Correlation of Fish Assemblages with Habitat and Environmental Variables in a Headwater Stream Section of Lijiang River, China
Abstract
1. Introduction
2. Materials and Methods
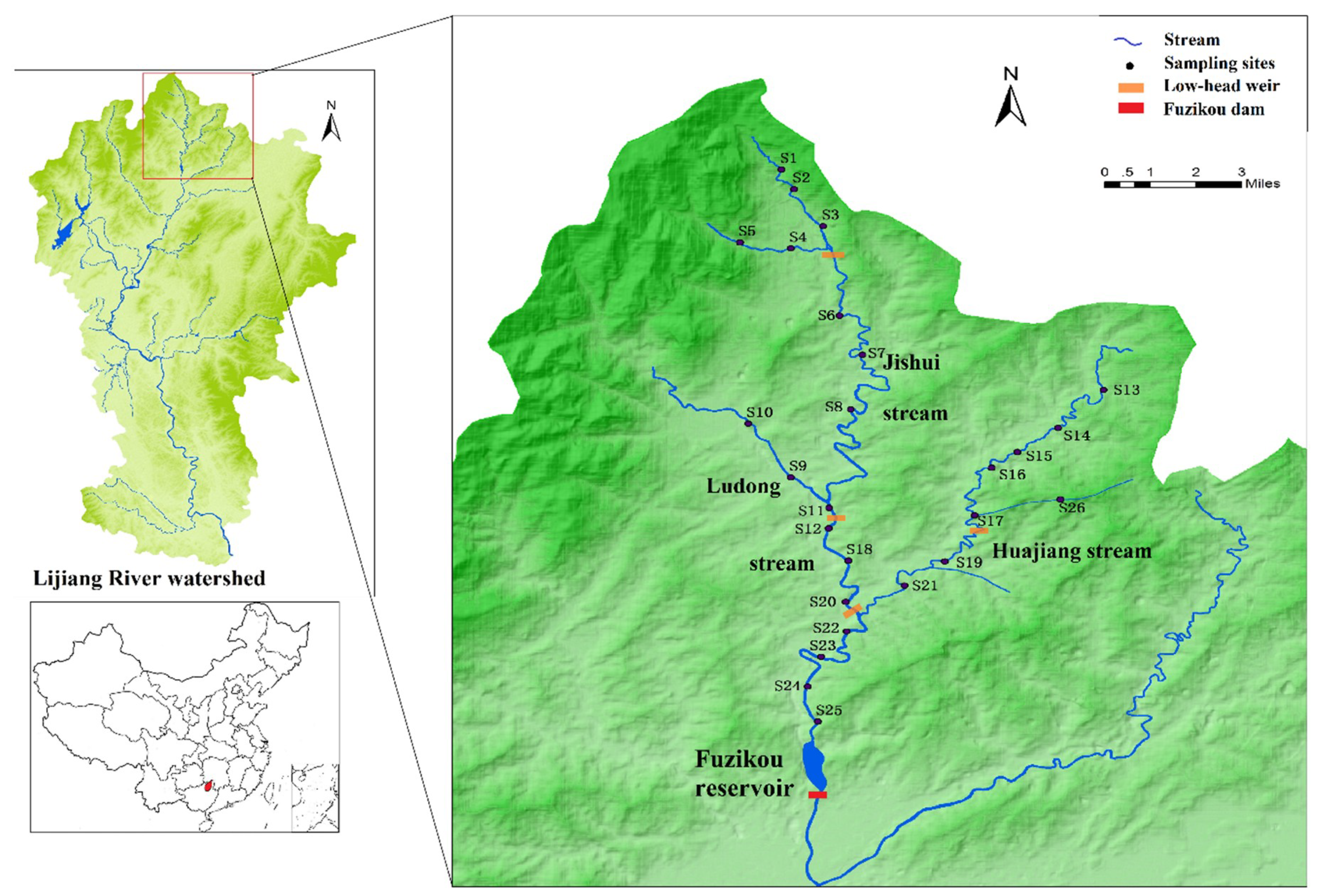
2.1. Study Area
2.2. Classification of Habitat Types
2.3. Field Sample Collection
2.4. Data Analyses
3. Results
3.1. Environmental Variables of the Stream Habitats
3.2. Fish Assemblage Composition
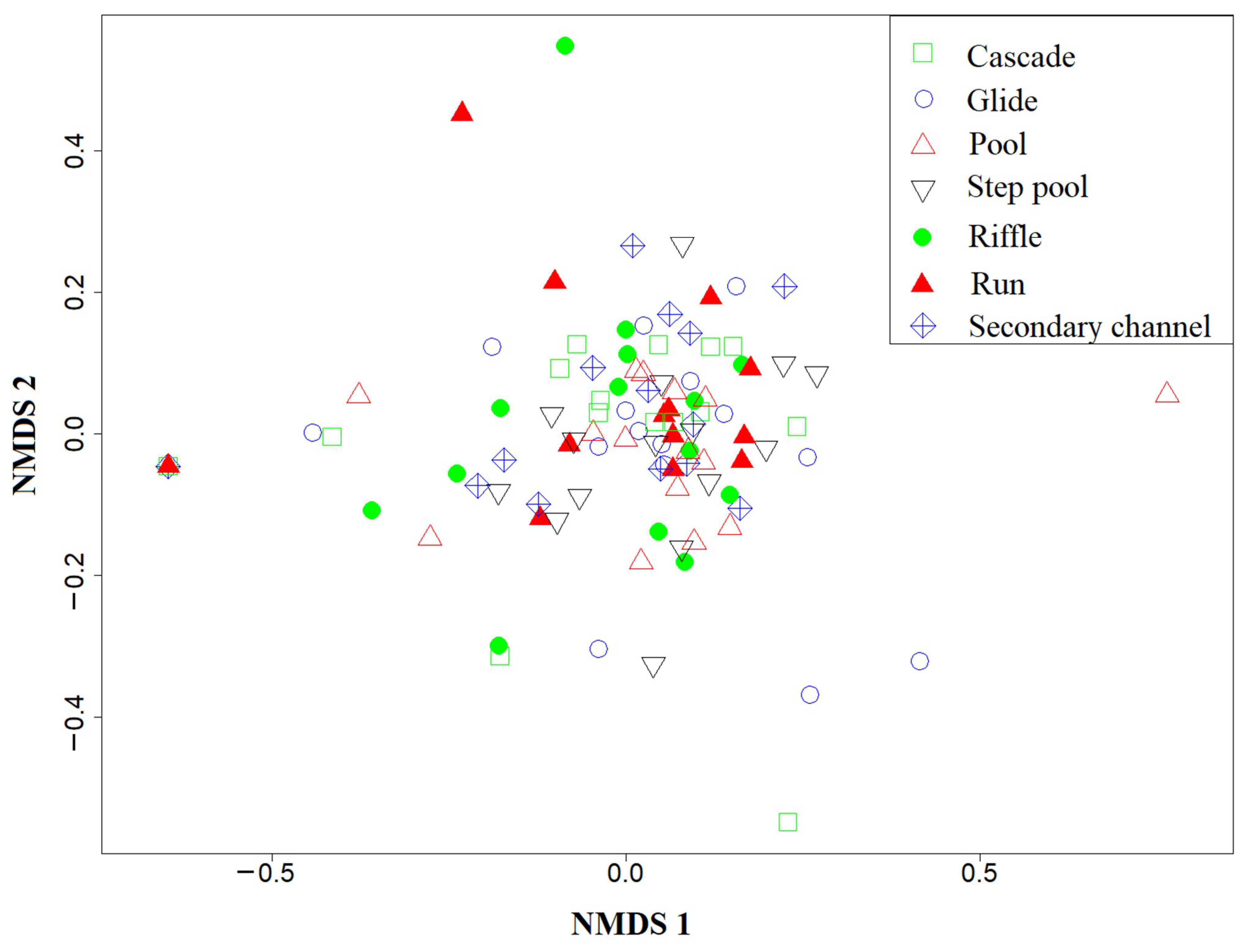
3.3. Correlation between Fish Assemblage and Habitat Types
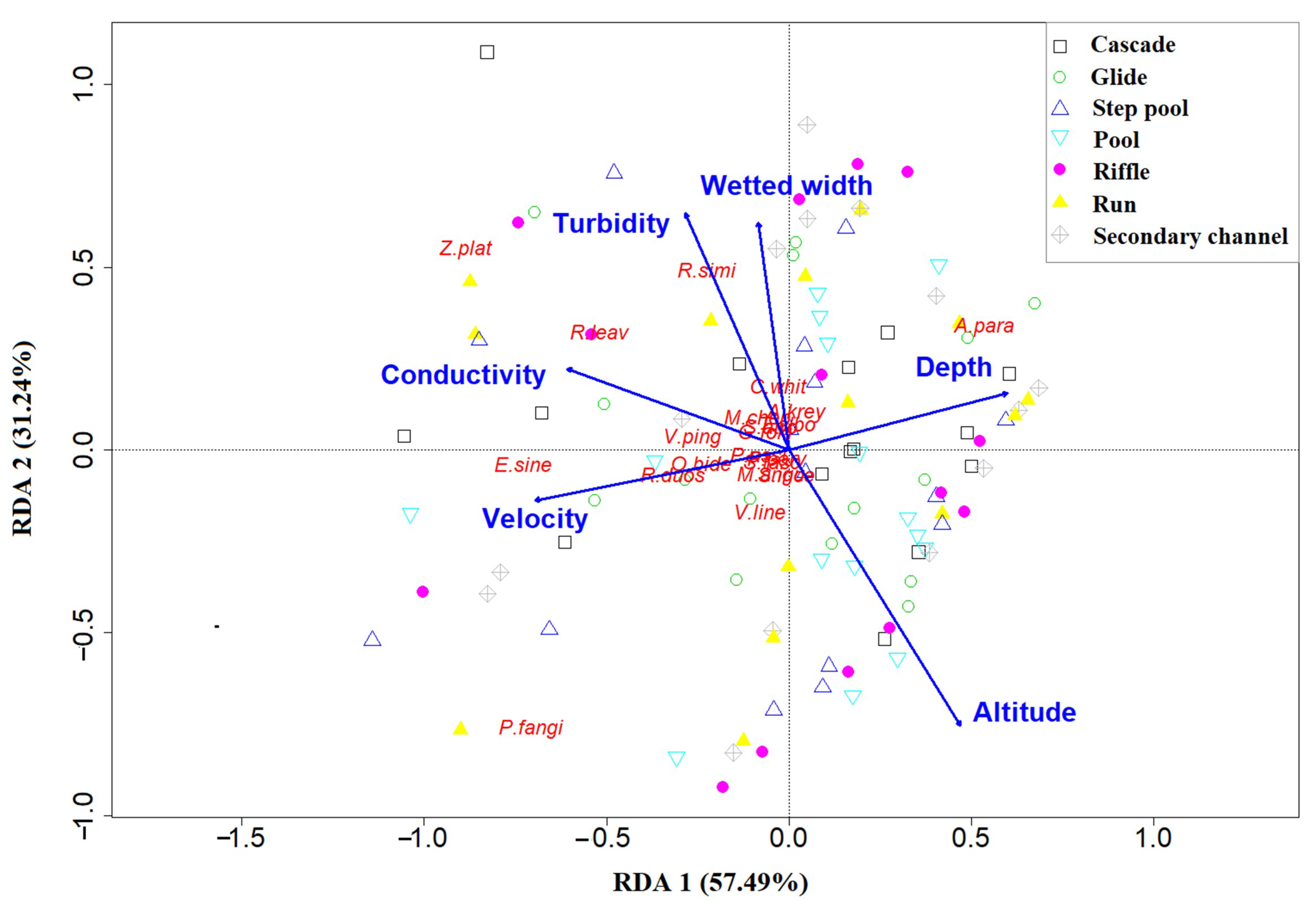
