The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
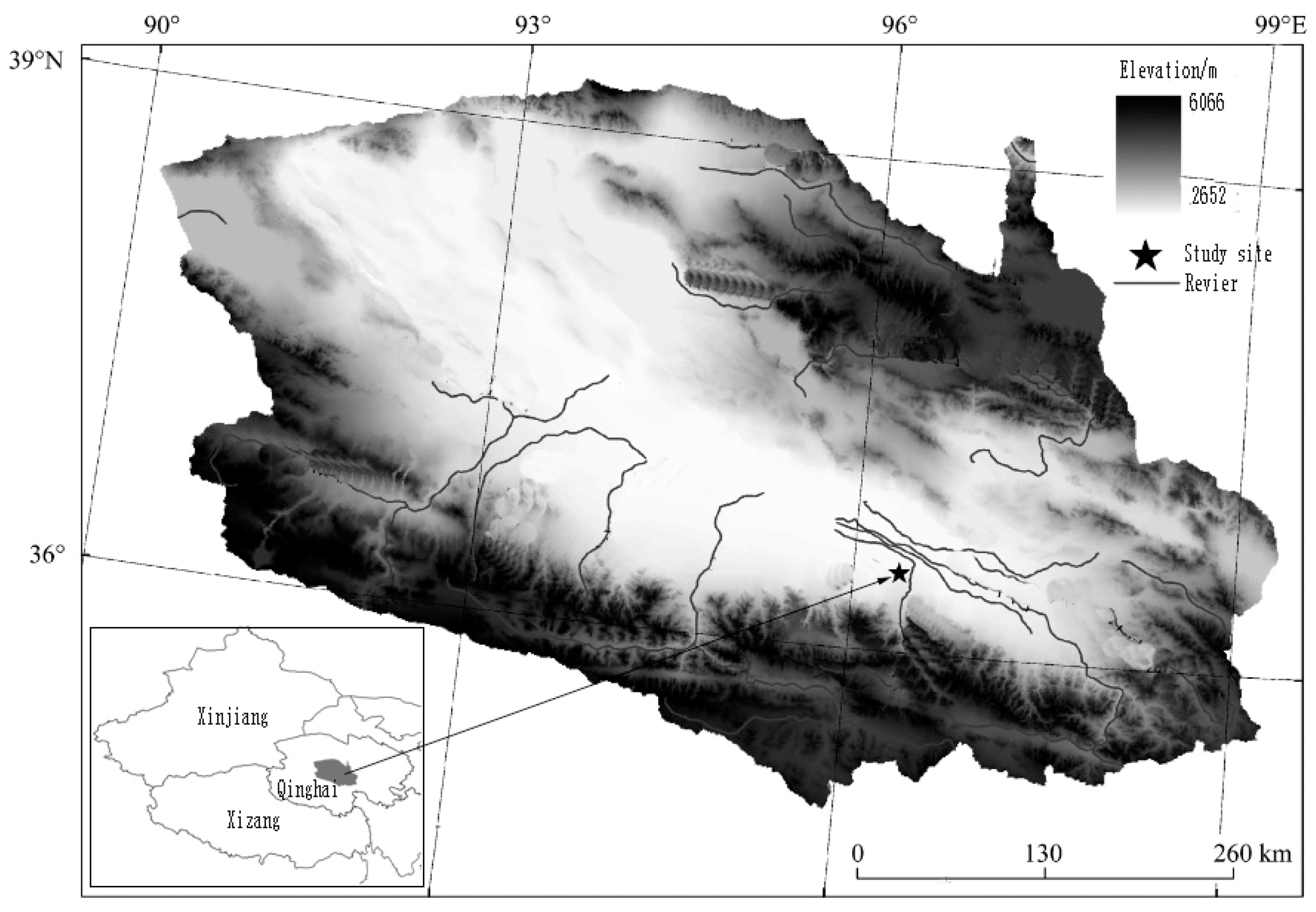
2.1. Study Sites
2.2. Experimental Design
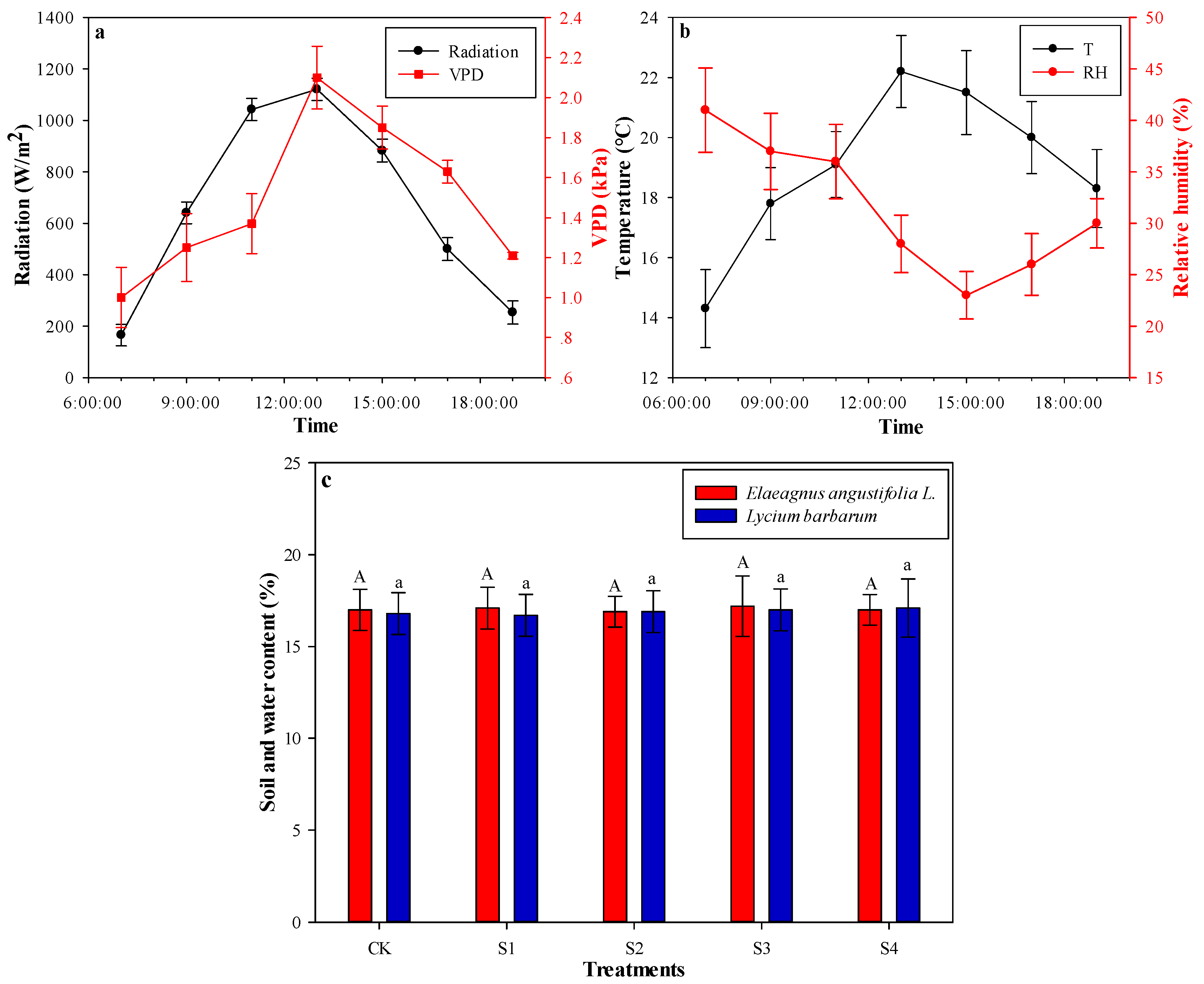
2.3. Determination of Meteorological Parameters
2.4. Determination of Physiological and Biochemical Indicators
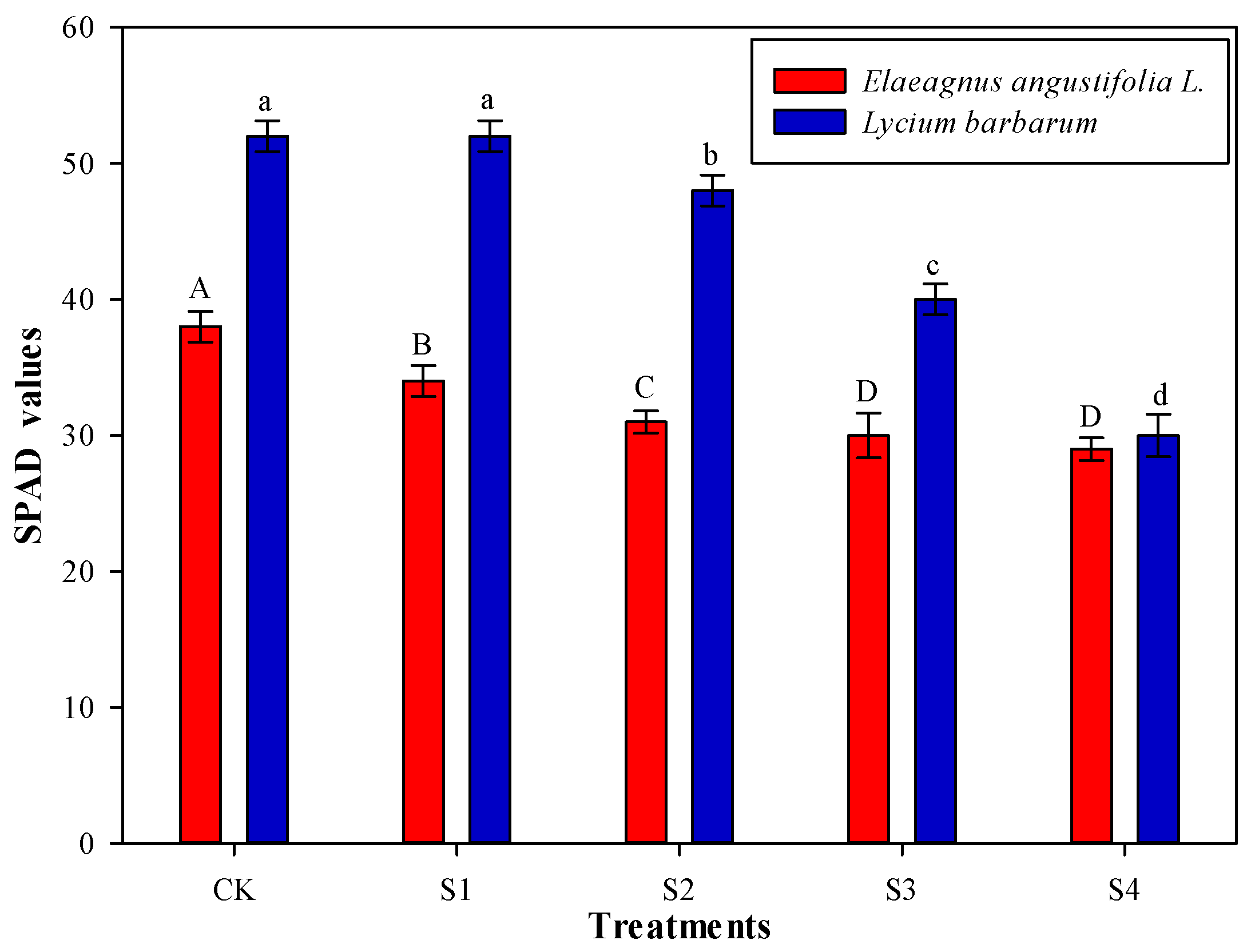
2.4.1. Leaf SPAD Value
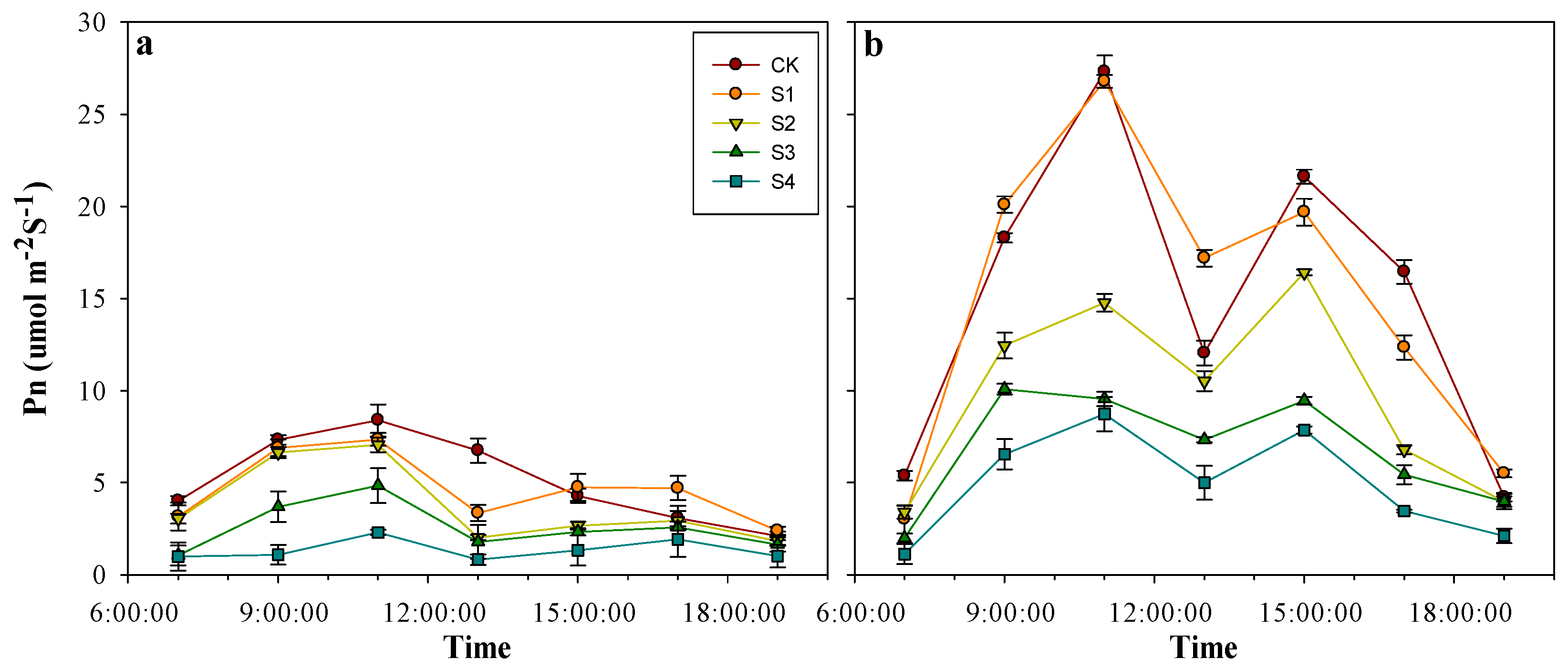
2.4.2. Photosynthetic and Transpiration Processes
2.4.3. Measurement of Light Response
2.5. Statistical Analysis
3. Results and Discussion
3.1. Basic Meteorological Characteristics
3.2. Effects of Salt Stress on Chlorophyll Content of Shrubs
3.3. Effects of Salt Stress on Photosynthesis
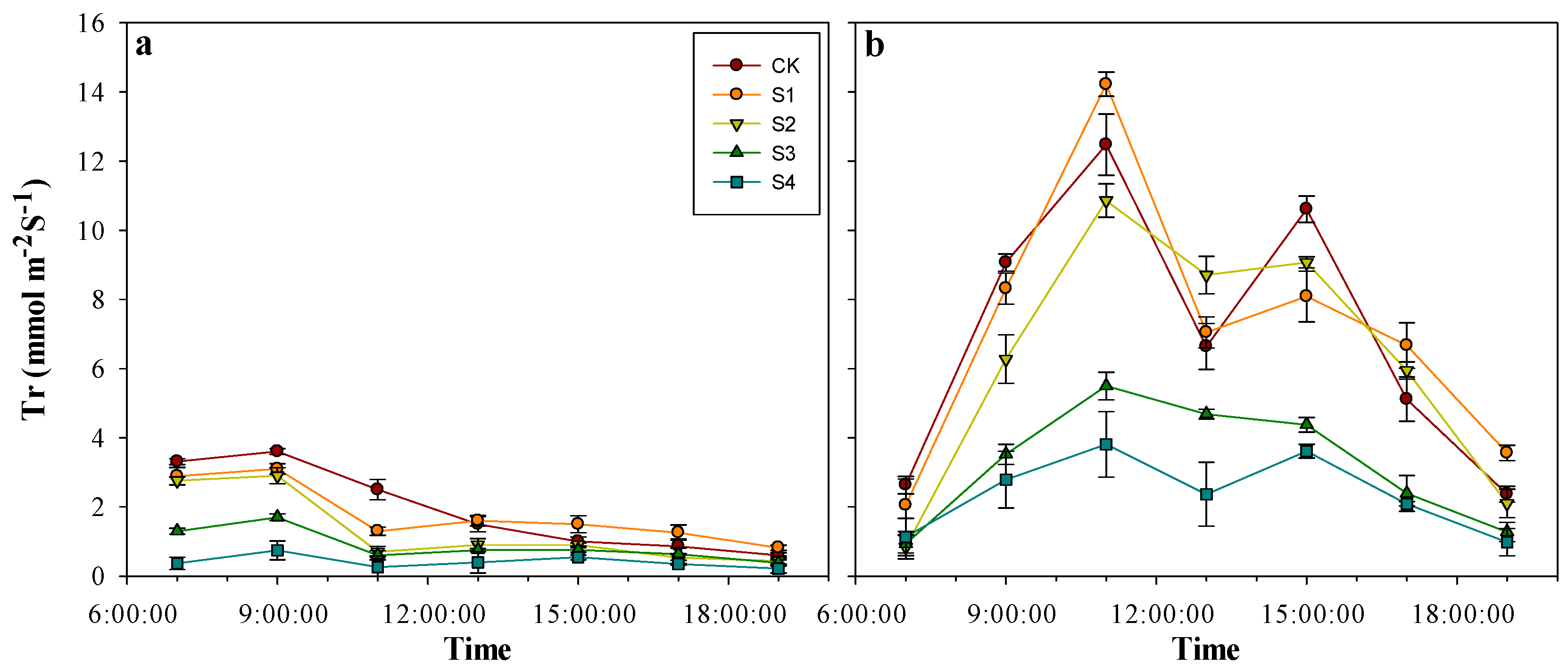
3.4. Effects of Salt Stress on Transpiration
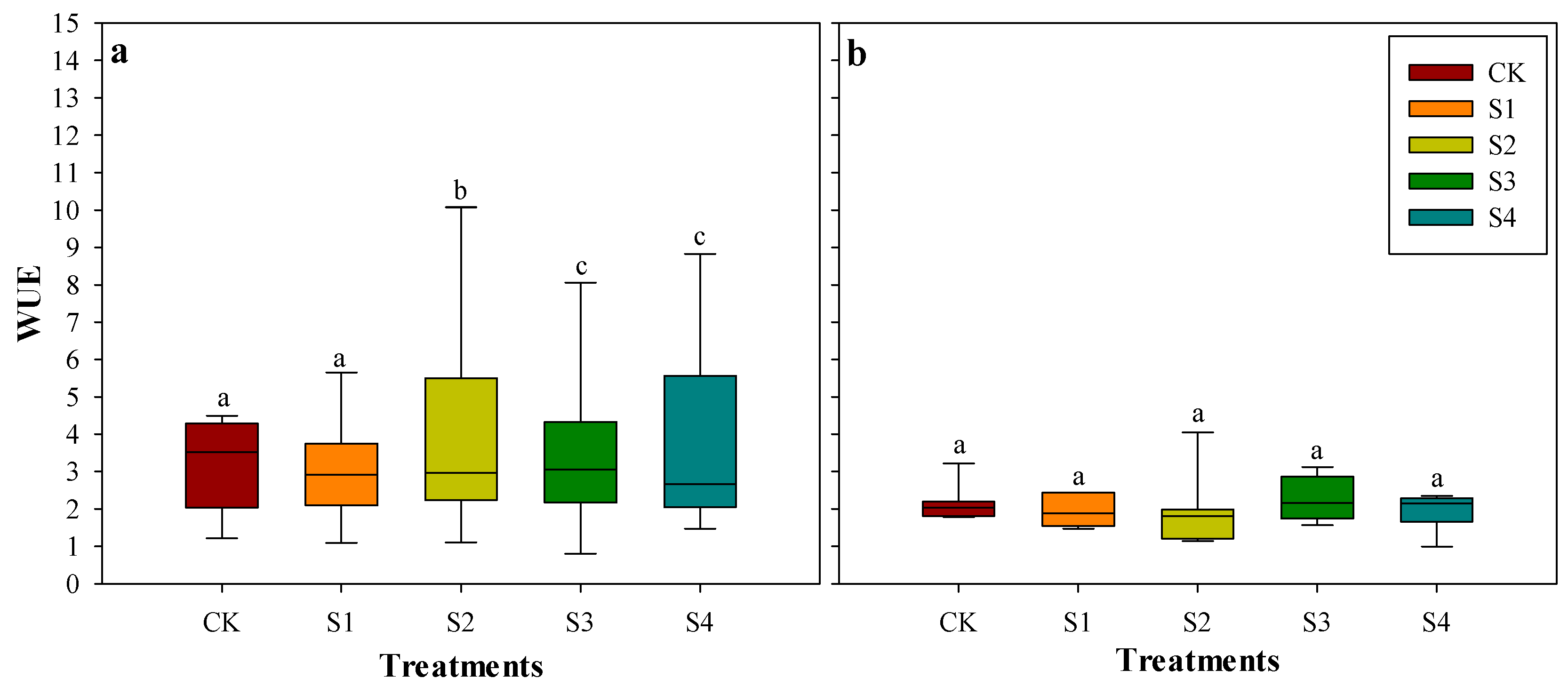
3.5. Effects of Salt Stress on Water Use Efficiency
3.6. Evaluation of the Effect of Saline Stress on E. angustifolia and L. barbarum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jesus, J.; Castro, F.; Niemelä, A.; Borges, M.T.; Danko, A.S. Evaluation of the impact of different soil salinization processes on organic and mineral soils. Water Air Soil Pollut. 2015, 226, 1–12. [Google Scholar] [CrossRef]
- Satoshi, Y. Responses of crops to soil salinization in south Baja California, Mexico. J. Plant Nutr. 2008, 31, 1800–1810. [Google Scholar] [CrossRef]
- Zhou, H.; Li, W. The effects of oasis ecosystem hydrological processes on soil salinization in the lower reaches of the tarim river, China. Ecohydrology 2013, 6, 1009–1020. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Hashemi, H.; Bouri, S. Soil salinization and critical shallow groundwater depth under saline irrigation condition in a Saharan irrigated land. Arabian J. Geosci. 2017, 10, 301. [Google Scholar] [CrossRef]
