Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermogravimetric Analysis
2.3. Kinetic Study
3. Results
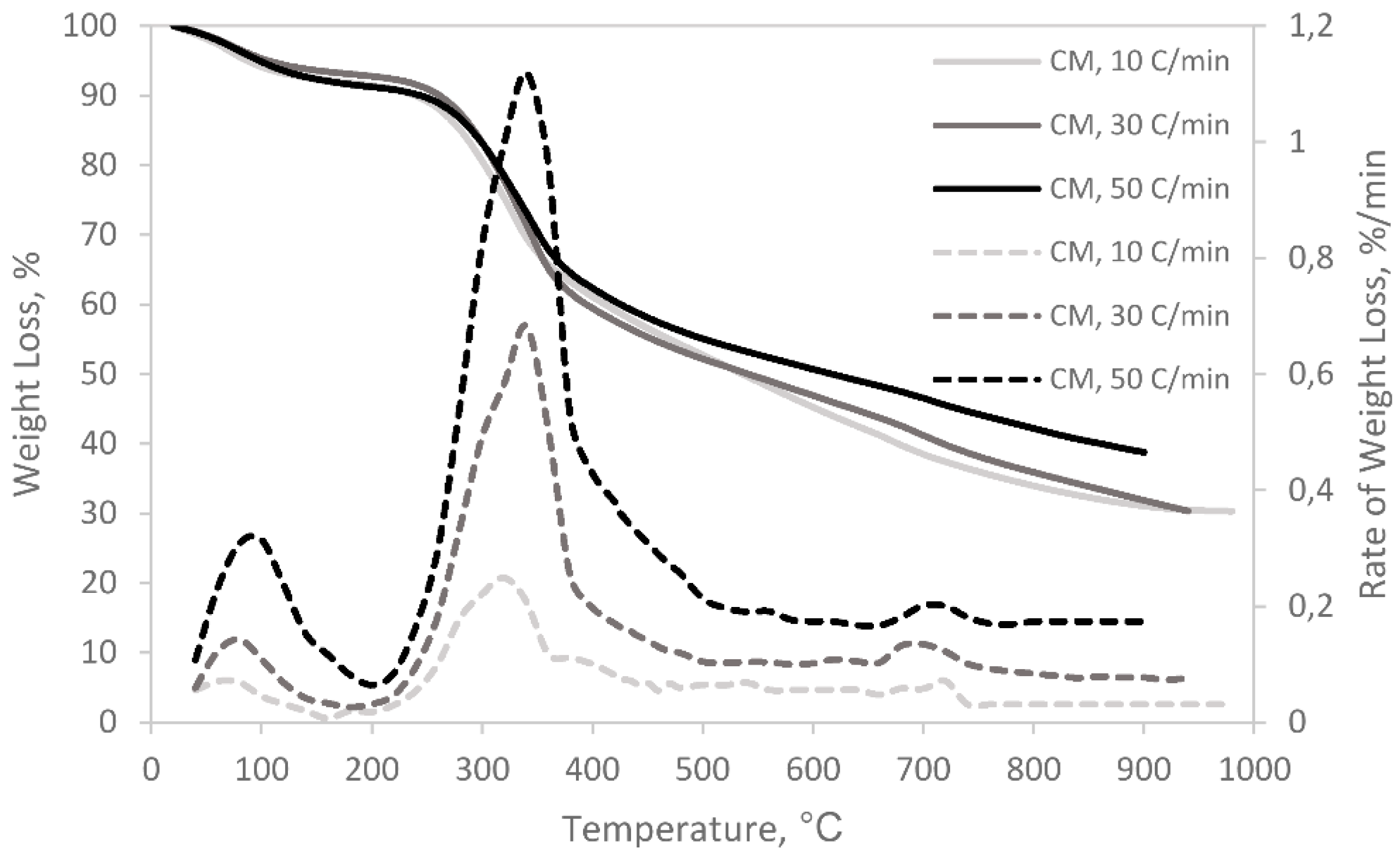
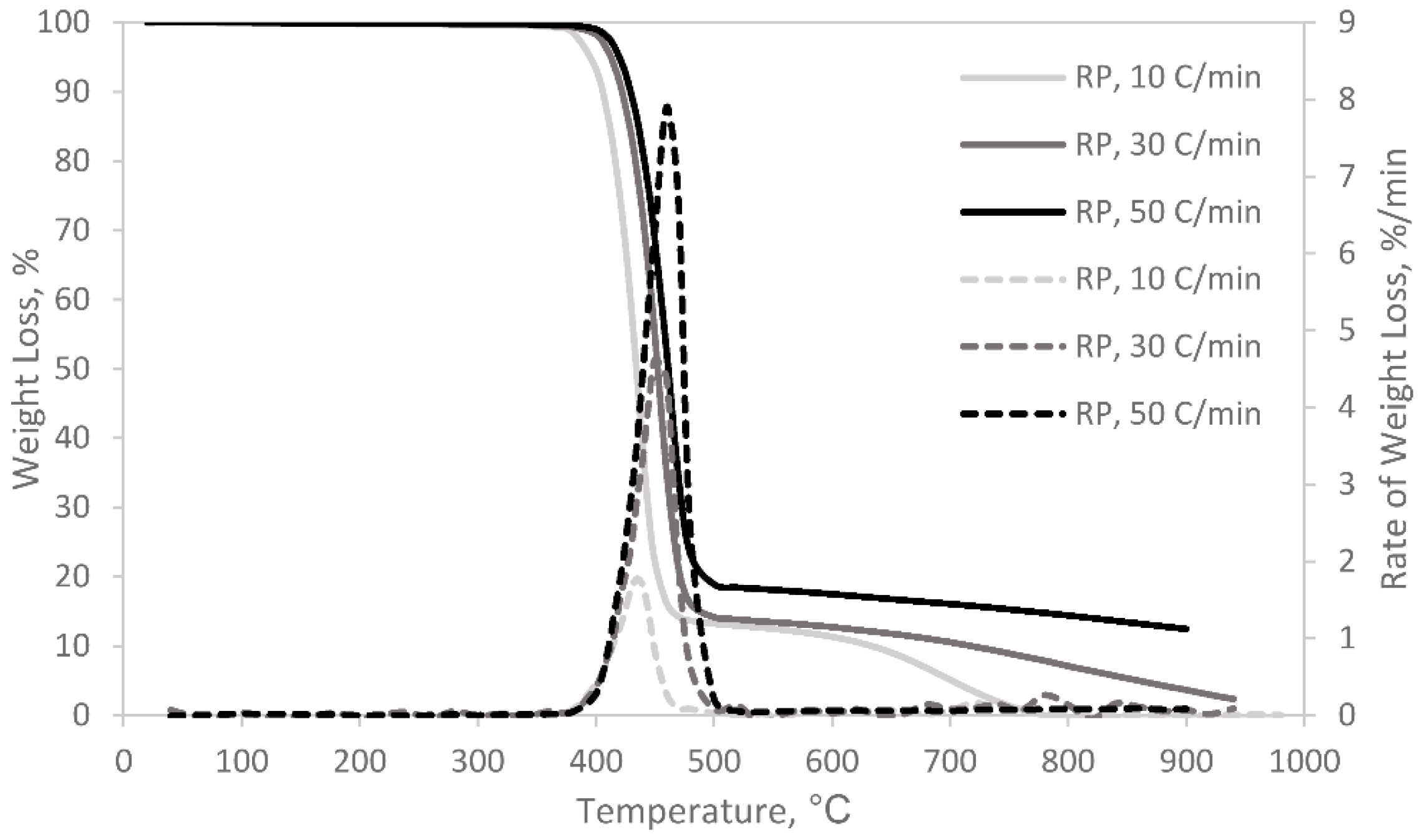