3.4. Relationship between Fish Assemblage and Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newson, M.D.; Newson, C.L. Geomorphology, ecology and river channel habitat: Mesoscale approaches to basin-scale challenges. Prog. Phys. Geogr. 2000, 24, 195–217. [Google Scholar] [CrossRef]
- Zeni, J.O.; Casatti, L. The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 2014, 726, 259–270. [Google Scholar] [CrossRef]
- Karr, J.R.; Dudley, D.R. Ecological perspectives on water quality goals. Environ. Manag. 1981, 5, 55–68. [Google Scholar] [CrossRef]
- Van Liefferinge, C.; Simoens, I.; Vogt, C.; Cox, T.J.S.; Breine, J.; Ercken, D.; Goethals, P.; Belpaire, C.; Meire, P. Impact of habitat diversity on the sampling effort required for the assessment of river fish communities and IBI. Hydrobiologia 2010, 644, 169–183. [Google Scholar] [CrossRef]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Schlosser, I.J. Assessing Biological Integrity in Running Waters: A Method and Its Rationale; Illinois Natural History Survey: Champaigne, IL, USA, Special Publication 5; 1986; p. 26. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadable Rivers: Periphyton, Benthic Invertebrates and Fish, 2rd ed.; EPA 841-B-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. Available online: https://www.krisweb.com/biblio/gen_usepa_barbouretal_1999_rba.pdf (accessed on 20 December 2018).