- Schofield, R.V.; Kirkby, M.J. Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Glob. Biogeochem. Cycles. 2003, 17. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Wang, M.; Xie, B.; Fu, Y.; Dong, C.; Hui, L.; Guang, H.L.; Liu, H. Effects of different elevated CO2 concentrations on chlorophyll contents, gas exchange, water use efficiency, and psii activity on C3 and C4 cereal crops in a closed artificial ecosystem. Photosynth. Res. 2015, 126, 351–362. [Google Scholar] [CrossRef]
- Berguerand, N.; Marinkovic, J.; Vilches, T.B.; Thunman, H. Use of alkali-feldspar as bed material for upgrading a biomass-derived producer gas from a gasifier. Chem. Eng. J. 2016, 295, 80–91. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, G.L.; Zhang, Y.M. Dune-scale distribution pattern of herbaceous plants and their relationship with environmental factors in a saline-alkali desert in central Asia. Sci. Total Environ. 2017, 576, 473–480. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; López-Beltrán, F.; Valencia, C.; Franco, J.M. Effect of an alkali treatment on the development of cellulose pulp-based gel-like dispersions in vegetable oil for use as lubricants. Tribol. Int. 2018, 123. [Google Scholar] [CrossRef]
- Peng, M.; Jia, H.; Wang, Q. The effect of land use on bacterial communities in saline-alkali soil. Curr. Microbiol. 2017, 74, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alemán, F.; González-Jasso, E.; Pless, R.C. Use of alternative alkali chlorides in RT and PCR of polynucleotides containing G quadruplex structures. Analytical Biochem. 2018, 543, 43. [Google Scholar] [CrossRef] [PubMed]