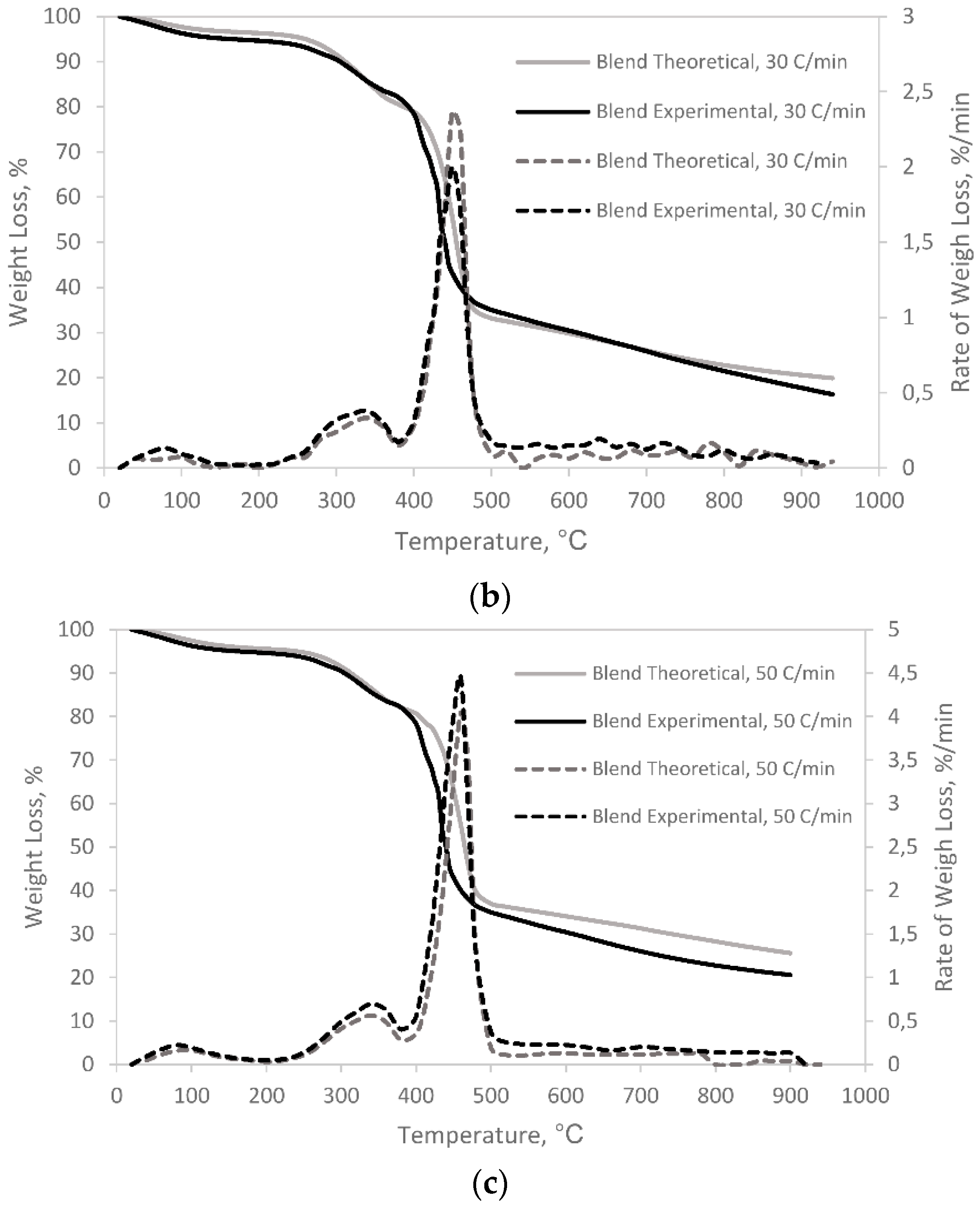
3.1. Pyrolysis and Co-Pyrolysis of Cattle Manure (CM) and Recycled Polyester
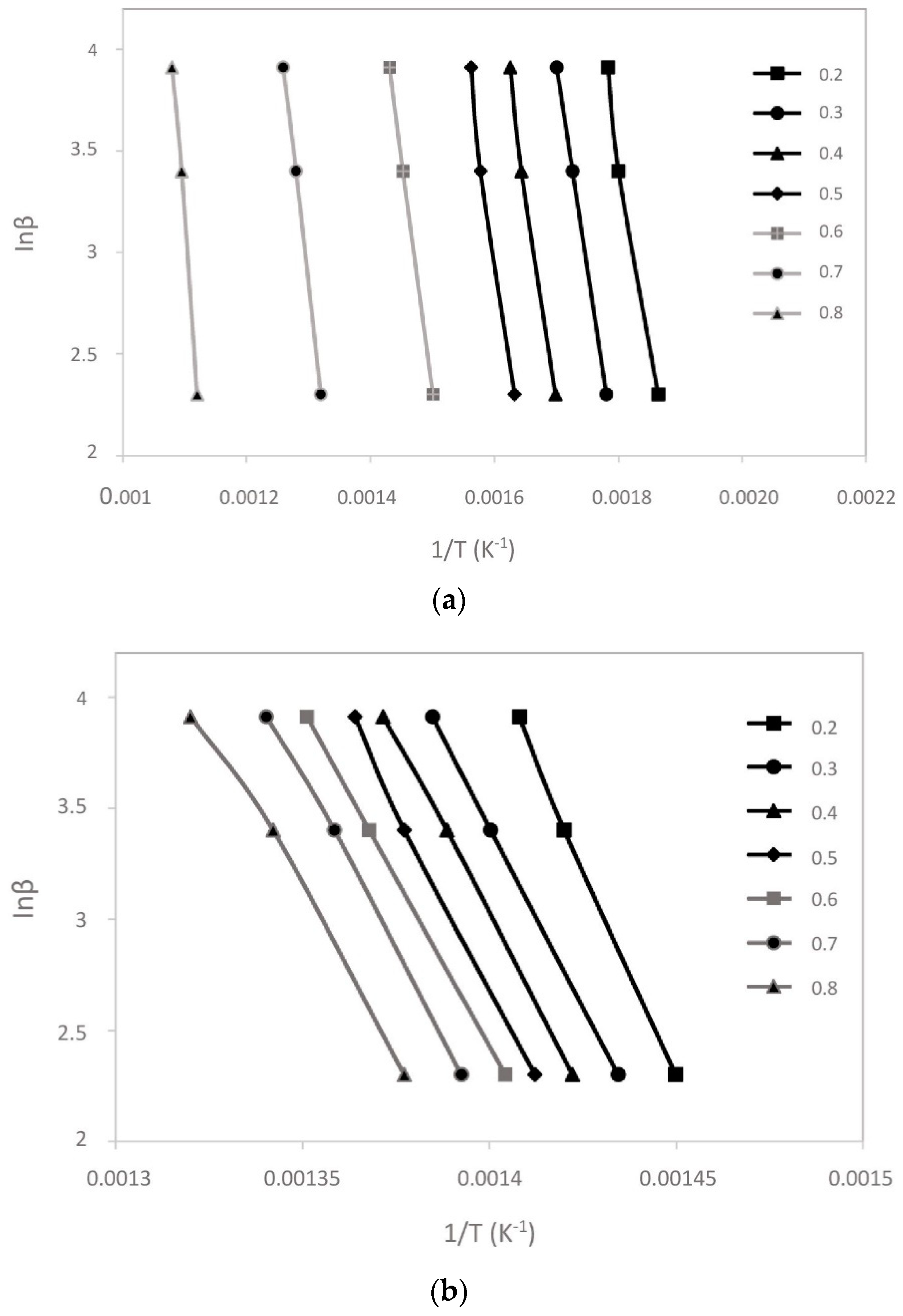
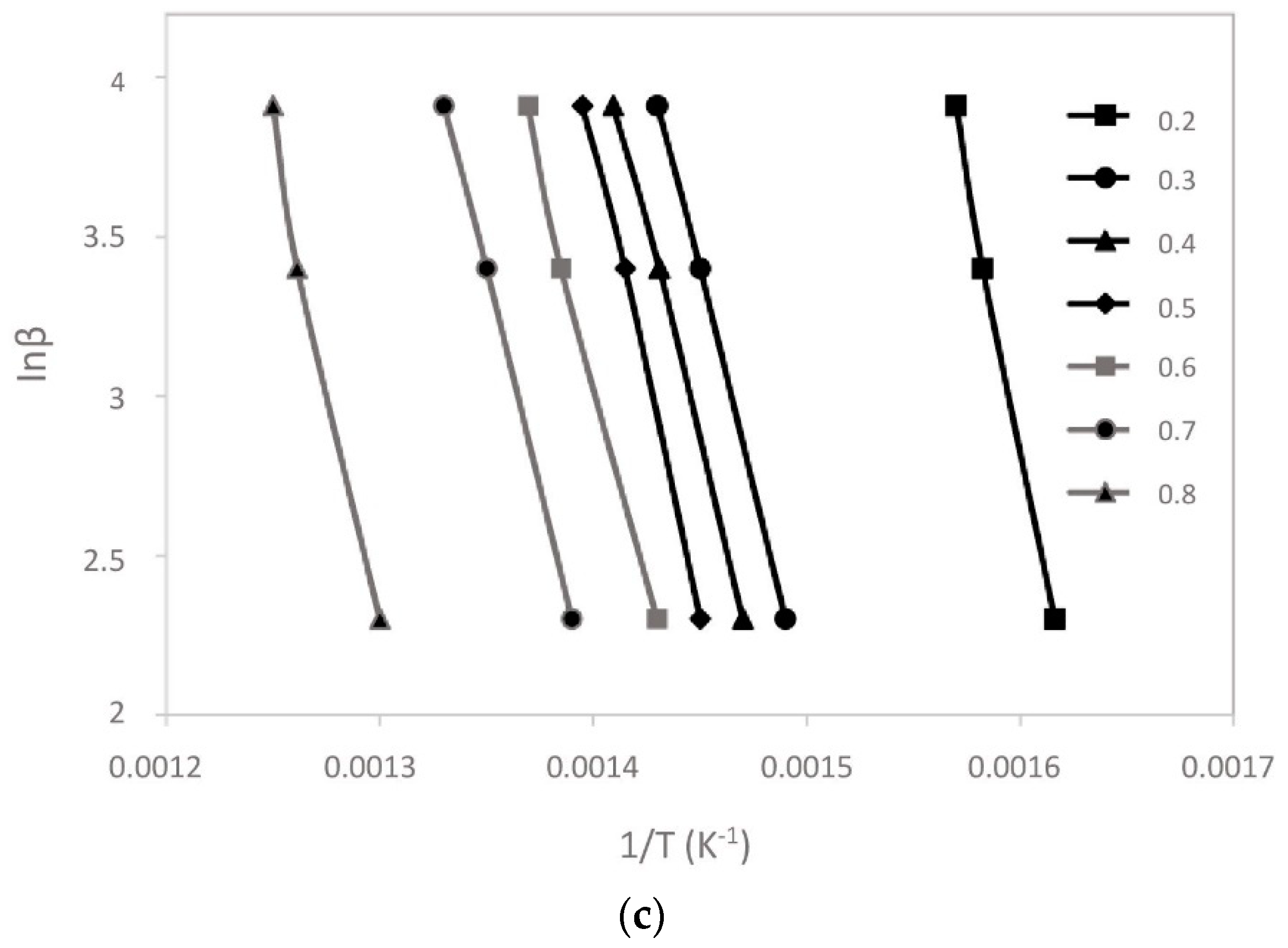