- Wang, L.; Seelbach, P.W.; Hughes, R.M. Introduction to landscape influences on stream habitats and biological assemblages. Am. Fish. Soc. Symp. 2006, 48, 1–23. [Google Scholar]
- Fialho, A.P.; Oliveira, L.G.; Tejerina-Garro, F.L.; de Mérona, B. Fish-habitat relationship in a tropical river under anthropogenic influences. Hydrobiologia 2008, 598, 315–324. [Google Scholar] [CrossRef]
- Gorman, O.T.; Karr, J.R. Habitat Structure and Stream Fish Communities. Ecology 1978, 59, 507–515. [Google Scholar] [CrossRef]
- Lau, J.K.; Lauer, T.E.; Weinman, M.L. Impacts of channelization on stream habitats and associated fish assemblages in East Central Indiana. Am. Midl. Nat. 2006, 156, 319–330. [Google Scholar] [CrossRef]
- Diana, M.; Allan, J.D.; Infante, D. The influence of physical habitat and land use on stream fish assemblages in southeastern Michigan. Am. Fish. Soc. Symp. 2006, 48, 359–374. [Google Scholar]
- Mossop, K.D.; Moran, N.P.; Chapple, D.G.; Wong, B.B.M. Connectivity and habitat type shape divergent dispersal behavior in a desert-dwelling fish. Landsc. Ecol. 2017, 32, 1065–1078. [Google Scholar] [CrossRef]
- Yu, S.L.; Lee, T.W. Habitat preference of the stream fish, Sinogastromyzon pulinensis (Homalopteridae). Zool. Stud. 2002, 41, 183–187. [Google Scholar]
- Blanck, A.; Tedesco, P.A.; Lamouroux, N. Relationships between life-history strategies of European freshwater fish species and their habitat preferences. Freshw. Biol. 2007, 52, 843–859. [Google Scholar] [CrossRef]
- Jones, N.E.; Tonn, W.M.; Scrimgeour, G.J.; Katopodis, C. Productive capacity of an artificial stream in the Canadian Arctic: Assessing the effectiveness of fish habitat compensation. Can. J. Fish Aquat. Sci. 2003, 60, 849–863. [Google Scholar] [CrossRef]
- Mohseni, O.; Stefan, H.G.; Eaton, J.G. Global warming and potential changes in fish habitat in US streams. Clim. Chang. 2003, 59, 389–409. [Google Scholar] [CrossRef]
- Raven, P.J.; Holmes, N.T.H.; Vaughan, I.P.; Dawson, F.H.; Scarlett, P. Benchmarking habitat quality: Observations using River Habitat Survey on near-natural streams and rivers in northern and western Europe. Aquat. Conserv. -Mar. Freshw. Ecosyst. 2010, 20, S13–S30. [Google Scholar] [CrossRef]
- Wood, J.L.A.; Belmar-Lucero, S.; Hutchings, J.A.; Fraser, D.J. Relationship of habitat variability to population size in a stream fish. Ecol. Appl. 2014, 24, 1085–1100. [Google Scholar] [CrossRef]
- Zheng, B.H.; Zhang, Y.; Li, Y.B. Study of indicators and methods for river habitat assessment of Liao River Basin. Acta Sci. Circumstantiae 2007, 27, 928–936. (In Chinese) [Google Scholar]
- Wang, J.H.; Tian, J.H.; Lv, X.G. Assessment of stream habitat quality in Naoli River Watershed, China. Acta Ecol. Sin. 2010, 30, 481–486. (In Chinese) [Google Scholar]
- Wang, Q.; Yuan, X.Z.; Liu, H.; Zhang, Y.W. Rapid assessment model for mountain stream habitat and its application. J. Hydraul. Eng. 2011, 42, 928–933. (In Chinese) [Google Scholar]
- Chu, L.; Wang, W.J.; Zhu, R.; Yan, Y.Z.; Chen, Y.F.; Wang, L.Z. Variation in fish assemblages across impoundments of low-head dams in headwater streams of the Qingyi River, China: Effects of abiotic factors and native invaders. Environ. Biol. Fishes 2015, 98, 101–112. [Google Scholar] [CrossRef]
- Li, Y.R.; Tao, J.; Chu, L.; Yan, Y.Z. Effects of anthropogenic disturbances on alpha and beta diversity of fish assemblages and their longitudinal patterns in subtropical streams, China. Ecol. Freshw. Fish 2018, 27, 433–441. [Google Scholar] [CrossRef]
- Fu, C.Z.; Wu, J.H.; Chen, J.K.; Qu, Q.H.; Lei, G.C. Freshwater fish biodiversity in the Yangtze River basin of China: Patterns, threats and conservation. Biodivers. Conserv. 2003, 12, 1649–1685. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, L.L.; Sato, T.; Zou, L.M.; Jiang, K.; Yahara, T.; Kano, Y. Distribution pattern, threats and conservation of fish biodiversity in the East Tiaoxi, China. Environ. Biol. Fishes 2013, 96, 519–533. [Google Scholar] [CrossRef]
- Kang, B.; Huang, X.X.; Yan, Y.Z.; Yan, Y.R.; Lin, H.D. Continental-scale analysis of taxonomic and functional fish diversity in the Yangtze river. Glob. Ecol. Conserv. 2018, 15, e00442. [Google Scholar] [CrossRef]
- Raat, A.J.P. Ecological rehabilitation of the Dutch part of the River Rhine with special attention to the fish. River Res. Appl. 2001, 17, 131–144. [Google Scholar] [CrossRef]