- Czinkota, I.; Issa, I.; Rétháti, G.; Kovács, B. Determination of the behaviour and the transport parameters of chromium in soil-water systems. Agrokémia És Talajtan. 2007, 55, 287–294. [Google Scholar] [CrossRef]
- Patel, A.D.; Panchal, N.S.; Pandey, I.B.; Pandey, A.N. Growth, water status and nutrient accumulation of seedlings of jatropha curcas l. (euphorbiaceae) in response to soil salinity. Anales De Biología 2010, 52, 59–71. [Google Scholar] [CrossRef]
- Zhou, H.; Li, W. Effect of water resource on soil salinization of oasis in the lower reaches of tarim river, China. Proced. Environ. Sci. 2011, 11, 925–933. [Google Scholar] [CrossRef]
- Laplante, S.R.; Fader, L.D.; Fandrick, K.R.; Fandrick, D.R.; Hucke, O.; Kemper, R.; Miller, S.P.F.; Edwards, P.J. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011, 54, 7005. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cong, Y.; Hussain, N.; Wang, Y.; Liu, Z.; Jiang, L.; Liang, Z.; Chen, K. The remodeling of seedling development in response to long-term magnesium toxicity and regulation by aba-della signaling in arabidopsis. Plant Cell Physiol. 2014, 55, 1713–1726. [Google Scholar] [CrossRef]
- Patel, N.T.; Pandey, A.N. Effect of soil salinity on growth, water status and nutrient accumulation in seedlings of suaeda nudiflora (chenopodiaceae). Anales De Biología 2009, 31, 61–70. [Google Scholar] [CrossRef]
- Fraga, T.I.; Carmona, F.D.C.; Anghinoni, I.; Marcolin, E. Attributes of irrigated rice and soil solution as affected by salinity levels of the water layer atributos de arroz irrigado e da solução do solo influenciados por níveis de salinidade da lâmina de água de irrigação. Revista Brasileira De Ciência Do Solo 2010, 34, 1049–1057. [Google Scholar] [CrossRef]