3.2. Kinetic Analysis
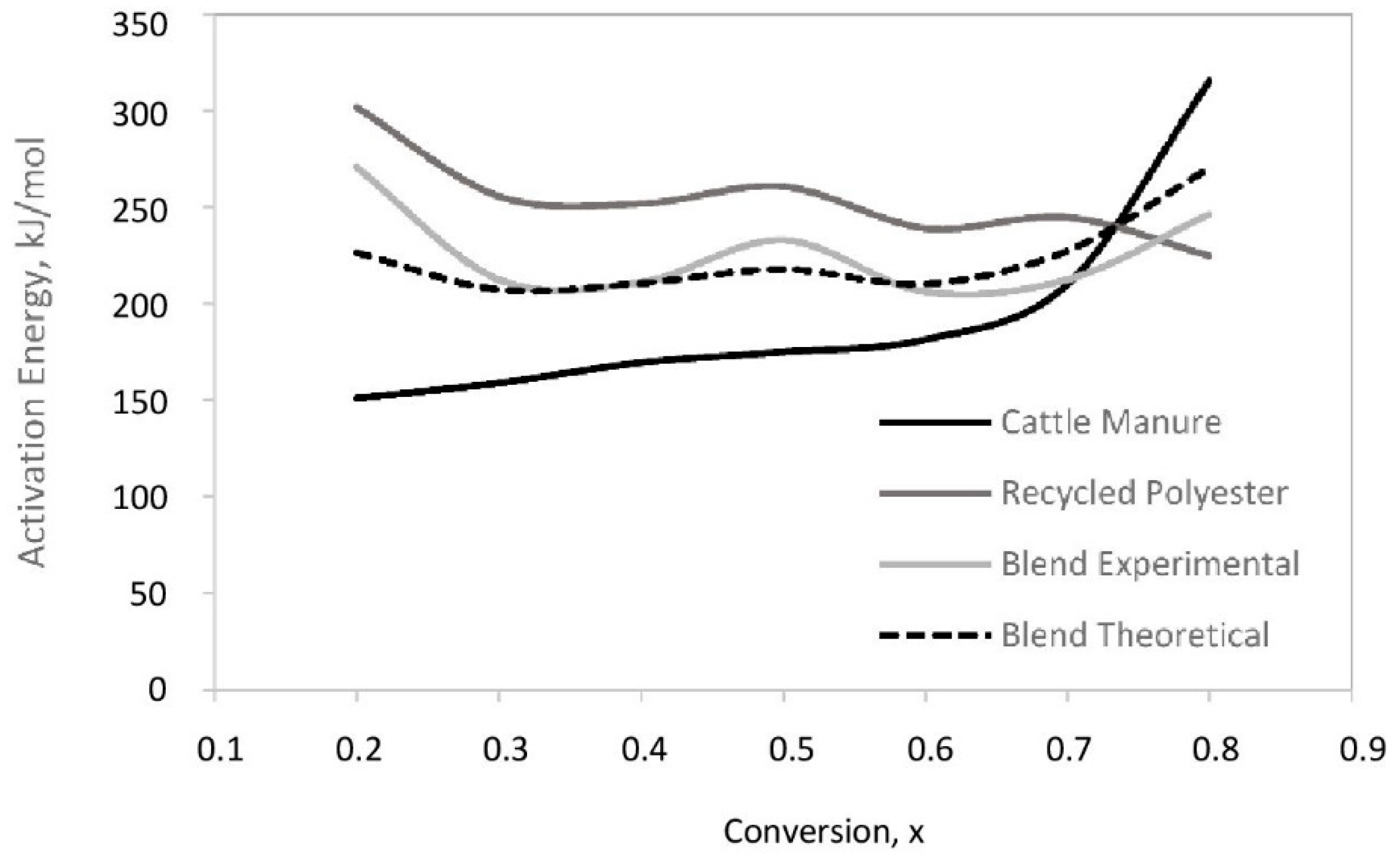
3.3. Pyrolysis Thermodynamics
4. Discussion
5. Conclusions
- (1)
- TGA plots demonstrate the existence of synergistic effect during co-pyrolysis of cattle manure and recycled polyester.
- (2)
- The apparent activation energies (Ea) of cattle manure, recycled polyester, and their blend were calculated by FWO method as 194.62, 254.22, and 227.21 kJ/mol, respectively.
- (3)
- The average ΔG value of the blend (174.62 kJ/mole) shows highly available energy to be considered as a feedstock for green energy production.
