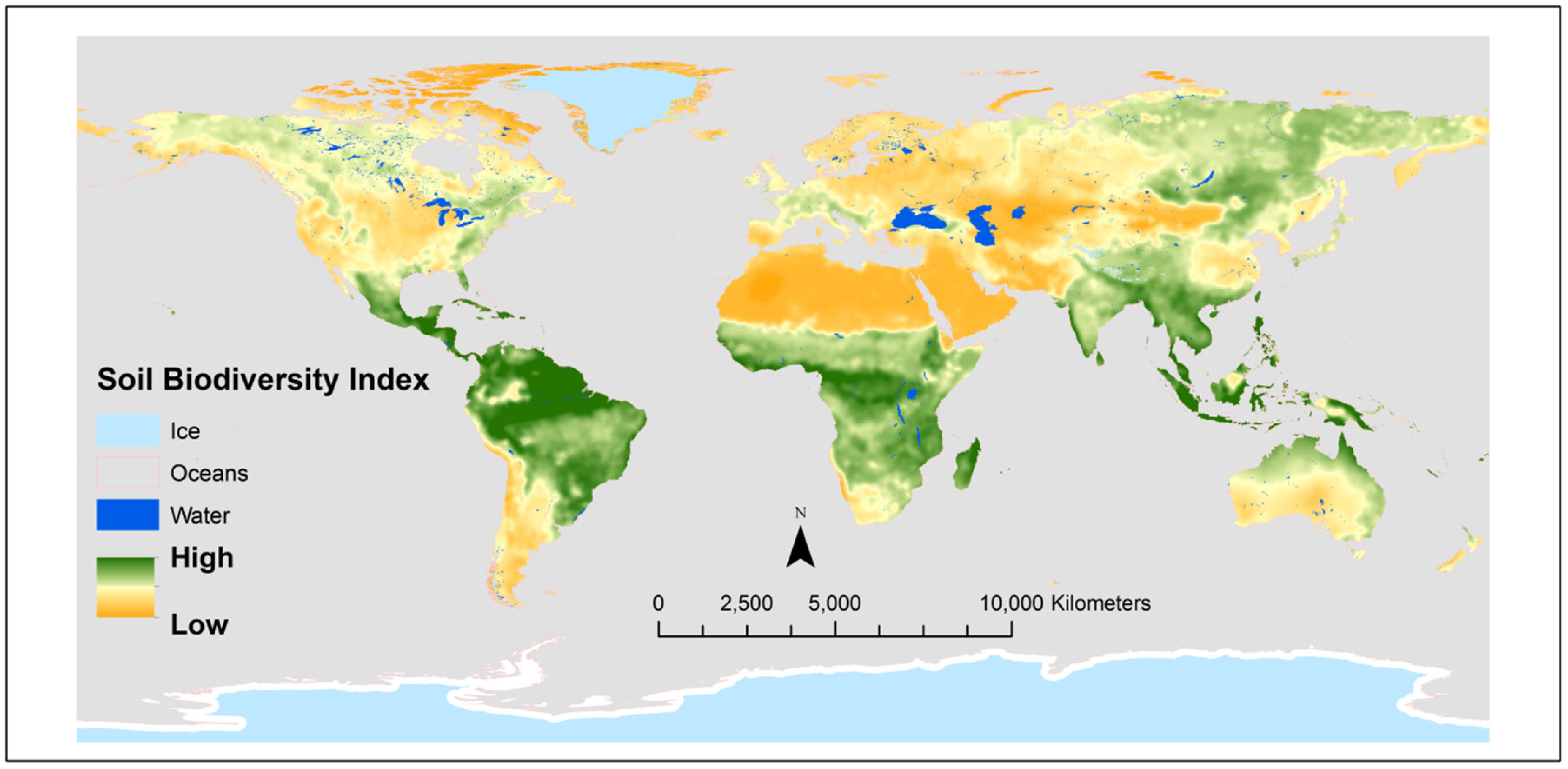
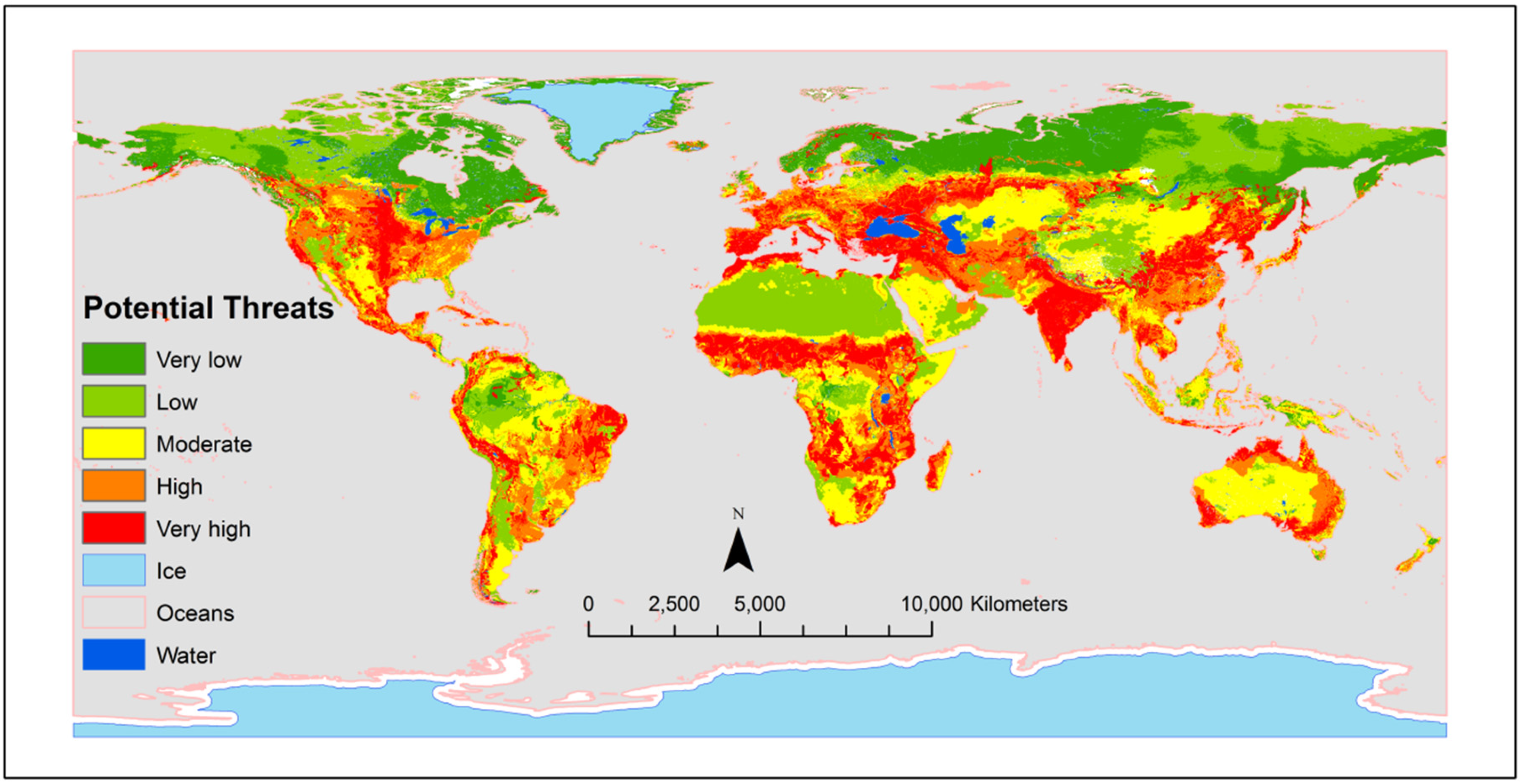


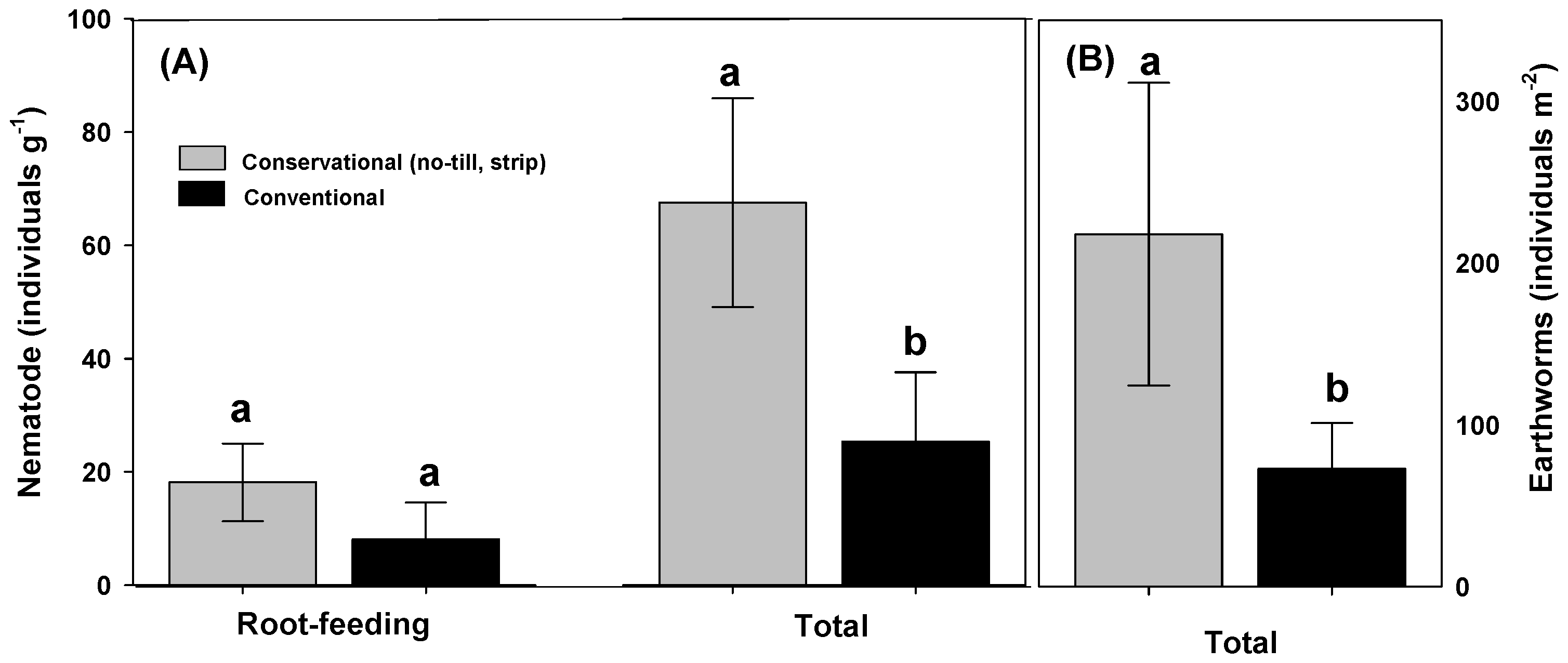
| Cultural Practices | Crops | Hypothesis | Target Pathogens | Responses | References |
|---|---|---|---|---|---|
| Crop rotation | Barley (Hordeum vulgare), rye grass (Lolium perenne) canola rape seed (Brassica napus) and potato (Solanum tuberosum). | Crop rotation (3 years) with potato will reduce Rhizoctonia infection. | Rhizoctonia spp. | The disease was reduced 15–50%. | Larkin et al. [124]. |
| Grafting | Cucumber (Cucumis sativus). | Grafting cucumber plants onto different rootstocks (a non-chemical method) reduce infection of root-knot nematodes. | Meloidogyne spp. | Plants grafted onto “Strong Tosa” rootstock had higher total number of fruits (20) and yield (5.4 kg) compared to other rootstocks or non-grafted plants in soil infected by root-knot nematodes. | Goreta Ban et al. [125]. |
| Tillage, crop rotations and crop residue | Maize (Zea mays) and wheat (Triticum aestivum). | Technical innovations (zero tillage, crop rotations, and residue management) could improve the productivity and biophysical sustainability of sub-tropical highland cropping systems. | Parasitic nematode (Pratylenchus thornei) root rot (Cochliobolus sativus). | A maize-wheat rotation decreased the incidence of maize root rot up to 30%. The incidence of root disease was lower in wheat than in maize. In maize, both non-parasitic and parasitic nematodes increased under zero tillage. | Govaerts et al. [126]. |
| Intercropping system | Faba bean (Vicia faba). | Disease control is influenced by intercropping wheat varieties, suggesting differences of root exudates as factors that affected soil-borne diseases. | Fusarium oxysporum. | Shoot biomass of faba bean increased by 13–17%, while disease index decrease faba bean fusarium wilt by 48%. | Dong et al. [127]. |