- Darlington, A.M.; Jarisz, T.A.; Dewaltkerian, E.L.; Roy, S.; Sun, K.; Azam, M.S. Separating the ph-dependent behavior of water in the stern and diffuse layers with varying salt concentration. J. Phys. Chem. C. 2017, 121. [Google Scholar] [CrossRef]
- Aryal, D.; Ganesan, V. Reversal of salt concentration dependencies of salt and water diffusivities in polymer electrolyte membranes. Acs Macro Lett. 2018, 7, 739–744. [Google Scholar] [CrossRef]
- Caruso, C.; Maucieri, C.; Berruti, A.; Borin, M.; Barbera, A.C. Responses of Different Panicum miliaceum L. Genotypes to Saline and Water Stress in a Marginal Mediterranean Environment. Agronomy 2018, 8, 8. [Google Scholar] [CrossRef]
- Maucieri, C.; Caruso, C.; Bona, S.; Borin, M.; Barbera, A.C.; Cavallaro, V. Influence of salinity and osmotic stress on germination process in an old sicilian landrace and a modern cultivar of Triticum Durum Desf. Cereal Res. Commun. 2018, 46, 253–262. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Relationship between extractable chlorophyll content and spad values in three varieties of kacip fatimah under greenhouse conditions. J. Plant Nutr. 2013, 36, 1366–1372. [Google Scholar] [CrossRef]
- Anand, M.H.; Byju, G. Chlorophyll meter and leaf colour chart to estimate chlorophyllcontent, leaf colour, and yield of cassava. Photosynthetica 2008, 46, 511–516. [Google Scholar] [CrossRef]
- Wang, X.; Geng, S.; Ma, Y.; Shi, D.; Yang, C.; Wang, H. Growth, photosynthesis, solute accumulation, and ion balance of tomato plant under sodium- or potassium-salt stress and alkali stress. Agron. J. 2015, 65, 927–928. [Google Scholar] [CrossRef]
