Seasonal Net Carbon Exchange in Rotation Crops in the Temperate Climate of Central Lithuania
Abstract
:1. Introduction
2. Materials and Methods
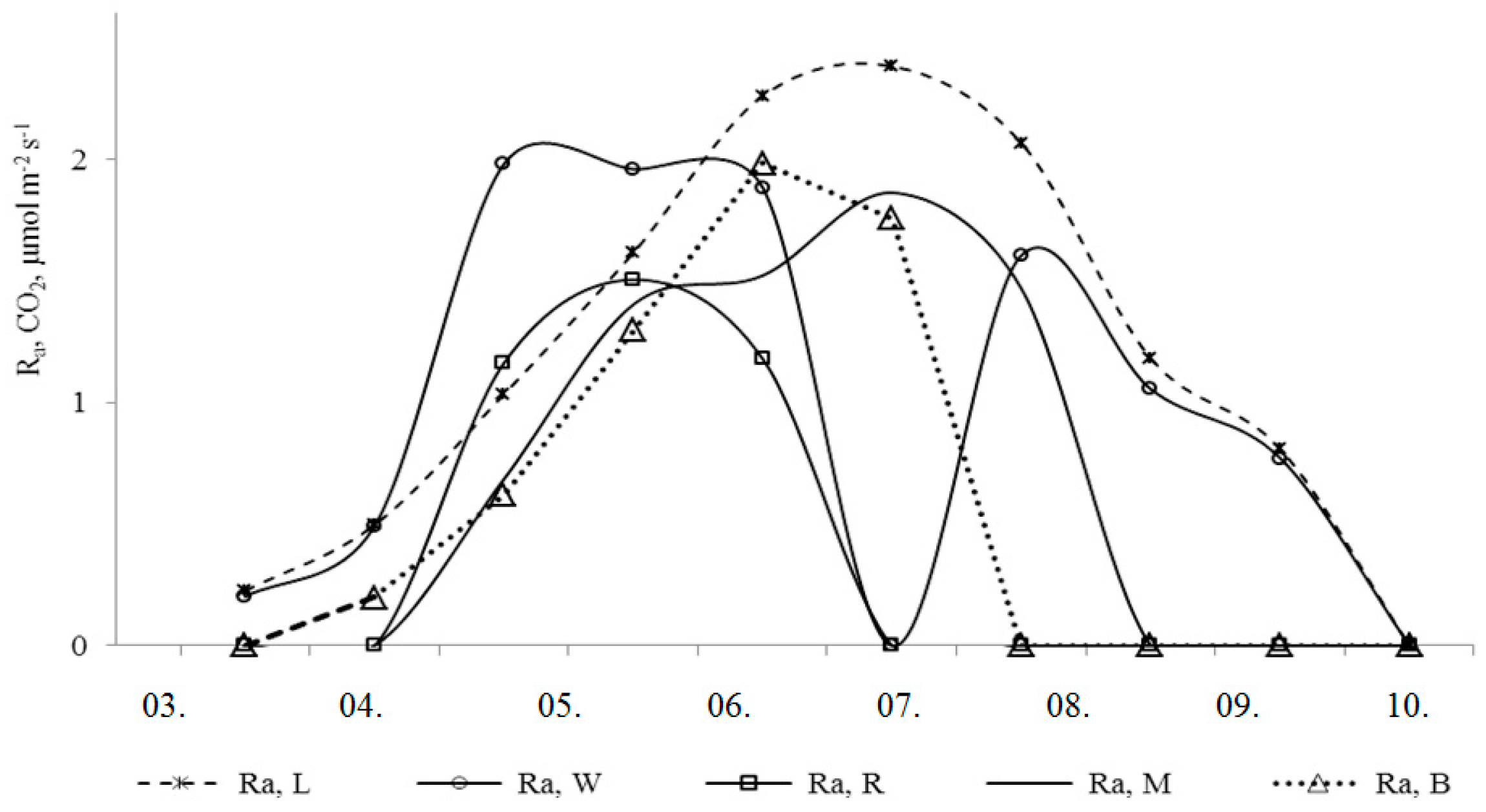
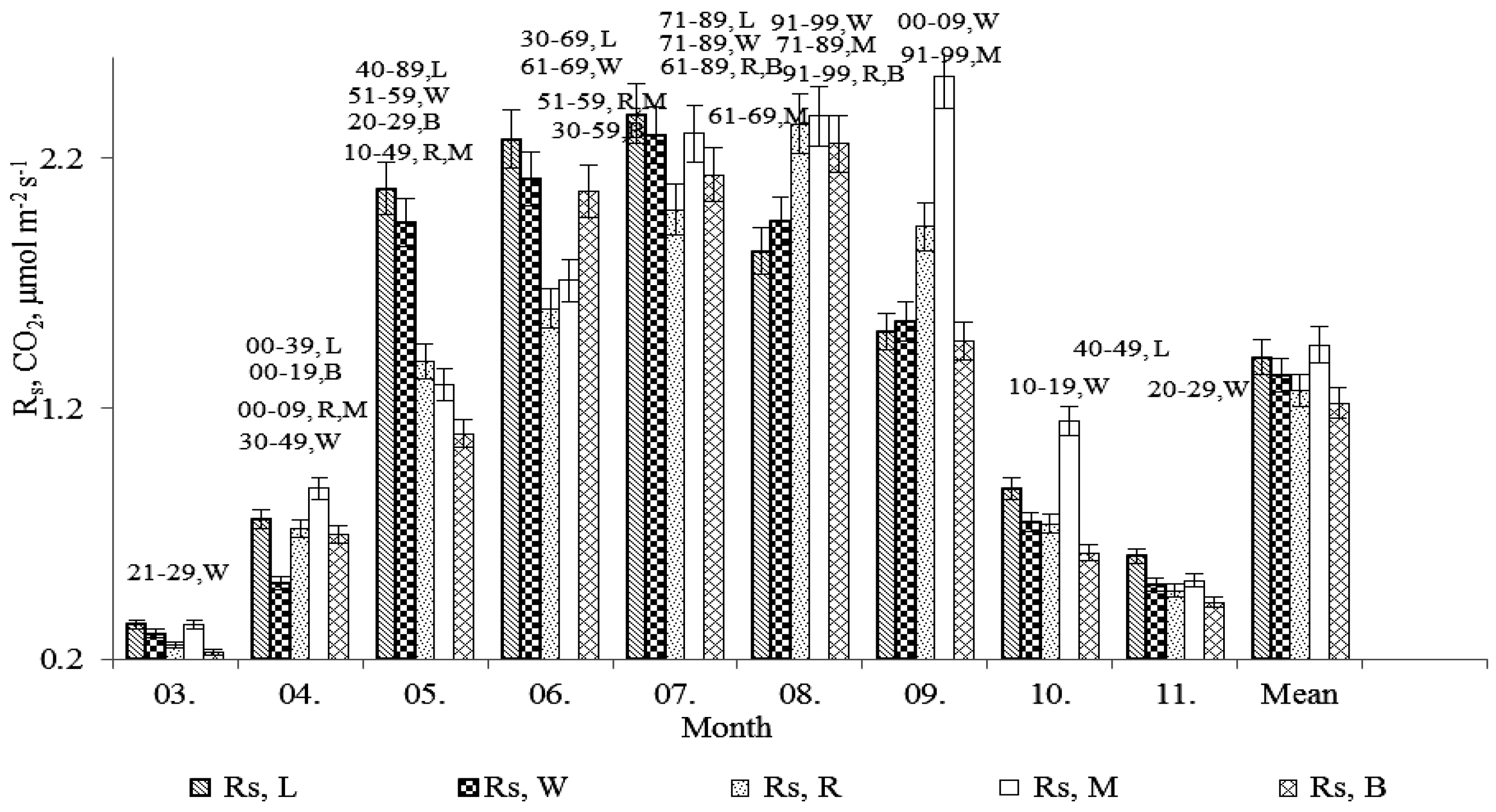
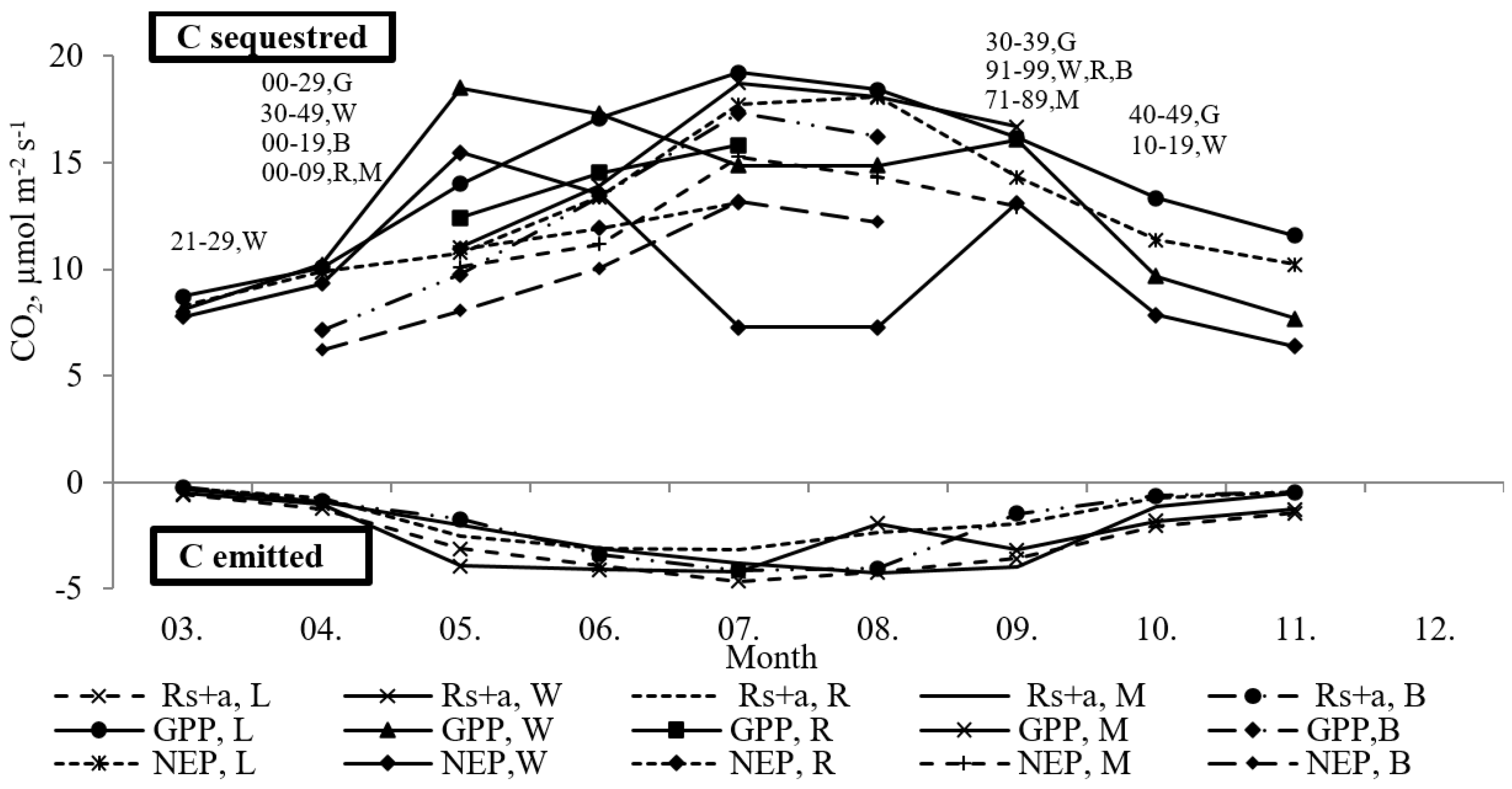
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, P.; Martino, D.; Ciais, Z.; Gwary, D.; Janzen, H.; Kumar, P. Policy and technological constraints to implementation of greenhouse gas mitigation options in agriculture. Agric. Ecosyst. Environ. 2007, 118, 6–28. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Databases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Available online: http://faostat.fao.org (accessed on 15 December 2018).
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Academic Press Elsevier: New York, NY, USA, 2013. [Google Scholar]
- European Environment Agency (EEA). The European Environment—State and Outlook 2010: Synthesis; European Environment Agency: Copenhagen, Denmark, 2010; p. 228. [Google Scholar]
- LR Ministry of Environment, National Policy of Climate Change Management. LR Ministry of Environment, Vilnius, 2012, p. 80. Available online: http://www.am.lt (accessed on 10 December 2018).