| Physical method (steam soil disinfection) | Tobacco (Nicotiana tabacum). | Injecting or diffusing hot water vapor into soil will reduce Rhizoctonia infection. | Rhizoctonia solani. | Dry heat (70 to 80 °C for 2 h) and chemical (NaCl) treatments reduced inoculum levels on trays up to 45% compared to controls. | Gutierrez et al. [128]. |
| Soil organic residue | Broccoli (Brassica oleracea). | Broccoli residues mowed and allowed to dry on the soil surface for several days reduce disease incidence. | Verticillium dahlia. | The number of propagules after two broccoli crops was reduced by 94%. Disease incidence and severity were reduced by 50% by broccoli treatments. | Xiao et al. [129]. |
| Physical method (soil solarization) and biological control | Tomato (Lycopersicon esculentum). | Soil solarization in combination with the fungal antagonistic Gilocladium virenes has the potential to manage southern blight diseases. | Sclerotium rolfsii. | Solarized soil amended with Gilocladium virenes reduced the disease incidence by 49%. | Ristaino et al. [130]. |
| Biological control | Pepper (Capsicum annuum). | Antagonistic microbes (Trichoderma harzianum) can lead to competition and reduction of disease levels. | Phytophthora capsici. | Leaf inoculation of infected pepper with Trichoderma significantly reduced necrosis legion (28 mm) compared to control. | Sid et al. [131]. |
| Biological control (antimicrobial activity of essential oils) | Lavender (Lavandula angustifolia), anise (Pimpinella anisum) chamomile (Matricaria recutita), fennel (Foeniculum vulgare), geranium (Pelargonium graveolens), oregano (Origanum vulgare), parsley (Petroselinum crispum) and sage (Salvia officinalis). | Antimicrobial activity of several essential oils can inhibit pathogens infection under in vitro conditions. | Verticillium fungicola var. fungicola, Mycogone perniciosa, and Cladobotryum sp. | A 100% inhibition of plant pathogens was achieved by oregano and geranium oil extracts at 0.32 μL mL−1 of air after 4-day exposure. | Tanović et al. [132]. |