- Bergmann, I.; Geiß-Brunschweiger, U.; Hagemann, M.; Schoor, A. Salinity tolerance of the chlorophyll b -synthesizing cyanobacterium prochlorothrix hollandica, strain sag 10.89. Microb. Ecol. 2008, 55, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Gouveianeto, A.S.; Silva, E.A.J.; Oliveira, R.A.; Moura, D.S.; Cunha, P.C.; Costa, E.B.; Câmara, T.J.R.; Willadino, L.G. Water deficit and salt stress diagnosis through led induced chlorophyll fluorescence analysis in jatropha curcas l. oil plants for biodiesel. J. Fluoresc. 2011, 22, 623–630. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, P.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 2008, 46, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L. Land exploitation resulting in soil salinization in a desert–oasis ecotone. Catena. 2013, 100, 50–56. [Google Scholar] [CrossRef]
- Silva, E.N.D.; Ribeiro, R.V.; Ferreirasilva, S.L.; Viégas, R.A.; Silveira, J.A.G. Salt stress induced damages on the photosynthesis of physic nut young plants. Scientia Agricola. 2011, 68, 62–68. [Google Scholar] [CrossRef]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4, photosynthesis do for C3, plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Andriani, A.; Tachibana, S.; Itoh, K. Effects of saline-alkaline stress on benzo[a]pyrene biotransformation and ligninolytic enzyme expression by Bjerkandera adusta, SM46. World J. Microbiol. Biotechnol. 2016, 32, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mercedes-Martín, R.; Rogerson, M.R.; Brasier, A.T.; Vonhof, H.B.; Prior, T.J.; Fellows, S.M.; Reijmer, J.J.G.; Billing, I.; Pedleya, H.M. Growing spherulitic calcite grains in saline, hyperalkaline lakes: Experimental evaluation of the effects of Mg-clays and organic acids. Sediment. Geol. 2016, 335, 93–102. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Sharma, P.C.; Sharma, D.K.; Ravikiran, K.T.; Singh, Y.P.; Mishra, V.K.; Burman, D.; Maji, B.; Mandal, S.; Sarangi, S.K.; et al. Identification of mega-environments and rice genotypes for general and specific adaptation to saline and alkaline stresses in India. Sci. Rep. 2017, 7, 7968. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Kolganova, T.V.; Khijniak, T.V.; Jones, B.E.; Kublanov, I.V. Diversity of cultivated aerobic poly-hydrolytic bacteria in saline alkaline soils. Peerj 2017, 2017, 3796. [Google Scholar] [CrossRef]
- Noormets, A.; Sôber, A.; Pell, E.J.; Dickson, R.E.; Podil, G.K.; Sôber, J.; Isebrands, J.G.; Karnosky, D.F. Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (populus tremuloides michx.) clones exposed to elevated CO2 and/or O3. Plant Cell Environ. 2010, 24, 327–336. [Google Scholar] [CrossRef]
- Varonea, L.; Ribas-Carbob, M.; Cardonac, C.; Galléb, A.; Medranob, H.; Gratania, L.; Flexasb, J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Botany. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Yue, L.J.; Li, S.X.; Ma, Q.; Zhou, X.R.; Wu, G.Q.; Bao, A.K.; Zhang, J.L.; Wang, S.M. NaCl stimulates growth and alleviates water stress in the xerophyte zygophyllum xanthoxylum. J. Arid Environ. 2012, 87, 153–160. [Google Scholar] [CrossRef]
- Farissi, M.; Faghire, M.; Bargaz, A.; Bouizgaren, A.; Makoudi, B.; Ghoulam, C. Growth, nutrients concentrations, and enzymes involved in plants nutrition of alfalfa populations under saline conditions. J. Agric. Sci. Technol. 2014, 16, 301–314. [Google Scholar] [CrossRef]
- Jiao, Y.; Bai, Z.; Xu, J.; Zhao, M.; Khan, Y.; Hu, Y.; Shi, L. Metabolomics and its physiological regulation process reveal the salt-tolerant mechanism in Glycine soja seedling roots. Plant Physiol. Biochem. 2018, 126, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Kang, J.G.; Li, J.; Lee, D.B.; Park, C.W.; Kim, J.D. Evaluation of salt-tolerance plant for improving saline soil of reclaimed land. Korean J. Soil Sci. Fertil. 2007, 40, 173–180. [Google Scholar]