Funding
Acknowledgments
Conflicts of Interest
References
- Teixeira, T.R.; Ribeiroa, C.A.A.S.; Santos, A.R.; Marcattic, G.E.; Lorenzon, A.S.; Castro, N.L.M.; Domingues, G.F.; Leite, H.G.; Menezes, S.J.M.C.; Mota, P.H.S.; et al. Forest Biomass Power Plant Installation Scenarios. Biomass Bioenerg. 2018, 108, 35–47. [Google Scholar] [CrossRef]
- Tchapda, A.H.; Pisupati, S.V. A Review of Thermal Co-Conversion of Coal and Biomass/Waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Meng, H.; Wu, Z.; Zhao, J. Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng. 2017, 111, 834–846. [Google Scholar] [CrossRef]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle Manure Pyrolysis: Kinetic and Thermodynamic Analysis with Isoconversional Methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- Akyürek, Z. Potential of biogas energy from animal waste in the Mediterranean Region of Turkey. J. Energy Syst. 2018, 2, 159–167. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Mihaescu, L.; Negreanu, G.; Pana, C.; Pisa, I.; Cernat, A.; Ciupageanu, D.A. Experimental Investigations of Innovative Biomass Energy Harnessing Solutions. Energies 2018, 11, 3469. [Google Scholar] [CrossRef]
- Beringer, T. Bioenergy production potential of global biomass plantations under environmental and agricultural constraints. GCB Bioenerg. 2011, 3, 299–312. [Google Scholar] [CrossRef]
- Graham, J.P.; Nachman, K.E. Managing Waste from confined animal feeding operations in the United States: The need for sanitary reform. J. Water Health 2010, 8, 646–670. [Google Scholar] [CrossRef] [PubMed]
- Carlin, N.; Annamalai, K.; Sweeten, J.; Mukhtar, S. Thermo-Chemical Conversion Analysis on Dairy Manure-Based Biomass Through Direct Combustion. Int. J. Green Energy 2007, 4, 133–159. [Google Scholar] [CrossRef]
- Philip, J.M.; Aravind, U.K.; Aravindakumar, C.T. Chapter 5 Use of Antibiotics in Animals and Its Possible Impacts in the Environment. In Handbook of Research on Social Marketing and Its Influence on the Animal Origin Food Product Consumption; Bogueva, D., Marinova, D., Raphaely, T., Eds.; IGI Global: Hershey, PA, USA, 2018; pp. 77–91. [Google Scholar]
- Kaygusuz, K.; Şekerci, T. Biomass for efficiency and sustainability energy utilization in Turkey. J. Eng. Res. App. Sci. 2016, 5, 332–341. [Google Scholar]
- Das, P.; Twari, P. Valorization of packaging plastic waste by slow pyrolysis. Resources. Conserv. Recycl. 2018, 128, 69–77. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste—A review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Association of Plastic Manufacturers Europe (APME). An Analysis of European Plastics Production, Demand and Waste Data, 2017. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/plastics_the_facts_2017_final_for_website_one_page.pdf (accessed on 12 April 2019).
- Turkish Plastic Industry Research, Development and Education Foundation (Pagev), Packaging waste of Turkey. Available online: https://www.pagev.org/turkiye-de-plastik-geri-donusumu-avrupa-nin-odaginda (accessed on 5 March 2019).
- Lee, S.R.; Lee, J.; Lee, T.; Cho, S.H.; Oh, J.I.; Kim, H.; Tsang, D.C.W.; Kwon, E.E. Carbon Dioxide Assisted Thermal Decomposition of Cattle Manure. Sci. Total Environ. 2018, 615, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.E.F.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Volpe, M.; D’Anna, C.; Messineo, S.; Volpe, R.; Messineo, A. Sustainable Production of Bio-Combustibles from Pyrolysis of Agro-Industrial Wastes. Sustainability 2014, 6, 7866–7882. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Zhang, L.; Zhao, Y.; Zhang, Z.; Xie, X.; Qiua, P.; Chen, G.; Pei, J. Thermogravimetric analysis and kinetics of the co-pyrolysis of coal blends with corn stalks. Thermochim. Acta 2018, 659, 59–65. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Islam, M.A.; Asif, M.; Hameed, B.H. Pyrolysis kinetics of raw and hydrothermally carbonized Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour. Technol. 2015, 179, 227–233. [Google Scholar] [CrossRef]