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Terrestrial Nutrient Cycling, in Principles of Terrestrial Ecosystem Ecology; Chapin, F.S., Ed.; Springer: New York, NY, USA, 2002; p. 398. [Google Scholar]
- Abiven, S.; Menassero, S.; Chenu, C. The effect of organic inputs over time on soil aggregate stability: A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Randerson, J.T.; Chapin, F.S.; Harden, J.; Neff, J.C.; Harmon, M.E. Net ecosystem production: A comprehensive measure of net carbon accumulation by ecosystems. Ecol. Appl. 2002, 12, 937–947. [Google Scholar] [CrossRef]
- Zhao, Q.R.; Chen, Q.H.; Xiao, Y.T.; Tian, G.Q.; Chu, X.L.; Liu, Q.M. Saving forests through development? Fuelwood consumption and the energy-ladder hypothesis in rural Southern China. Transform. Bus. Econ. 2017, 16, 199–219. [Google Scholar]
- Heinemeyer, A.; McNamara, N. Comparing the closed static versus the closed dynamic chamber flux methodology: Implications for soil respiration studies. Plant Soil 2011, 346, 145–151. [Google Scholar] [CrossRef]
- Toma, P.; Massari, S.; Miglietta, P.P. Natural resource use efficiency and economic productivity. In Life Cycle Approaches to Sustainable Regional Development; Massari, S., Ed.; Routledge, Taylor and Francis Group: Abingdon, UK, 2016; pp. 143–148. [Google Scholar]
- Woodwell, G.M.; Whittaker, R.H. Primary production in terrestrial communities. Am. Zool. 1968, 8, 19–30. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Boulton, T.W.; Miller, R.M. Carbon dynamics of aggregate-associated organic matter estimated by carbon-13 natural abundance. Soil Sci. Soc. 2007, 60, 801–807. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Vina, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.; Leavitt, B.; Ciganda, V.; Burba, G.G.; Suyker, A.E.; et al. Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Baležentienė, L.; Kusta, A. Reducing greenhouse gas emissions in grassland ecosystems of the Central Lithuania: Multi-criteria evaluation on a basis of the ARAS Method. Sci. World J. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Verchot, L.V.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Liatukas, Ž.; Ronis, A.; Ruzgas, V. Leaf area index suitability for screening of winter wheat (Triticum aestivum L.) selection of breeding lines. Zemdirb.-Agric. 2009, 96, 3–15. [Google Scholar]
- Song, M.L.; Peng, J.; Wang, J.L.; Dong, L. Better resource management: An improved resource and environmental efficiency evaluation approach that considers undesirable outputs. Resour. Conserv. Recycl. 2018, 128, 197–205. [Google Scholar] [CrossRef]
- Aertsens, J.; de Nocker, L.; Gobin, A. Valuing the carbon sequestration potential for European agriculture. Land Use Policy 2013, 31, 584–594. [Google Scholar] [CrossRef]
- Ali, M.K.; Ahmad, W.; Malhi, S.S.; Atta, B.M.; Zia, M.H.; Ghafoor, A. Potential of carbon dioxide biosequestration of saline-sodic soils during amelioration under rice-wheat land use. Commun. Soil Sci. Plant Anal. 2013, 44, 2625–2635. [Google Scholar] [CrossRef]
- Janssens, I.A.; Freibauer, A.; Ciais, P.; Smith, P.; Nabuurs, G.J.; Folberth, G.; Schlamadinger, B.; Hutjes, R.W.A.; Ceulemans, R.; Schulze, E.-D.; et al. Europe’s terrestrial biosphere absorbs 7 to 12% of European anthropogenic CO2 emissions. Science 2003, 300, 1538–1542. [Google Scholar] [CrossRef]
- Li, J.; Zhao, B.; Li, X.; Jiang, R.B.; So, H.B. Effects of long-term combined application of organic and mineral fertilizers on microbial biomass, soil enzyme activities and soil fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar] [CrossRef]
- Wood, S.; Sebastian, K.; Scherr, S.J. Pilot Analysis of Global Ecosystems; International Food Policy Research Institute and World Resources Institute: Washington, DC, USA, 2000; p. 125. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2014; Update 2015; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Braunschweig, Germany, 2001; p. 158. [Google Scholar]