- Dudgeon, D. River rehabilitation for conservation of fish biodiversity in monsoonal Asia. Ecol. Soc. 2005, 10, 15. [Google Scholar] [CrossRef]
- Pokharel, K.K.; Basnet, K.B.; Majupuria, T.C.; Baniya, C.B. Correlations between fish assemblage structure and environmental variables of the Seti Gandaki River Basin, Nepal. J. Freshw. Ecol. 2018, 33, 31–43. [Google Scholar] [CrossRef]
- Taylor, C.M. A large-scale comparative analysis of riffle and pool fish communities in an upland stream system. Environ. Biol. Fishes 2000, 58, 89–95. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Batalha, D.C.; Collares-Pereira, M.J. Gradients in stream fish assemblages across a Mediterranean landscape: Contributions of environmental factors and spatial structure. Freshw. Biol. 2002, 47, 1015–1031. [Google Scholar] [CrossRef]
- Kautza, A.; Sullivan, S.M.P. Relative effects of local- and landscape-scale environmental factors on stream fish assemblages: Evidence from Idaho and Ohio, USA. Fundam. Appl. Limnol. 2012, 180, 259–270. [Google Scholar] [CrossRef]
- Meyer, J.L.; Strayer, D.L.; Wakkace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The contribution of headwater streams to biodiversity in river networks. J. Am. Water Resour. Assoc. 2007, 43, 86–103. [Google Scholar] [CrossRef]
- Yang, Y. Discussion on ecological and environmental water supplement target flow of Lijiang River. Guangxi Water Resour. Hydropower Eng. 2012, 3, 7–10. (In Chinese) [Google Scholar]
- Li, R.; Chen, Q.; Tonina, D.; Cai, D. Effects of upstream reservoir regulation on the hydrological regime and fish habitats of the Lijiang River, China. Ecol. Eng. 2015, 76, 75–83. [Google Scholar] [CrossRef]
- Li, R.; Chen, Q.; Ye, F. Modelling the impacts of reservoir operations on the downstream riparian vegetation and fish habitats in the Lijiang River. J. Hydroinformatics 2011, 13, 229–244. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Z.Q.; Zhu, Z.J.; Yan, J. Species composition, trend biodiversity variation and conservation of the fish in Lijiang River (in China). Environ. Biol. Fishes 2018, 101, 675–685. [Google Scholar] [CrossRef]
- Liang, R.F.; Liu, C. Investigation and research on water environment background value of Lijiang River. Guangxi Water Resour. Hydropower Eng. 1984, 2, 44–52. (In Chinese) [Google Scholar]
- Guo, C.Q.; Fang, R.J.; Dai, J.F. Evolution and Prediction of Water Resources and Water Environment in the Upper Reaches of Lijiang River Basin; China Water Power Press: Beijing, China, 2011; p. 351. ISBN 9787508491172. (In Chinese) [Google Scholar]
- Han, Y.Q. Studies on fish species diversity and evolution trend in Lijiang River. J. Hydroecology 2010, 3, 132–135. (In Chinese) [Google Scholar]
- Anonymous. Freshwater fishes of Guangxi, China, 2rd ed.; Guangxi People’s Publishing House: Nanning, China, 2006; p. 535. ISBN 9787219057674. (In Chinese) [Google Scholar]
- Zheng, C.Y. Fishes of Pearl River; Science Press: Beijing, China, 1989; p. 438. ISBN 9787030008657. (In Chinese) [Google Scholar]
- Harrelson, C.C.; Rawlins, C.L.; Potyondy, J.P. Stream Channel Reference Sites: An Illustrated Guide to Field Technique; Gen. Tech. Rep. RM-245; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1994; p. 61. Available online: https://www.fs.fed.us/rm/pubs_rm/rm_gtr245.pdf (accessed on 20 December 2018).
- Walag, A.M.P.; Canencia, M.O.P. Physico-chemical parameters and macrobenthic invertebrates of the intertidal zone of Gusa, Cagayan de Oro city, Philippines. Adv. Enivronmental Sci. Int. J. Bioflux Soc. 2016, 8, 71–82. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Hoboken, NJ, USA, 2003; p. 264. ISBN 9780632056330. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2011, 26, 32–46. [Google Scholar]
- Imai, N.; Seino, T.; Aiba, S.; Takyu, M.; Titin, J.; Kitayama, K. Effects of selective logging on tree species diversity and composition of Bornean tropical rain forests at different spatial scales. Plant Ecol. 2012, 213, 1413–1424. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, L.L.; Zou, L.M.; Kano, Y.; Sato, T.; Yahara, T. Spatial and temporal variation of fish assemblages and their associations to habitat variables in a mountain stream of north Tiaoxi River, China. Environ. Biol. Fishes 2012, 93, 403–417. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambride, UK, 2003; p. 110. ISBN 9780511076619. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 2 July 2018).