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in brassica crops. Breed Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Talei, D.; Yusop, M.K.; Kadir, M.A.; Abdullah, M.P.; Valdiani, A. Response of king of bitters (andrographis paniculata nees.) seedlings to salinity stress beyond the salt tolerance threshold. Aust. J. Crop Sci. 2012, 6, 1059–1067. [Google Scholar]
| Species | Height (cm) | DBH (cm) | CK | S1 | S2 | S3 | S4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | EC | NaCl | EC | NaCl | EC | NaCl | EC | NaCl | EC | |||
| Elaeagnus angustifolia | 57.94 ± 0.78 | 1.27 ± 0.08 | 0 | 0 | 100 | 47 | 200 | 95 | 300 | 145 | 400 | 196 |
| Lycium barbarum | 40.28 ± 0.49 | 1.26 ± 0.06 | ||||||||||
| Species | T | SPAD | Pn | Gs | Ci | Tr | Pnmax | AQY | RD | LCP | LSP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elaeagnus angustifolia L. | T | 1 | - | - | - | - | - | - | - | - | - | - |
| SPAD | −0.99 ** | 1 | - | - | - | - | - | - | - | - | - | |
| Pn | −0.99 ** | 0.99 ** | 1 | - | - | - | - | - | - | - | - | |
| Gs | −0.99 ** | 0.99 ** | 0.99 ** | 1 | - | - | - | - | - | - | - | |
| Ci | −0.99 ** | 0.99 ** | 0.99 ** | 0.99 ** | 1 | - | - | - | - | - | - | |
| Tr | −0.11 | 0.7 | 0.04 | −0.006 | −0.012 | 1 | - | - | - | - | - | |
| Pnmax | 0.98 ** | −0.99 ** | −0.99 ** | −0.99 ** | −0.99 ** | −0.07 | 1 | - | - | - | - | |
| AQY | 0.98 ** | −0.95 ** | −0.96 ** | −0.96 ** | −0.95 ** | −0.15 | −0.91 ** | 1 | - | - | - | |
| RD | 0.73 * | −0.67 * | −0.68 * | −0.70 * | −0.67 * | −0.13 | −0.91 ** | −0.68 * | 1 | - | - | |
| LCP | −0.48 | 0.53 | 0.55 | 0.57 | 0.58 | −0.79 | 0.56 | 0.76 * | 0.58 | 1 | - | |