| Bio-fertilizer (Glomus mosseae), inorganic Fertilizers | Tomato. | Competition for nutrients will lead to a reduction of pathogen population. | Bacterial wilt (Ralstonia solanacearum) and Fusarium wilt (Fusarium oxysporum). | Tomato wilt severity was reduced by 25% with Glomus mosseae relative to the control. | Taiwo et al. [133]. |
| Chemical control | Geranium (Pelargonium hortorum). | Using bactericides in irrigation water will protect geranium plants from Bacterial wilt. | Bacterial wilt (Ralstonia solanacearum). | Phosphoric acid inhibited in vitro growth of R. solanacearum. K and phosphoric acid salt (K-Phite) were very effective in protecting plants from infection at 6 × 106 CFU/g soil. | Norman et al. [134]. |
| Crop | Soil Type | Study Period (Year) | Nutrient | Response | Reference |
|---|---|---|---|---|---|
| Citrus (Citrus × sinensis) | Clay soil (Oxisols soil, 50% Clay, 20% silt; 30% sand). | 6 | N | The organic system had 2 ton ha−1 more N (p < 0.05) stocked at 0–100 cm than in conventional system. | Escanhoela et al. [152]. |
| Wheat (Triticum aestivum) maize (Zea mays) rotation | Sandy loam soil (aquic inceptisol). | 18 | N | Organic soil had 5–22% more N (p < 0.05) than conventional. | Gong et al. [136]. |
| Wheat, potatoes (Solanum tuberosum), and clover (Trifolium sp.) | Clay soil. | 21 | P, K, Ca2+, Mg2+ | Organic farming had higher (p < 0.05) Ca2+, and Mg2+ than conventional. Organic (mg kg−1): 16 P, 90 K, 2100 Ca2+, 144 Mg2+. Conventional (mg kg−1): 14 P, 95 K, 1700 Ca2+, 94 Mg2+. | Mäder et al. [137]. |