- Zannikos, F.; Kalligeros, S.; Anastopoulos, G.; Lois, E. Converting biomass and waste plastic to solid fuel briquettes. J. Renew. Energy 2013, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Zhu, X.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S. Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass with plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrog. Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic Effects in Steam Gasification of Combined Biomass and Plastic Waste Mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, X.; Li, S.; Mikulcic, H.; Beseni, T.; Deng, S.; Vujanovic, M.; Tan, H.; Kumfer, B.M. Synergistic effects during co-pyrolysis of biomass and plastic: Gas, tar, soot, char products and thermogravimetric study. J. Energy Inst. 2019, 92, 108–117. [Google Scholar] [CrossRef]
- Xiang, Z.; Liang, J.; Morgan, H.M., Jr.; Liu, Y.; Mao, H.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, X. Synergistic enhancement of product quality through fast co-pyrolysis of acid pretreated biomass and waste plastic. Energy Convers Manag. 2018, 164, 629–638. [Google Scholar] [CrossRef]
- Duangchan, A.; Samart, C. Tertiary recycling of PVC-containing plastic waste by copyrolysis with cattle manure. Waste Manag. 2008, 28, 2415–2421. [Google Scholar] [CrossRef]
- Ansah, E.; Wang, L.; Shahbazi, A. Thermogravimetric and calorimetric characteristics during co-pyrolysis of municipal solid waste components. Waste Manag. 2016, 56, 196–206. [Google Scholar] [CrossRef]
- Aboulkas, A.; Elharfi, K.; El Bouadili, A. Non-isothermal kinetic studies on co-processing of olive residue and polypropylene. Energy Convers Manag. 2008, 49, 3666–3671. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Gonçalves, M.; Barbosa, R.; Gulyurtlu, I. Toxicity of char residues produced in the co-pyrolysis of different wastes. Waste Manag. 2010, 30, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Brebu, M.; Ucar, S.; Vasile, C.; Yanik, J. Co-pyrolysis of pine cone with synthetic polymers. Fuel 2010, 89, 1911–1918. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.Y.; Zhang, G. Interactions of municipal solid waste components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyroly. 2014, 108, 19–25. [Google Scholar] [CrossRef]
- Özyuğuran, A.; Yaman, S.; Küçükbayrak, S. Prediction of calorific value of biomass based on elemental analysis. Int. Adv. Res. Eng. J. 2018, 2, 254–260. [Google Scholar]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Chen, G.; He, S.H.; Cheng, Z.; Guan, Y.; Yan, B.; Ma, W.; Leung, D.Y.C. Comparison of Kinetic Analysis Methods in Thermal Decomposition of Cattle Manure by Thermogravimetric Analysis. Bioresour. Technol. 2017, 243, 69–77. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Nipattummakul, N.; Gupta, A.K. Characteristics of Syngas from Co-gasification of Polyethylene and Woodchip. Appl. Energy 2011, 88, 165–174. [Google Scholar] [CrossRef]
- D’Almeida, A.L.F.S.; Barreto, D.W.; Calado, V.; D’Almeida, J.R.M. Thermal analysis of less common lignocellulose fibers. J. Therm. Anal. Calorim 2008, 91, 405–408. [Google Scholar] [CrossRef]
- Fernandez-Lopez, I.M.; Gamero, L.P.; Lopez-Gonzalez, D.; Avalos-Ramirez, A.; Valverde, R.J.; Sanchez-Silva, L. Life cycle assessment of swine and dairy manure: Pyrolysis and combustion processes. Bioresour Technol. 2015, 182, 184–192. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Lang, X.; Ren, X.; Fan, S. Evaluation of agricultural residues pyrolysis under non-isothermal conditions: Thermal behaviors, kinetics, and thermodynamics. Bioresour Technol. 2017, 241, 340–348. [Google Scholar] [CrossRef]
- Larraín, T.; Carrier, M.; Radovic, L.R. Structure-reactivity relationship in pyrolysis of plastics: A comparison with natural polymers. J. Anal. Appl. Pyrol. 2017, 126, 346–356. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, J. Preparation of high adsorption performance activated carbon by pyrolysis of waste polyester. J. Mater. Sci. 2018, 53, 5458–5466. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Q.; Sun, K.; Chi, Y.; Yan, J. Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Bioresour Technol. 2018, 249, 16–23. [Google Scholar] [CrossRef]