- Gu, D.E.; Ma, G.M.; Zhu, Y.J.; Xu, M.; Luo, D.; Li, Y.Y.; Wei, H.; Mu, X.D.; Luo, J.R.; Hu, Y.C. The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 2015, 59, 1–7. [Google Scholar] [CrossRef]
- Gu, D.E.; Yu, F.D.; Xu, M.; Wei, H.; Mu, X.D.; Luo, D.; Yang, Y.X.; Pan, Z.; Hu, Y.C. Temperature effects on the distribution of two invasive tilapia species (Tilapia zillii and Oreochromis niloticus) in the rivers of South China. J. Freshw. Ecol. 2018, 33, 521–534. [Google Scholar] [CrossRef]
- Shuai, F.M.; Li, X.H.; Li, Y.F.; Jie, L.; Yang, J.P.; Lek, S. Forecasting the invasive potential of Nile tilapia (Oreochromis niloticus) in a large subtropical river using a univariate approach. Fundam. Appl. Limnol. 2015, 187, 165–176. [Google Scholar] [CrossRef]
- Favrot, S.D.; Jonasson, B.C.; Peterson, J.T. Fall and winter microhabitat use and suitability for spring chinook Salmon parr in a US Pacific Northwest River. Trans. Am. Fish. Soc. 2018, 147, 151–171. [Google Scholar] [CrossRef]
- Espírito-Santo, H.M.V.; Zuanon, J. Temporary pools provide stability to fish assemblages in Amazon headwater streams. Ecol. Freshw. Fish 2017, 2, 475–483. [Google Scholar] [CrossRef]
- Neely, D.A.; Conway, K.W.; Mayden, R.L. Erromyzon yangi, a new hillstream loach (Teleostei: Balitoridae) from the Pearl River drainage of Guangxi Province, China. Ichthyol. Explor. Freshw. 2007, 18, 97–102. [Google Scholar]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Jähnig, S.C.; Lorenz, A.; Hering, D. Hydromorphological parameters indicating differences between single- and multiple-channel mountain rivers in Germany, in relation to their modification and recovery. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 1200–1216. [Google Scholar]
- Helfield, J.M.; Engström, J.; Michel, J.T.; Nilsson, C.; Jansson, R. Effects of River Restoration on Riparian Biodiversity in Secondary Channels of the Pite River, Sweden. Environ. Manag. 2012, 49, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.P.; Suen, J.P. The importance of providing multiple-channel sections in dredging activities to improve fish habitat environments. Water 2016, 8, 36. [Google Scholar] [CrossRef]
- Pennock, C.A.; Cathcart, C.N.; Hedden, S.C.; Weber, R.E.; Gido, K. Fine-scale movement and habitat use of a prairie stream fish assemblage. Oecologia 2018, 186, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Schumann, D.A.; Howell, J.; Graeb, B.D.S.; Bertrand, K.N.; Klumb, R.A. Seasonality, floods and droughts structure larval fish assemblages in prairie rivers. Ecol. Freshw. Fish 2018, 27, 389–397. [Google Scholar] [CrossRef]
- Walrath, J.D.; Dauwalter, D.C.; Reinke, D. Influence of stream condition on habitat diversity and fish assemblages in an impaired upper Snake River Basin Watershed. Trans. Am. Fish. Soc. 2016, 145, 821–834. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The effects of weirs on structural stream habitat and biological communuities. J. Appl. Ecol. 2011, 48, 1450–1460. [Google Scholar] [CrossRef]
- Smith, S.C.F.; Meiners, S.J.; Hastings, R.P.; Thomas, T.; Colombo, R.E. Low-head dam impacts on habitat and the functional composition of fish commuities. River Res. Appl. 2017, 33, 680–689. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wu, Z.Q.; Huang, L.L.; Feng, W.L.; Ding, Y. Species composition and diversity of fish in the upper reach of Lijiang River. Sichuan J. Zool. 2015, 34, 126–132. (In Chinese) [Google Scholar]
| Habitat Types | Feature | |
|---|---|---|
| Lentic habitat | Pool | Gentle slope, deep water (>40 cm), smooth surface, very low velocity (<10 cm/s), often connected with riffle and glide. The substrate consists of fine sand and cobble (<15 cm). |
| Step pool | Deep water (>40 cm), very low velocity (<10 cm/s), located in the larger gradient of the river, often form a step-pool pattern, water disorder with little spray splashing. The substrate consists of fine sand and boulders (>20cm) mostly. | |