| Artichoke (Cynara cardunculus) | Clay soil (hyperthermic Aridic Calciustolls). | 2 | NO3−, P, K, Ca2+, Mg2+, S, Na | Organic soil had lower NO3−, P, K and Mg+2 and higher Ca+2 and Na than conventional. NO3−, P, K, Mg+2, Ca+2 and Na were significant at p < 0.05. Organic (mg kg−1): 5 NO3−-N, 34 P, 588 K, 11200 Ca2+, 263 Mg2+, 15.8 S, 64 Na. Conventional (mg kg−1): 22 NO3-N, 62 P, 669 K, 10800 Ca2+, 307 Mg+2, 16.3 S, 28 Na. | Leskovar and Othman [16]. |
| Cashew (Anacardium occidentale) | Loamyskeletal, mixed isohyperthermic Ustic Haplohumults. | 5 | N | Available N in organic was higher (p < 0.05, 435 kg ha−1) than conventional (402 kg ha−1). | Mangalassery et al. [148]. |
| Cowpea (Vigna unguiculata) | Loamy soil. | 4 | N, P, K, Ca2+, Mg2+, Fe, Mn, Zn and Cu | Organic farming increased available P, K, Fe, and reduced total N compared to conventional. N, P, K, Fe were significant at p < 0.05. Organic: (73 N, 111 P, 359 K kg h−1), (3500 Ca2+, 1200 Mg2+, 80 Fe, 17 Mn2+, 5.5 Zn, 1.3 Cu mg kg−1. Conventional: (86 N, 96 P, 192 K kg h−1), (2400 Ca2+, 900 Mg2+, 70 Fe, 15 Mn2+, 4.3 Zn, 1.2 Cu mg kg−1). | Suja et al. [153]. |
| Broccoli (Brassica oleracea), lettuce (Lactuca sativa), potato (Solanun tuberosum), and carrot (Dancus carota) | Loamy soil (Xerofluvent). | 5 | Fe, Mn2+, Zn and Cu | The available nutrients in organic were statistically similar to conventional fields. | Maqueda et al. [154]. |
| Conservational Tillage vs. Conventional | ||||
|---|---|---|---|---|
| Crop | Irrigation Water Applied | Total Increase in WUE | Total Increase in Net Returns in U $ S | Reference |
| Maize | Reduced irrigation water by 25%. | 16%. | $281 ha−1 | Jat et al. [169]. |
| Rice-Wheat | Reduced irrigation water by 12–20%. | 1.4 kg grain per m3 input water compared to 0.75 for conventional. | $184–280 ha−1 | Jat et al. [174]. |