- Amthor, J.S.; Baldocchi, D.D. Terrestrial higher plant respiration and net primary production. In Terrestrial Global Productivity; Roy, J., Saugier, B., Mooney, H.A., Eds.; Academic Press: San Diego, CA, USA, 2001; p. 573. [Google Scholar]
- Ruiz-Vera, U.M.; Siebers, M.; Gray, S.B.; Drag, D.W.; Rosenthal, D.M.; Kimball, B.A.; Ort, D.R.; Bernacchi, C.J. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 2013, 162, 410–423. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies. Modeling and validation in the context of precision agriculture. Remote. Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Berry, J.A.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Rochette, P.; Desjardins, R.L.; Pattey, E.; Lessard, R. Crop net carbon dioxide exchange rate and radiation use efficiency in soybean. Agron. J. 1995, 87, 22–28. [Google Scholar] [CrossRef]
- Ciais, P.; Gervois, S.; Vuichard, N.; Piao, S.L.; Viovy, N. Effects of land use change and management on the European cropland carbon balance. Glob. Chang. Biol. 2011, 17, 320–338. [Google Scholar] [CrossRef]
- Leith, H.; Whittaker, R.H. Primary Productivity of the Biosphere; Springer: New York, NY, USA, 2008; p. 215. [Google Scholar]
- Lehuger, S.; Gabrielle, B.; Cellier, P.; Loubet, B.; Roche, R.; Béziat, P.; Ceschia, E.; Wattenbach, M. Predicting the net carbon exchanges of crop rotations in Europe with an agroecosystem model. Agric. Ecosyst. Environ. 2011, 139, 363–383. [Google Scholar] [CrossRef]
| Agroecosystem | Area, ha | Crop Fertilising |
|---|---|---|
| Ley (L) | 22.86 | Ammonium nitrate, 150 kg ha−1 (N 51 kg ha−1); |
| in 2nd yr. autumn—manure 50 t ha−1 | ||
| Wheat (W) | 13.7 | NPK 8-20-30, 200 kg ha−1 |
| Ammonium nitrate, 140 kg ha−1 (N 48 kg ha−1) | ||
| Rapeseed (R) | 47.59 | Ammonium sulphate, 300 kg ha−1 (N 63 kg ha−1) |
| Ammonium nitrate 100 kg ha−1 (N 34 kg ha−1) | ||
| Maize (M) | 46.68 | NPK 8-20-30. 280 kg ha−1 |
| Ammonium sulphate, 300 kg ha−1 (N 63 kg ha−1) | ||
| Ammonium nitrate, 170 kg ha−1 (N 58 kg ha−1) | ||
| Barley (B) | 14.51 | NPK 8-20-30, 200 kg ha−1 |
| Ammonium nitrate, 160 kg ha−1 (N 54 kg ha−1) | ||
| Ammonium nitrate, 120 kg ha−1 (N 41 kg ha−1) | ||
| Crop rotation | Ley 1-yr. + Ley 2-yr. + winter wheat + maize + spring rapeseed + barley with ley undercrop | |
| Year/Month | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2.81 | 1.62 | 4.15 | 2.86 | ||||||
| 2014 | 1.83 | 0.78 | 2.03 | 1.13 | 0.82 | 2.02 | 1.98 | 1.61 | 4.15 | 1.81 |
| 2015 | 3.21 | 2.15 | 1.30 | 0.35 | 1.34 | 0.11 | 1.32 | 1.96 | 3.46 | 1.68 |
| 2016 | 2.94 | 1.85 | 0.77 | 1.62 | 2.93 | 2.18 | 0.6 | 2.69 | 1.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baležentienė, L.; Mikša, O.; Baležentis, T.; Streimikiene, D. Seasonal Net Carbon Exchange in Rotation Crops in the Temperate Climate of Central Lithuania. Sustainability 2019, 11, 1966. https://doi.org/10.3390/su11071966
Baležentienė L, Mikša O, Baležentis T, Streimikiene D. Seasonal Net Carbon Exchange in Rotation Crops in the Temperate Climate of Central Lithuania. Sustainability. 2019; 11(7):1966. https://doi.org/10.3390/su11071966
Chicago/Turabian StyleBaležentienė, Ligita, Ovidijus Mikša, Tomas Baležentis, and Dalia Streimikiene. 2019. "Seasonal Net Carbon Exchange in Rotation Crops in the Temperate Climate of Central Lithuania" Sustainability 11, no. 7: 1966. https://doi.org/10.3390/su11071966
APA StyleBaležentienė, L., Mikša, O., Baležentis, T., & Streimikiene, D. (2019). Seasonal Net Carbon Exchange in Rotation Crops in the Temperate Climate of Central Lithuania. Sustainability, 11(7), 1966. https://doi.org/10.3390/su11071966