| Slow-flow habitat | Glide | Low gradient, shallow water (<30 cm), slow water flow (10–30 cm/s), smooth surface with little turbulence. The substrate consists of cobble (<15 cm) mostly. |
| Run | Slightly deep water (20–40 cm), higher velocity than pool but still belongs to slow-flow (10–30 cm/s), smooth surface without stirring. The substrate consists of fine sand and cobble (<15 cm). | |
| Secondary channel | Low gradient, slow flow (10–30 cm/s), located in the multiple channel sections and isolated from main channel. The substrate consists of fine sand and cobble (<15 cm). | |
| Fast-flow habitat | Riffle | High gradient, shallow water (20–30 cm), high velocity (30–60 cm/s), water disorder with white splashes. The substrate consists of fine sand and cobble (<15 cm). |
| Cascade | High gradient turbulence, shallow water (20–30 cm), very fast flow (>60 cm/s), water disorder with white spray splashing. The substrate consists of fine sand and cobble (<15 cm) mostly. | |
| Habitat Types | Velocity (cm/s) | Substrate Size (cm) | Depth (cm) | Conductivity (μS/cm) | Dissolved Oxygen (mg/L) | pH | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|
| Pool | 6.78 ± 2.09 a | 12.52 ± 5.42 a | 49.06 ± 15.95 a | 64.37 ± 48.57 | 8.60 ± 0.67 | 7.88 ± 0.89 | 2.53 ± 3.80 |
| Step pool | 8.35 ± 1.24 a | 23.54 ± 8.69 b | 46.36 ± 16.05 a | 57.36 ± 56.05 | 8.19 ± 0.27 | 7.87 ± 1.15 | 0.82 ± 0.52 |
| Glide | 20.52 ± 7.50 a | 10.15 ± 3.73 a | 24.28 ± 9.26 b | 82.33 ± 51.78 | 8.76 ± 0.46 | 8.11 ± 0.86 | 2.94 ± 4.51 |
| Run | 16.60 ± 4.64 a | 17.67 ± 6.27 b | 28.65 ± 10.31 b | 28.22 ± 10.32 | 8.28 ± 0.42 | 7.28 ± 0.67 | 1.30 ± 1.35 |
| Secondary channel | 18.17 ± 6.46 a | 10.67 ± 2.10 a | 33.12 ± 12.91 b | 59.51 ± 40.35 | 7.75 ± 1.55 | 7.53 ± 0.70 | 1.33 ± 0.86 |
| Riffle | 37.42 ± 7.94 b | 11.67 ± 4.59 a | 27.29 ± 10.20 b | 56.40 ± 38.38 | 8.54 ± 0.49 | 7.78 ± 0.78 | 2.94 ± 4.75 |
| Cascade | 96.38 ± 34.15 c | 12.23 ± 3.45 a | 30.16 ± 12.73 b | 54.29 ± 28.81 | 8.60 ± 0.43 | 7.76 ± 0.47 | 4.94 ± 6.75 |
| Fish Species | Fish Individuals in Different Habitat Types (N) | Frequency of Occurrence | Relative Abundance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pool | Step Pool | Glide | Run | Secondary Channel | Riffle | Cascade | |||
| Cypriniformes | |||||||||
| Balitoridae | |||||||||
| Erromyzon sinensis (Chen, 1980) | 29 | 4 | 21 | 2 | 5 | 195 | 56 | 49.0% | 10.51% |
| Pseudogastromyzon fangi (Nichols, 1931) | 25 | 20 | 45 | 12 | 29 | 480 | 125 | 67.7% | 24.80% |
| Vanmanenia lineata (Fang, 1935) | 1 | 1 | 1 | 14 | 9 | 12.8% | 0.88% | ||
| Vanmanenia pingchowensis (Fang, 1935) | 1 | 4 | 64 | 17 | 23.5% | 2.90% | |||
| Cobitidae | |||||||||
| Misgurnus anguillicaudatus (Cantor, 1842) | 2 | 1 | 25 | 4 | 3 | 1 | 9.8% | 1.21% | |
| Cyprinidae | |||||||||
| Acrossocheilus kreyenbergii (Regan, 1908) | 11 | 2 | 2 | 3 | 1 | 7.8% | 0.64% | ||
| Acrossocheilus parallens (Nichols, 1931) | 208 | 24 | 40 | 34 | 36 | 27 | 12 | 69.6% | 12.84% |
| Carassius auratus (Linnaeus, 1758) | 1 | 1.0% | 0.03% | ||||||
| Cyprinus carpio (Linnaeus, 1758) | 2 | 3 | 2.0% | 0.17% | |||||
| Microphysogobio chenhsienensis (Fang, 1938) | 20 | 2 | 2 | 5 | 7.8% | 0.98% | |||
| Microphysogobio kiatingensis (Wu, 1930) | 3 | 2.0% | 0.10% | ||||||
| Onychostoma barbatulum (Pellegrin, 1908) | 1 | 1.0% | 0.03% | ||||||
| Onychostoma gerlachi (Peters, 1881) | 1 | 1.0% | 0.03% | ||||||
| Opsariichthys bidens (Günther, 1873) | 3 | 13 | 4 | 6 | 57 | 5 | 18.6% | 2.97% | |
| Parasinilabeo assimilis (Wu and Yao, 1977) | 3 | 1 | 12 | 6.9% | 0.54% | ||||
| Pseudorasbora parva (Temminck and Schlegel, 1846) | 1 | 3 | 2 | 6 | 5.9% | 0.40% | |||
| Rhodeus ocellatus (Kner, 1866) | 1 | 1.0% | 0.03% | ||||||