- Al-Juaidiyah, J. Pyrolysis kinetics of recycled polyesters. Int. J. Cloth. Sci. Technol. 2015, 24, 523–531. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrol. 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Daugaard, D.E.; Brown, R.C. Enthalpy for pyrolysis for several types of biomass. Energy Fuel. 2003, 17, 934–939. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Taqvic, H.S.T.; Elkamel, A.; Liu, C.G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef]
- Turmanova, S.C.; Genieva, S.D.; Dimitrova, A.S.; Vlaev, L.T. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Express Poly Lett. 2008, 2, 133–146. [Google Scholar] [CrossRef]

| Proximate Analysis (As Received Basis) | ||
| CM | RP | |
| Moisture,% | 7.75 | 0.62 |
| Volatile Matter,% | 54.55 | 87.19 |
| Fixed Carbon,% | 12.40 | 12.15 |
| Ash,% | 25.30 | 0.04 |
| Ultimate Analysis (Dry Basis) | ||
| C,% | 33.07 | 62.8 |
| H,% | 4.87 | 4.3 |
| N,% | 2.90 | 0.07 |
| S,% | 0.63 | 0.04 |
| O,% (by difference) | 58.53 | 32.79 |
| LHV (kJ/kg) | 11.20 | 20.57 |
| Sample | Heating Rate (°C/min) | Ti (°C) | Tmax (°C) | Tf (°C) | Rmax (%/min.mg) | Total Weight Loss,% |
|---|---|---|---|---|---|---|
| CM | ||||||
| 10 | 231.1 | 339.3 | 523.7 | 0.041 | 69.7 | |
| 30 | 236.3 | 344.6 | 557.1 | 0.110 | 67.4 | |
| 50 | 239.3 | 348.9 | 566.4 | 0.138 | 61.2 | |
| RP | ||||||
| 10 | 384.9 | 436.5 | 470.5 | 0.533 | 99.2 | |
| 30 | 401.8 | 457.8 | 499.5 | 0.609 | 89.9 | |
| 50 | 409.5 | 462.7 | 521.7 | 0.980 | 89.5 | |
| Blend (1:1 wt.%) | ||||||
| 10 | 223.9 | 433.4 | 522.4 | 0.102 | 83.3 | |
| 30 | 252.2 | 435.8 | 520.6 | 0.276 | 80.1 | |
| 50 | 261.8 | 436.4 | 502.9 | 0.419 | 73.3 | |
| Sample | Conversion % | Ea (kJ/mol) | A (s−1) | R2 | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol K) |
|---|---|---|---|---|---|---|---|
| CM | 0.2 | 151.16 | 6.31 × 1010 | 0.9935 | 146.07 | 178.20 | –52.46 |
| 0.3 | 159.04 | 3.12 × 1011 | 0.9958 | 153.95 | 145.26 | 14.18 | |
| 0.4 | 169.61 | 2.65 × 1012 | 0.9927 | 164.52 | 144.94 | 31.97 | |
| 0.5 | 175.15 | 8.12 × 1012 | 0.9986 | 170.06 | 144.77 | 41.28 | |
| 0.6 | 181.54 | 2.96 × 1013 | 0.9978 | 176.45 | 144.58 | 52.03 | |
| 0.7 | 210.14 | 9.41 × 1015 | 0.9946 | 205.05 | 155.57 | 80.79 | |
| 0.8 | 315.72 | 1.43 × 1025 | 0.9972 | 310.63 | 141.77 | 275.71 | |
| Average | 194.62 | 189.53 | 150.73 | ||||
| RP | 0.2 | 301.98 | 2.04 × 1025 | 0.9989 | 296.08 | 218.57 | 109.22 |
| 0.3 | 255.84 | 6.92 × 1016 | 1.0000 | 249.94 | 167.25 | 116.53 | |
| 0.4 | 251.89 | 3.50 × 1016 | 0.9995 | 245.99 | 167.34 | 110.83 | |
| 0.5 | 260.83 | 1.64 × 1017 | 0.9977 | 254.93 | 167.15 | 123.70 | |
| 0.6 | 239.19 | 3.85 × 1015 | 1.0000 | 233.29 | 167.65 | 92.51 | |
| 0.7 | 244.90 | 1.04 × 1016 | 0.9988 | 239.00 | 181.07 | 81.62 | |
| 0.8 | 224.93 | 3.23 × 1014 | 0.9942 | 219.03 | 168.00 | 71.90 | |
| Average | 254.22 | 248.32 | 176.72 | ||||
| Blend (1:1 wt.%) | 0.2 | 271.03 | 1.19 × 1018 | 0.9971 | 265.16 | 204.66 | 85.63 |
| 0.3 | 212.72 | 4.55 × 1013 | 0.9987 | 206.84 | 167.55 | 55.61 | |
| 0.4 | 211.37 | 3.59 × 1013 | 0.9981 | 205.49 | 167.59 | 53.64 | |
| 0.5 | 233.19 | 1.62 × 1015 | 0.9973 | 227.29 | 167.01 | 85.31 | |
| 0.6 | 206.35 | 1.49 × 1013 | 0.9956 | 200.47 | 167.74 | 46.33 | |
| 0.7 | 212.72 | 4.55 × 1013 | 0.9997 | 206.84 | 181.08 | 36.46 | |
| 0.8 | 218.65 | 1.70 × 1016 | 0.9903 | 240.78 | 166.70 | 104.85 | |
| Average | 227.71 | 221.84 | 174.62 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyürek, Z. Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy. Sustainability 2019, 11, 2280. https://doi.org/10.3390/su11082280
Akyürek Z. Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy. Sustainability. 2019; 11(8):2280. https://doi.org/10.3390/su11082280
Chicago/Turabian StyleAkyürek, Zuhal. 2019. "Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy" Sustainability 11, no. 8: 2280. https://doi.org/10.3390/su11082280
APA StyleAkyürek, Z. (2019). Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy. Sustainability, 11(8), 2280. https://doi.org/10.3390/su11082280