| Rice-Wheat | Reduced irrigation water by 16 to 18%. | 4.2%. | $49–96 ha−1 | Saharawat et al. [175]. |
| Wheat | Fuel consumption efficiency (energy) for irrigation was 21% higher. | 4.5 t grains per ha−1 compared to 4.109 t ha−1 for conventional. | Net income in zero-tillage was 33% higher than conventional. | Vivak et al. [176]. |
| Rice-Wheat | Reduced irrigation water 13 to 23%. | −5% (conventional was 5% higher). | $62 ha−1 for year 1; similar in year 2. Weed management incurred higher cost than with conventional systems. | Bhushan et al. [177]. |
| Wheat | No data. | 6.5–11.6 (kg ha−1/mm) compared with 4.05 to 7.5 for conventional. | No-tillage resulted in 520 kg ha−1 greater wheat grain yield than conventional. | Mohammad et al. [178]. |
| Wheat-Maize | Mean soil water storage measured at 0–2 m depth for no-tillage was 412 mm compared to 392 mm for conventional. | Not significant. | $57 ha−1. | Zhang et al. [179]. |
| Wheat-Maize | Reduced irrigation water consumption by 19%. | 24.6% for wheat and 15.9% for maize. | Wheat yield increased by 10.3% and maize yield by 17.4%. | Shao et al. [171]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Tahat, M.; M. Alananbeh, K.; A. Othman, Y.; I. Leskovar, D. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. https://doi.org/10.3390/su12124859
M. Tahat M, M. Alananbeh K, A. Othman Y, I. Leskovar D. Soil Health and Sustainable Agriculture. Sustainability. 2020; 12(12):4859. https://doi.org/10.3390/su12124859
Chicago/Turabian StyleM. Tahat, Monther, Kholoud M. Alananbeh, Yahia A. Othman, and Daniel I. Leskovar. 2020. "Soil Health and Sustainable Agriculture" Sustainability 12, no. 12: 4859. https://doi.org/10.3390/su12124859
APA StyleM. Tahat, M., M. Alananbeh, K., A. Othman, Y., & I. Leskovar, D. (2020). Soil Health and Sustainable Agriculture. Sustainability, 12(12), 4859. https://doi.org/10.3390/su12124859