| Squalidus atromaculatus (Nichols and Pope, 1927) | 1 | 3 | 12 | 2 | 4 | 1 | 4 | 12.8% | 0.91% |
| Zacco platypus (Temminck and Schlegel, 1846) | 31 | 12 | 49 | 5 | 54 | 369 | 103 | 55.9% | 21.00% |
| Nemacheilidae | |||||||||
| Oreonectes platycephalus (Günther, 1868) | 1 | 1 | 2.0% | 0.07% | |||||
| Schistura fasciolata (Nichols and Pope, 1927) | 1 | 1 | 3 | 14 | 3 | 13.7% | 0.74% | ||
| Schistura incerta (Nichols, 1931) | 2 | 6 | 1 | 7.8% | 0.30% | ||||
| Traccatichthys pulcher (Nichols and Pope, 1927) | 1 | 1.0% | 0.03% | ||||||
| Perciformes | |||||||||
| Cichlidae | |||||||||
| Oreochromis niloticus (Linnaeus, 1758) | 2 | 1.0% | 0.07% | ||||||
| Gobiidae | |||||||||
| Rhinogobius duospilus (Herre, 1935) | 11 | 3 | 18 | 3 | 9 | 47 | 19 | 49.0% | 3.71% |
| Rhinogobius filamentosus (Wu, 1939) | 2 | 1 | 1 | 3.0% | 0.14% | ||||
| Rhinogobius similis (Gill, 1859) | 7 | 45 | 3 | 28 | 25 | 35 | 31.4% | 4.82% | |
| Rhinogobius leavelli (Herre, 1935) | 10 | 3 | 26 | 3 | 10 | 77 | 54 | 48.0% | 6.17% |
| Percichthyidae | |||||||||
| Coreoperca whiteheadi (Boulenger, 1900) | 6 | 7 | 1 | 1 | 10 | 14.7% | 0.84% | ||
| Siniperca scherzeri (Steindachner, 1892) | 1 | 1.0% | 0.03% | ||||||
| Siniperca undulata (Fang and Chong, 1932) | 1 | 1.0% | 0.03% | ||||||
| Siluriformes | |||||||||
| Bagridae | |||||||||
| Tachysurus albomarginatus (Rendahl, 1928) | 6 | 1 | 4 | 2 | 1 | 17 | 3 | 13.7% | 1.15% |
| Tachysurus adiposalis (Oshima, 1919) | 1 | 1 | 2 | 3 | 4.9% | 0.24% | |||
| Siluridae | |||||||||
| Pterocryptis anomala (Herre, 1933) | 1 | 1 | 2.0% | 0.07% | |||||
| Pterocryptis cochinchinensis (Valenciennes, 1840) | 1 | 1.0% | 0.03% | ||||||
| Sisoridae | |||||||||
| Glyptothorax fokiensis (Rendahl, 1925) | 2 | 1 | 9 | 4 | 7.8% | 0.54% | |||
| Synbranchiformes | |||||||||
| Mastacembelidae | |||||||||
| Macrognathus aculeatus (Bloch, 1786) | 1 | 1 | 2.0% | 0.07% | |||||
| Abundance | 365 | 75 | 337 | 81 | 208 | 1442 | 460 | - | |
| Species richness | 23 | 10 | 22 | 16 | 23 | 27 | 19 | - | |
| Habitat Types | Species Richness | Fish Abundance | Fish Density (ind/m2) | Shannon Index (H’) |
|---|---|---|---|---|
| Pool | 4.23 ± 2.25 a | 16.59 ± 12.74 a | 0.34 ± 0.27 a | 0.93 ± 0.59 a |
| Step pool | 3.63 ± 1.77 a | 9.38 ± 8.60 a | 0.36 ± 0.35 a | 1.09 ± 0.40 a,b |
| Glide | 5.93 ± 2.19 a,b | 22.47 ± 11.97 a | 0.31 ± 0.14 a | 1.47 ± 0.37 b |
| Run | 5.00 ± 3.35 a,b | 13.50 ± 8.55 a | 0.25 ± 0.10 a | 1.09 ± 0.82 a,b |
| Secondary channel | 5.67 ± 3.20 a,b | 23.11 ± 13.62 a | 0.47 ± 0.28 a | 1.17 ± 0.56 a,b |
| Riffle | 6.89 ± 2.42 b | 51.50 ± 35.99 b | 1.15 ± 0.93 b | 1.33 ± 0.42 a,b |
| Cascade | 6.07 ± 2.70 b | 32.86 ± 25.74 b | 0.73 ± 0.44 b | 1.34 ± 0.44 a,b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Huang, L.; Wu, Z.; Mo, Y.; Zou, Q.; Wu, N.; Chen, Z. Correlation of Fish Assemblages with Habitat and Environmental Variables in a Headwater Stream Section of Lijiang River, China. Sustainability 2019, 11, 1135. https://doi.org/10.3390/su11041135
Huang J, Huang L, Wu Z, Mo Y, Zou Q, Wu N, Chen Z. Correlation of Fish Assemblages with Habitat and Environmental Variables in a Headwater Stream Section of Lijiang River, China. Sustainability. 2019; 11(4):1135. https://doi.org/10.3390/su11041135
Chicago/Turabian StyleHuang, Jian, Liangliang Huang, Zhiqiang Wu, Yuanmin Mo, Qi Zou, Naicheng Wu, and Zhongbing Chen. 2019. "Correlation of Fish Assemblages with Habitat and Environmental Variables in a Headwater Stream Section of Lijiang River, China" Sustainability 11, no. 4: 1135. https://doi.org/10.3390/su11041135
APA StyleHuang, J., Huang, L., Wu, Z., Mo, Y., Zou, Q., Wu, N., & Chen, Z. (2019). Correlation of Fish Assemblages with Habitat and Environmental Variables in a Headwater Stream Section of Lijiang River, China. Sustainability, 11(4), 1135. https://doi.org/10.3390/su11041135