| LSP | 0.64 * | −0.65 * | −0.62 * | −0.58 | −0.59 | −0.45 | −0.59 | −0.74 * | −0.59 | 0.29 | 1 | |
| Lycium barbarum | T | 1 | - | - | - | - | - | - | - | - | - | - |
| SPAD | −0.95 ** | 1 | - | - | - | - | - | - | - | - | - | |
| Pn | −0.93 ** | −0.93 ** | 1 | - | - | - | - | - | - | - | ||
| Gs | −0.84 * | −0.81 * | −0.94 ** | 1 | - | - | - | - | - | - | - | |
| Ci | 0.86 * | 0.81 * | 0.67 | 0.49 | 1 | - | - | - | - | - | - | |
| Tr | −0.89 * | −0.85 * | −0.9 * | −0.94 ** | 0.61 | 1 | - | - | - | - | - | |
| Pnmax | −0.9 ** | −0.9 ** | −0.95 ** | −0.95 ** | 0.61 | −0.96 ** | 1 | - | - | - | - | |
| AQY | −0.88 * | −0.9 ** | −0.95 ** | −0.93 ** | 0.66 | −0.9 * | −0.9 * | 1 | - | - | - | |
| RD | −0.84 * | −0.83 * | −0.84 * | −0.89 * | 0.6 | −0.97 ** | −0.95 ** | −0.84 * | 1 | - | - | |
| LCP | 0.71 | 0.87 * | 0.67 | 0.5 | −0.78 * | 0.59 | 0.66 | 0.7 | 0.64 | 1 | - | |
| LSP | −0.89 * | −0.74 * | −0.79 * | −0.73 | 0.69 | −0.79 | −0.74 | −0.71 | −0.69 * | 0.37 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, Z.; Li, Y.; He, K. The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China. Sustainability 2019, 11, 828. https://doi.org/10.3390/su11030828
Zhang T, Zhang Z, Li Y, He K. The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China. Sustainability. 2019; 11(3):828. https://doi.org/10.3390/su11030828
Chicago/Turabian StyleZhang, Tan, Zhenzhong Zhang, Yuanhang Li, and Kangning He. 2019. "The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China" Sustainability 11, no. 3: 828. https://doi.org/10.3390/su11030828
APA StyleZhang, T., Zhang, Z., Li, Y., & He, K. (2019). The Effects of Saline Stress on the Growth of Two Shrub Species in the Qaidam Basin of Northwestern China. Sustainability, 11(3), 828. https://doi.org/10.3390/su11030828




