Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Experiments
2.2.1. Calcination
2.2.2. Adsorption
2.3. Characterization
3. Results
3.1. Characterization
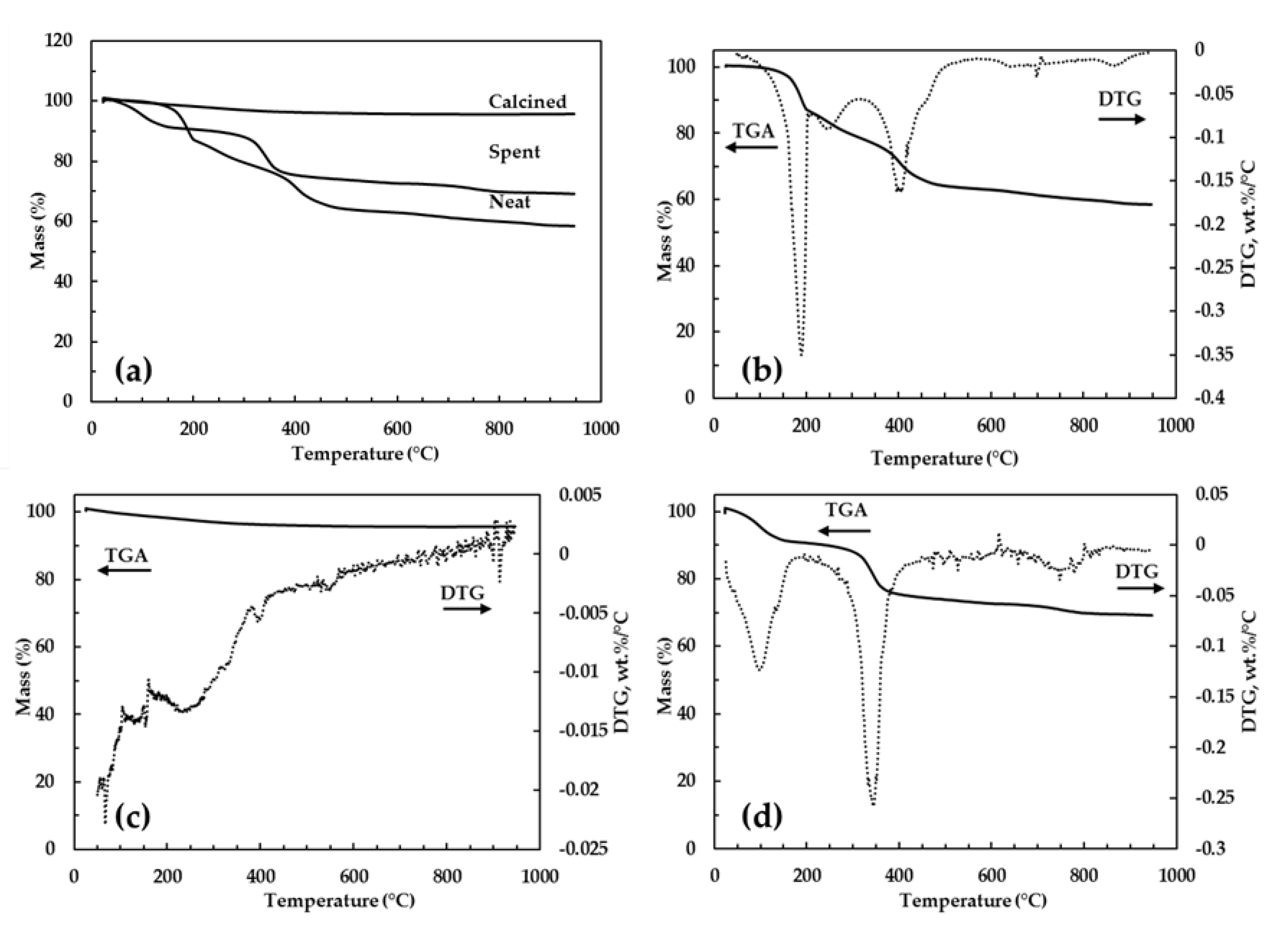
3.1.1. Thermal Analysis
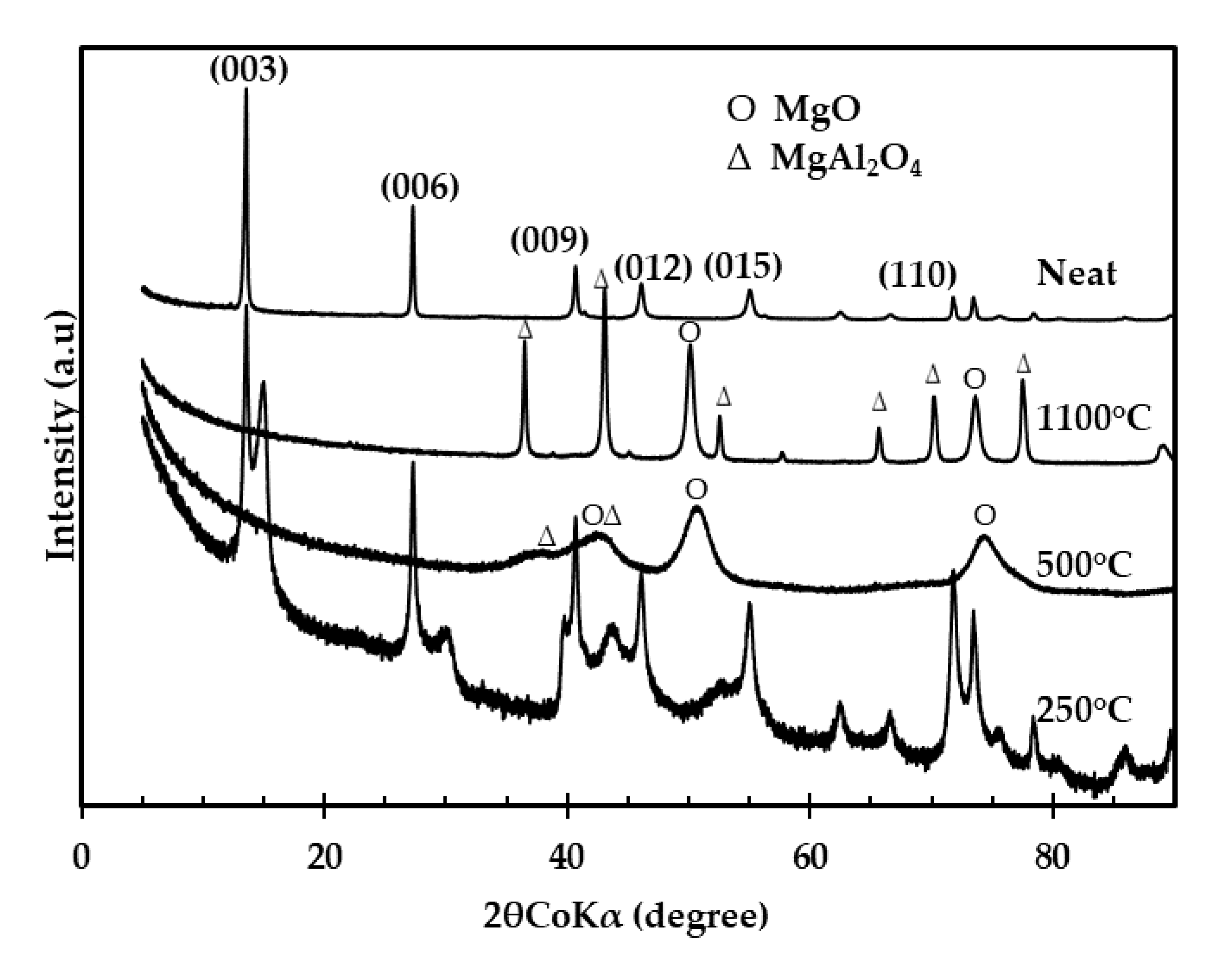
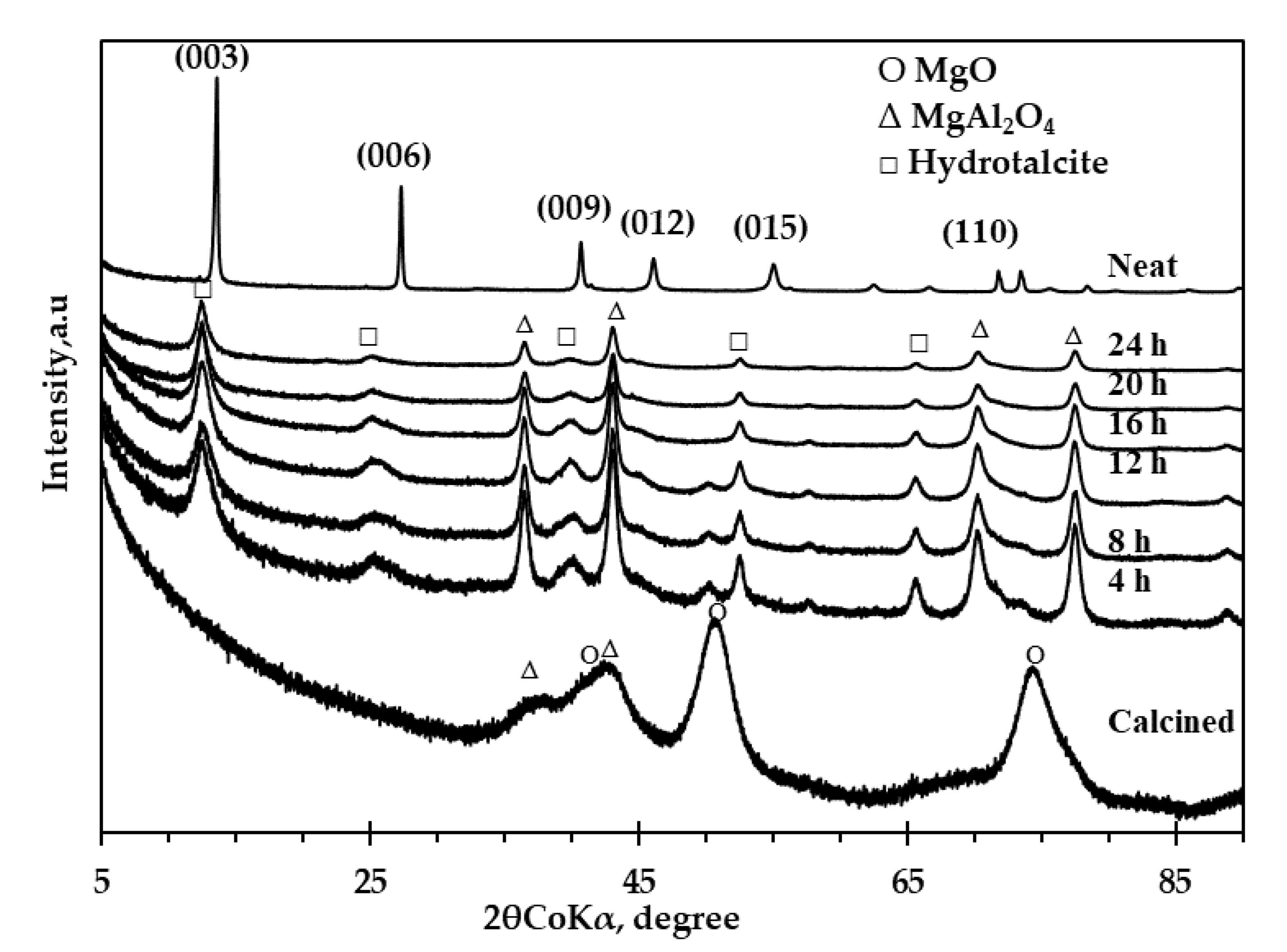
3.1.2. X-ray Diffraction (XRD) Spectra
3.1.3. Brunauer Emmett Teller Surface Area
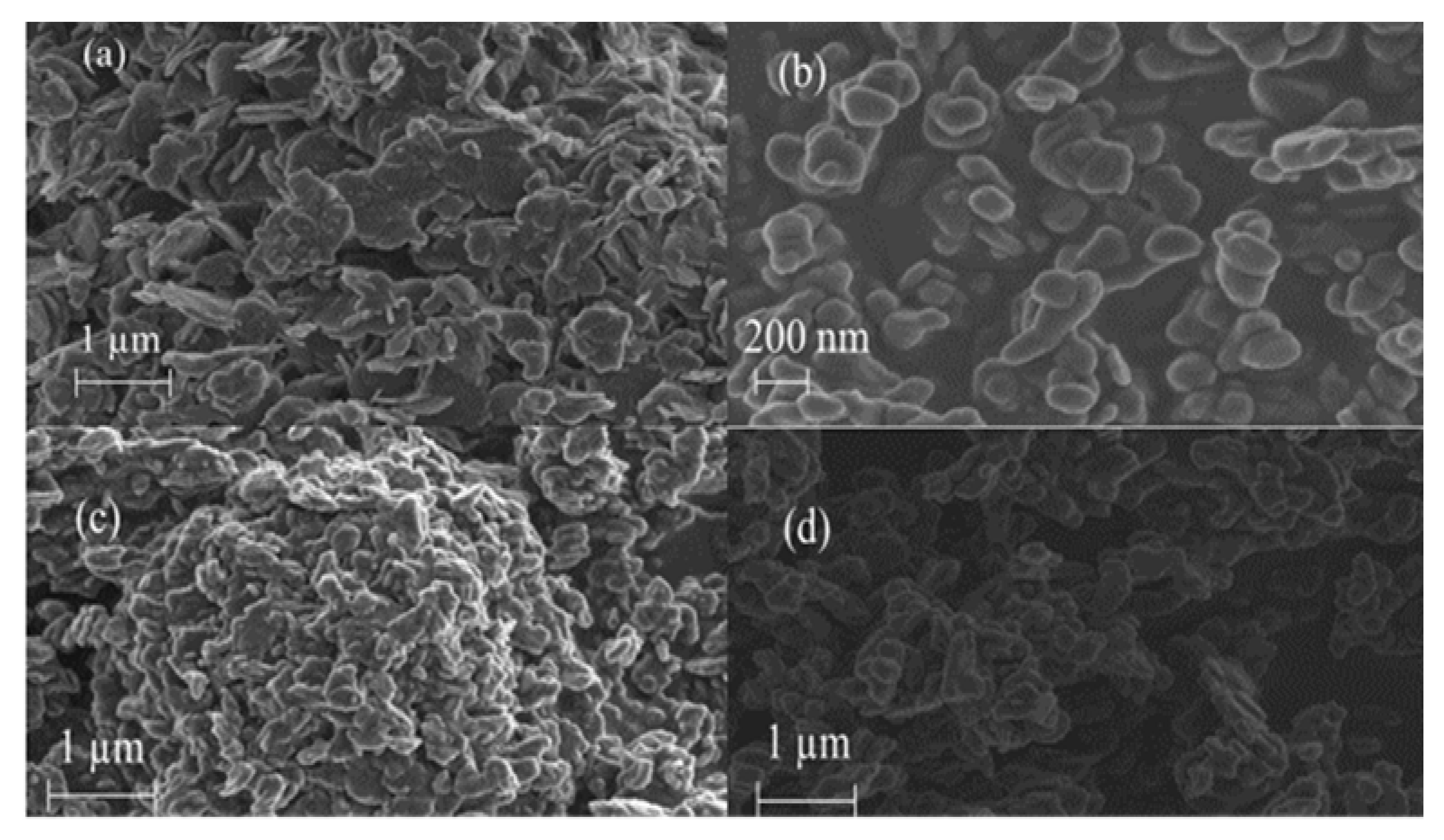
3.1.4. Scanning Electron Microscopy
3.2. Adsorption Results
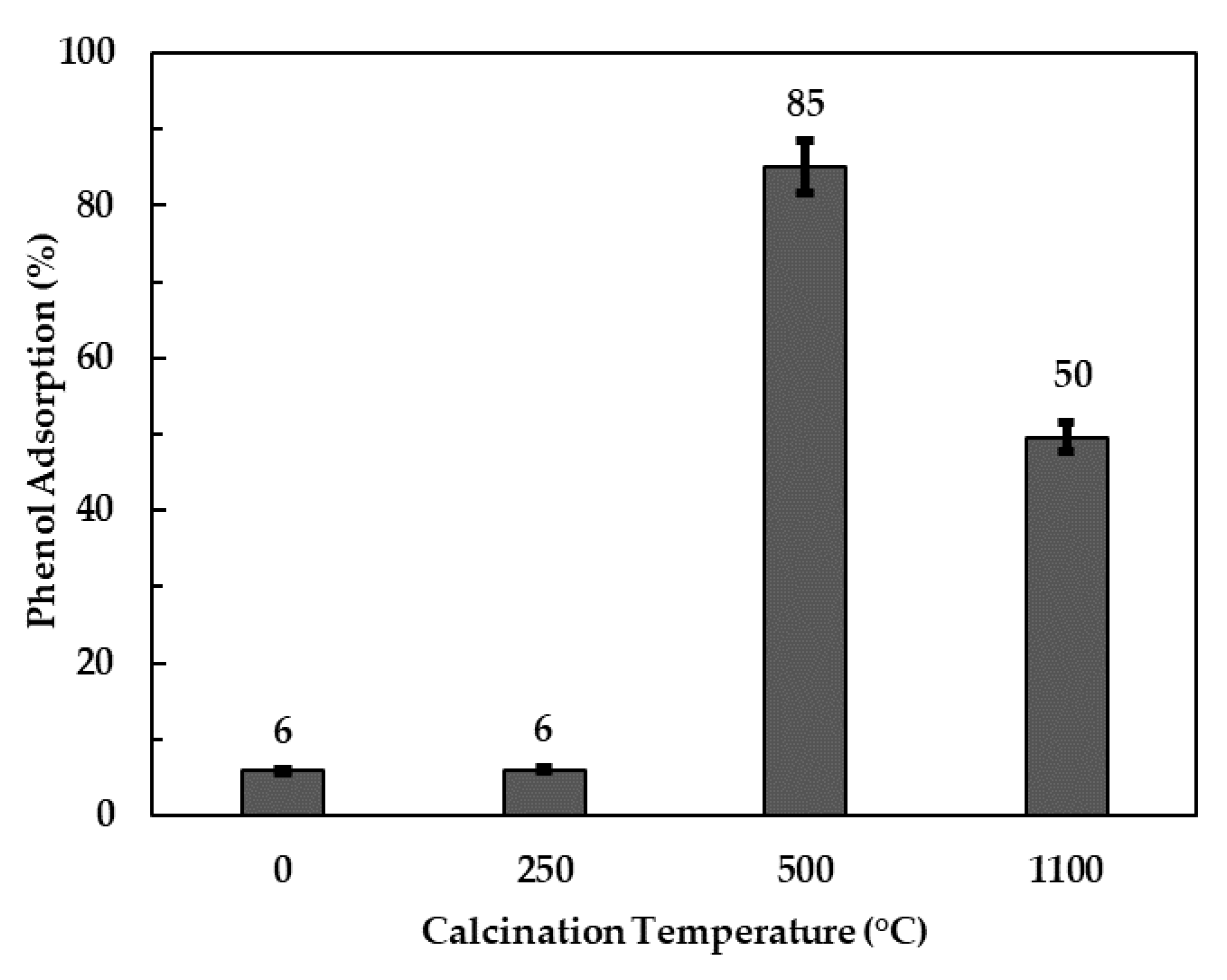
3.2.1. Effect of Calcination Temperature
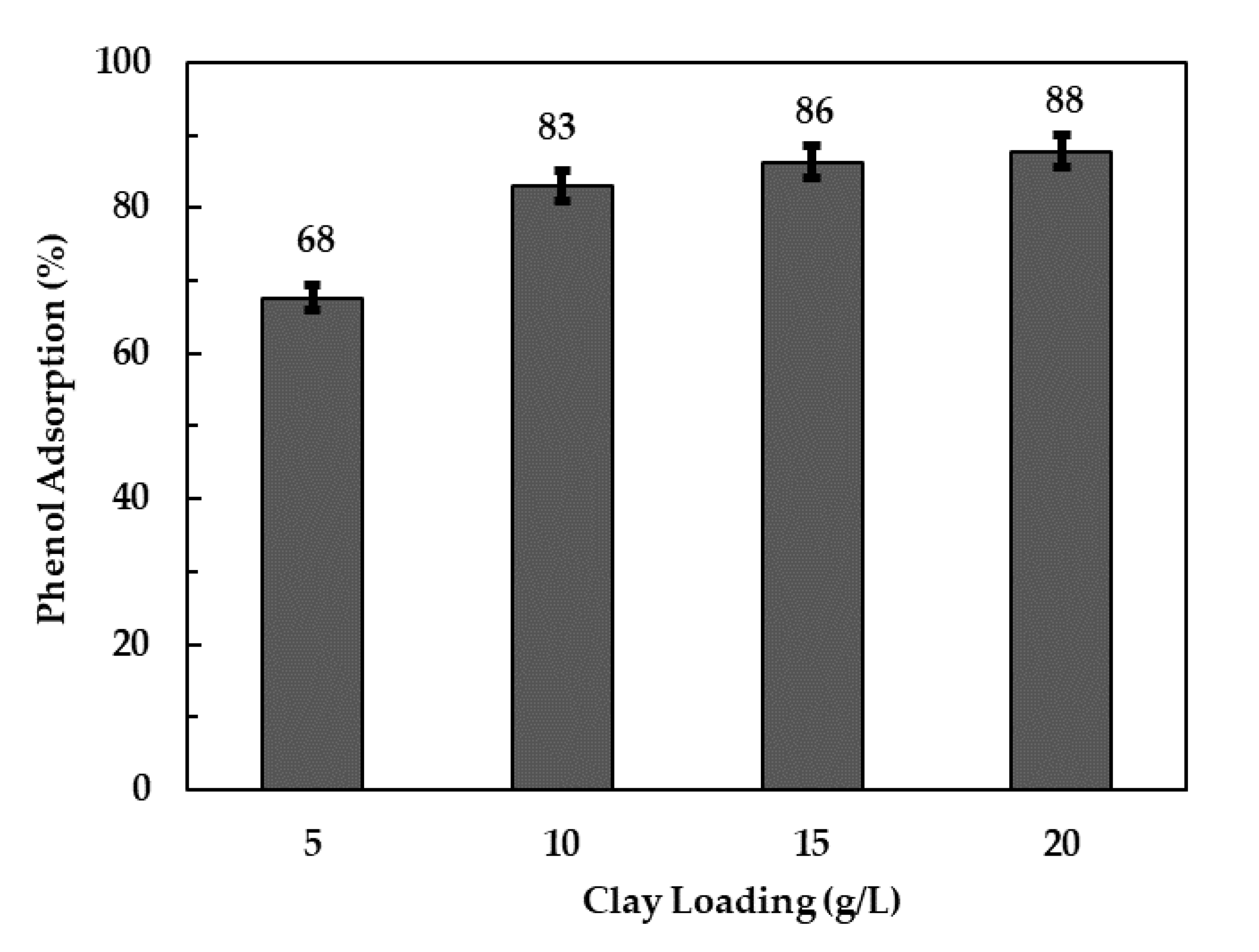
3.2.2. Effect of Adsorbent Loading
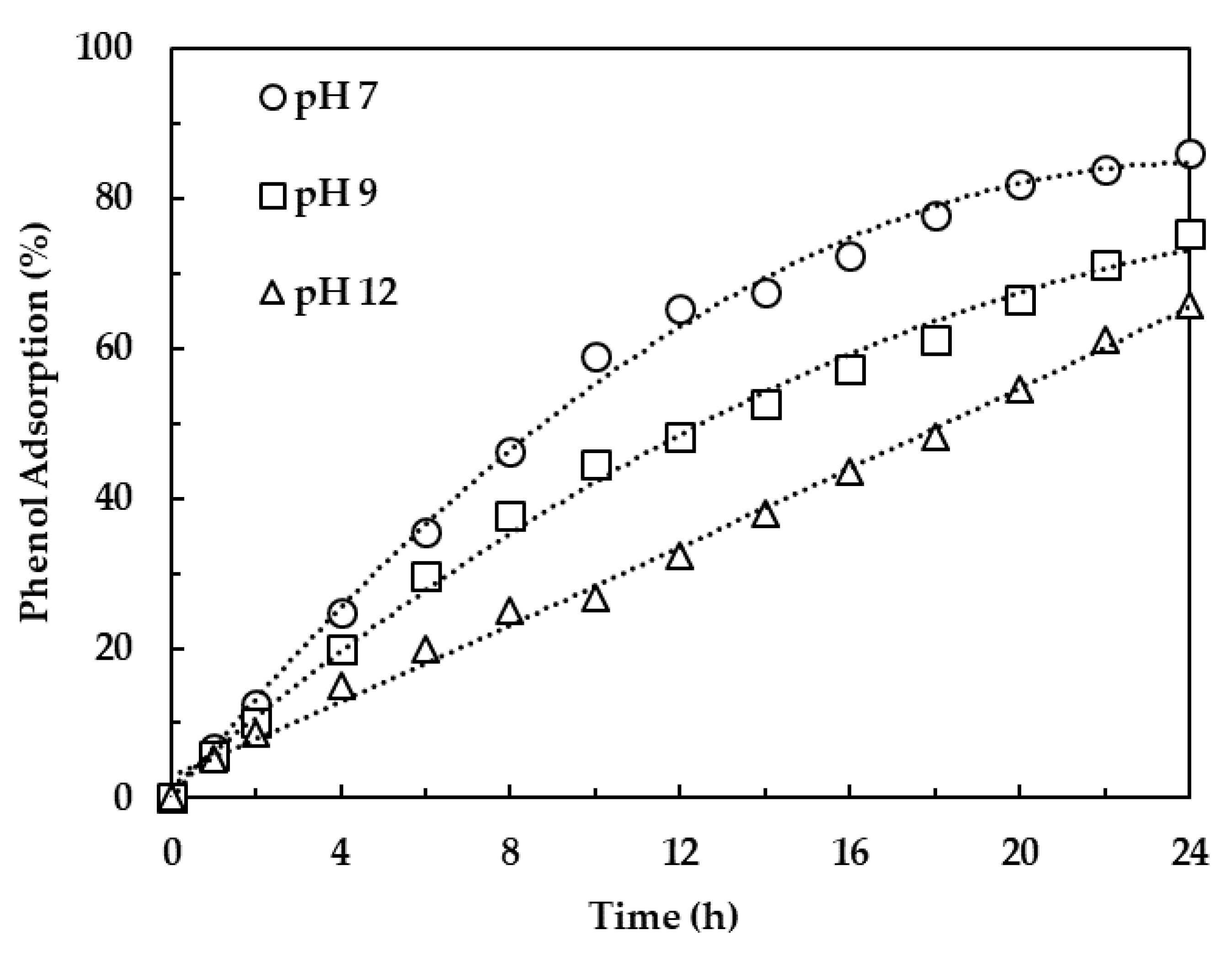
3.2.3. Effect of pH
3.2.4. Adsorption Isotherms
3.2.5. Adsorption Mechanism
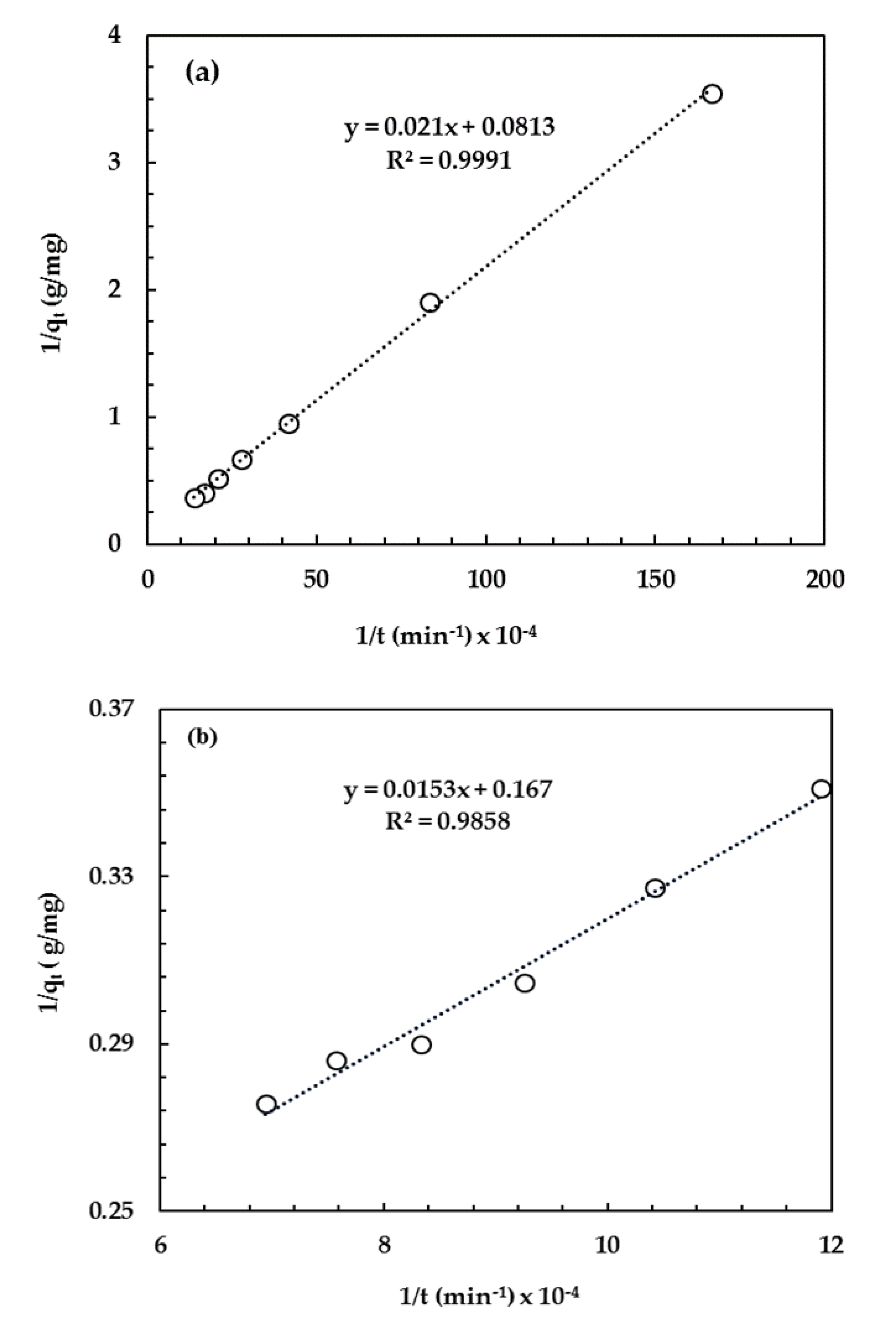
3.2.6. Adsorption Kinetics
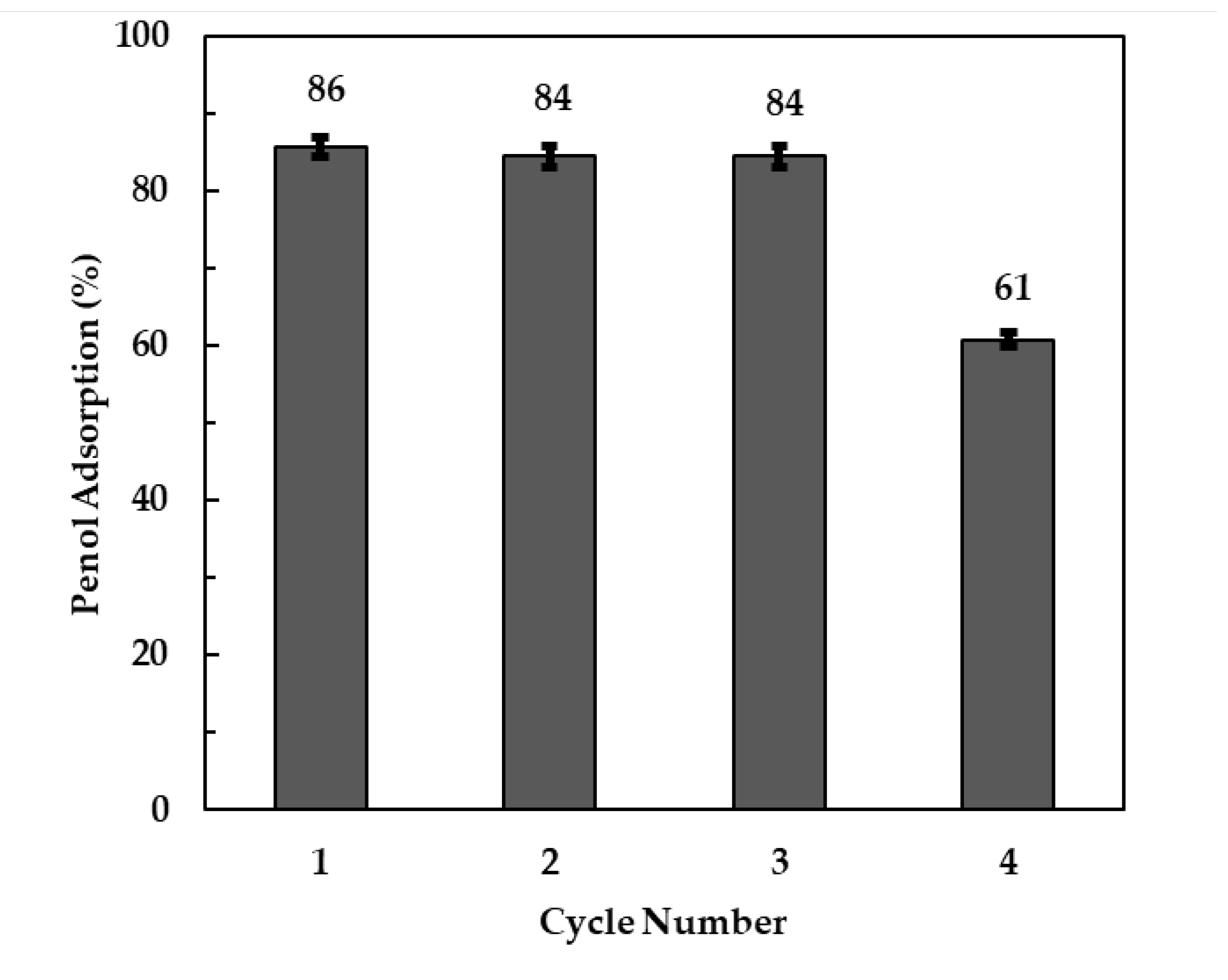
3.2.7. Clay Regeneration and Reusability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar Reddy, D.; Lee, S. Water pollution and treatment technologies. J. Environ. Anal. Toxicol. 2012, 2, e103. [Google Scholar] [CrossRef]
- Olujimi, O.; Fatoki, O.; Odendaal, J.; Okonkwo, J. Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: A need for more monitoring. Water SA 2010, 36. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Cresols: Health and Safety Guide; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Anderson, J. The environmental benefits of water recycling and reuse. Water Sci. Technol. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dolnicar, S.; Hurlimann, A. Drinking water from alternative water sources: Differences in beliefs, social norms and factors of perceived behavioural control across eight Australian locations. Water Sci. Technol. 2009, 60, 1433–1444. [Google Scholar] [CrossRef]
- Yasui, H.; Miyaji, Y. A novel approach to removing refractory organic compounds in drinking water. Water Sci. Technol. 1992, 26, 1503–1512. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.-H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Wang, L.; Li, Z. Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb (II) removal. Chem. Eng. J. 2016, 306, 619–628. [Google Scholar] [CrossRef]
- Capra, L.; Manolache, M.; Ion, I.; Stoica, R.; Stinga, G.; Doncea, S.M.; Alexandrescu, E.; Somoghi, R.; Calin, M.R.; Radulescu, I. Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets. Nanomaterials 2018, 8, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.; Wilson, M. A Hand Book of Determinative Methods in Clay Mineralogy; Blackie and Son Ltd.: New York, NY, USA, 1987; pp. 301–320. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Rozhina, E.; Konnova, S.; Kryuchkova, M.; Khaertdinov, N.; Fakhrullin, R. Organic-nanoclay composite materials as removal agents for environmental decontamination. RSC Adv. 2019, 9, 40553–40564. [Google Scholar] [CrossRef] [Green Version]
- Jayabalakrishnan, R.; Raja, S.M. Vermiculite Clay Mineral Barrier Treatment System for Chrome Tannery Effluent. J. Appl. Sci. 2007, 7, 1547–1550. [Google Scholar]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; da Costa, L.M.; da Silva, L.H.M.; Tronto, J.; Pinto, F.G. Removal of Acid Green 68: 1 from aqueous solutions by calcined and uncalcined layered double hydroxides. Appl. Clay Sci. 2013, 80, 189–195. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Moyo, L.; Focke, W.W.; Labuschagne, F.J.; Verryn, S. Layered double hydroxide intercalated with sodium dodecyl sulfate. Mol. Cryst. Liq. Cryst. 2012, 555, 51–64. [Google Scholar] [CrossRef]
- Labuschagne, F.J.W.J.; Wiid, A.; Venter, H.; Gevers, B.; Leuteritz, A. Green synthesis of hydrotalcite from untreated magnesium oxide and aluminum hydroxide. Green Chem. Lett. Rev. 2018, 11, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Liu, X.; Tian, W.; Yan, D.; Sun, X.; Lei, X. Adsolubilization of 2, 4, 6-trichlorophenol from aqueous solution by surfactant intercalated ZnAl layered double hydroxides. Chem. Eng. J. 2015, 279, 597–604. [Google Scholar] [CrossRef]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem. Eng. J. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Yu, S.; Hao, Y.; Yu, H.; Du, B. Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chem. Eng. J. 2014, 252, 38–46. [Google Scholar] [CrossRef]
- Laipan, M.; Fu, H.; Zhu, R.; Sun, L.; Steel, R.M.; Ye, S.; Zhu, J.; He, H. Calcined Mg/Al-LDH for acidic wastewater treatment: Simultaneous neutralization and contaminant removal. Appl. Clay Sci. 2018, 153, 46–53. [Google Scholar] [CrossRef]
- Jawad, A.; Liao, Z.; Zhou, Z.; Khan, A.; Wang, T.; Ifthikar, J.; Shahzad, A.; Chen, Z.; Chen, Z. Fe-MoS4: An effective and stable LDH-based adsorbent for selective removal of heavy metals. ACS Appl. Mater. Interfaces 2017, 9, 28451–28463. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Tabana, L.S.; Ledikwa, R.P.; Tichapondwa, S.M. Adsorption of Phenol from Wastewater Using Modified Layered Double Hydroxide Clay. Chem. Eng. Trans. 2019, 76, 1267–1272. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.-R.; Shi, J.-L. Construction of homogenous/heterogeneous hollow mesoporous silica nanostructures by silica-etching chemistry: Principles, synthesis, and applications. Acc. Chem. Res. 2014, 47, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, Z.; Sun, D.; Wu, T.; Li, Y. Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J. Ind. Eng. Chem. 2017, 49, 208–218. [Google Scholar] [CrossRef]
- Clariant. Available online: https://www.Clariant.com/en/solutions/products/2013/12/09/18/29/sorbacid (accessed on 14 January 2020).
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Haeussler, L.; Heinrich, G. Intercalation of Mg–Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Milanovic, N. Synthesis, Structural and Magnetic Properties of Layered Double Hydroxides; University of Oslo: Oslo, Norway, 2016. [Google Scholar]
- Monash, P.; Pugazhenthi, G. Utilization of calcined Ni-Al layered double hydroxide (LDH) as an Adsorbent for removal of methyl orange dye from aqueous solution. Environ. Prog. Sustain. Energy 2014, 33, 154–159. [Google Scholar] [CrossRef]
- Naseem, S.; Gevers, B.; Boldt, R.; Labuschagné, F.J.W.; Leuteritz, A. Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing tri-metal layered double hydroxides (LDHs) prepared by urea hydrolysis. RSC Adv. 2019, 9, 3030–3040. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, C.; Wei, Y.; Zhou, G.; Pan, A.; Wei, W.; Huang, B. Hollow LDH nanowires as excellent adsorbents for organic dye. J. Alloy. Compd. 2016, 687, 499–505. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Jaroniec, M. Template-free synthesis of hierarchical spindle-like γ-Al 2 O 3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010, 20, 4587–4594. [Google Scholar] [CrossRef]
- Hu, J.; Song, Z.; Chen, L.; Yang, H.; Li, J.; Richards, R. Adsorption properties of MgO (111) nanoplates for the dye pollutants from wastewater. J. Chem. Eng. Data 2010, 55, 3742–3748. [Google Scholar] [CrossRef]
- Chiou, C.T. Partition and Adsorption of Organic Contaminants in Environmental Systems; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Grover, A.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kim, K.-H. Zn-Al layered double hydroxides intercalated with surfactant: Synthesis and applications for efficient removal of organic dyes. J. Clean. Prod. 2019, 240, 118090. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@ carbon nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Piccin, J.; Dotto, G.; Pinto, L. Adsorption isotherms and thermochemical data of FD&C Red n 40 binding by chitosan. Braz. J. Chem. Eng. 2011, 28, 295–304. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Nethaji, S.; Sivasamy, A.; Mandal, A. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Sepehr, M.N.; Al-Musawi, T.J.; Ghahramani, E.; Kazemian, H.; Zarrabi, M. Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab. J. Chem. 2017, 10, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L.; Pode, R.; Hulka, I. Phenol adsorption using Aliquat 336 functionalized Zn-Al layered double hydroxide. Sep. Purif. Technol. 2018, 196, 82–95. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R. Sorption of atrazine on conventional and surface modified activated carbons. J. Colloid Interface Sci. 2006, 302, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Ni, Z.; Xia, S.; Liu, X.; Wang, Q. Removal of naphthol green B from aqueous solution by calcined layered double hydroxides: Adsorption property and mechanism studies. Chin. J. Chem. 2009, 27, 1767–1772. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Li, Y.-P.; Xie, M.; Xin, H.-Z. Sorption of an anionic dye by uncalcined and calcined layered double hydroxides: A case study. J. Hazard. Mater. 2005, 120, 163–171. [Google Scholar] [CrossRef]
- Alcaraz, J.J.; Arena, B.J.; Gillespie, R.D.; Holmgren, J.S. Solid base catalysts for mercaptan oxidation. Catal. Today 1998, 43, 89–99. [Google Scholar] [CrossRef]

| Adsorption Isotherm | ||
|---|---|---|
| Parameter | Freundlich | Langmuir |
| KL | - | 0.053 |
| QL | - | 12.1 |
| KF | 1.36 | - |
| n | 2.21 | - |
| R2 | 0.998 | 0.969 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabana, L.; Tichapondwa, S.; Labuschagne, F.; Chirwa, E. Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay. Sustainability 2020, 12, 4273. https://doi.org/10.3390/su12104273
Tabana L, Tichapondwa S, Labuschagne F, Chirwa E. Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay. Sustainability. 2020; 12(10):4273. https://doi.org/10.3390/su12104273
Chicago/Turabian StyleTabana, Lehlogonolo, Shepherd Tichapondwa, Frederick Labuschagne, and Evans Chirwa. 2020. "Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay" Sustainability 12, no. 10: 4273. https://doi.org/10.3390/su12104273
APA StyleTabana, L., Tichapondwa, S., Labuschagne, F., & Chirwa, E. (2020). Adsorption of Phenol from Wastewater Using Calcined Magnesium-Zinc-Aluminium Layered Double Hydroxide Clay. Sustainability, 12(10), 4273. https://doi.org/10.3390/su12104273







