Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Treatments
2.1.1. Growth Parameters
2.1.2. Monovalent Ion Content
2.1.3. Photosynthetic Pigments
2.1.4. Lipid Peroxidation and Non-Enzymatic Antioxidants
2.1.5. Proline Determination
2.1.6. Total Soluble Sugars Quantification
2.1.7. Statistical Analyses
3. Results
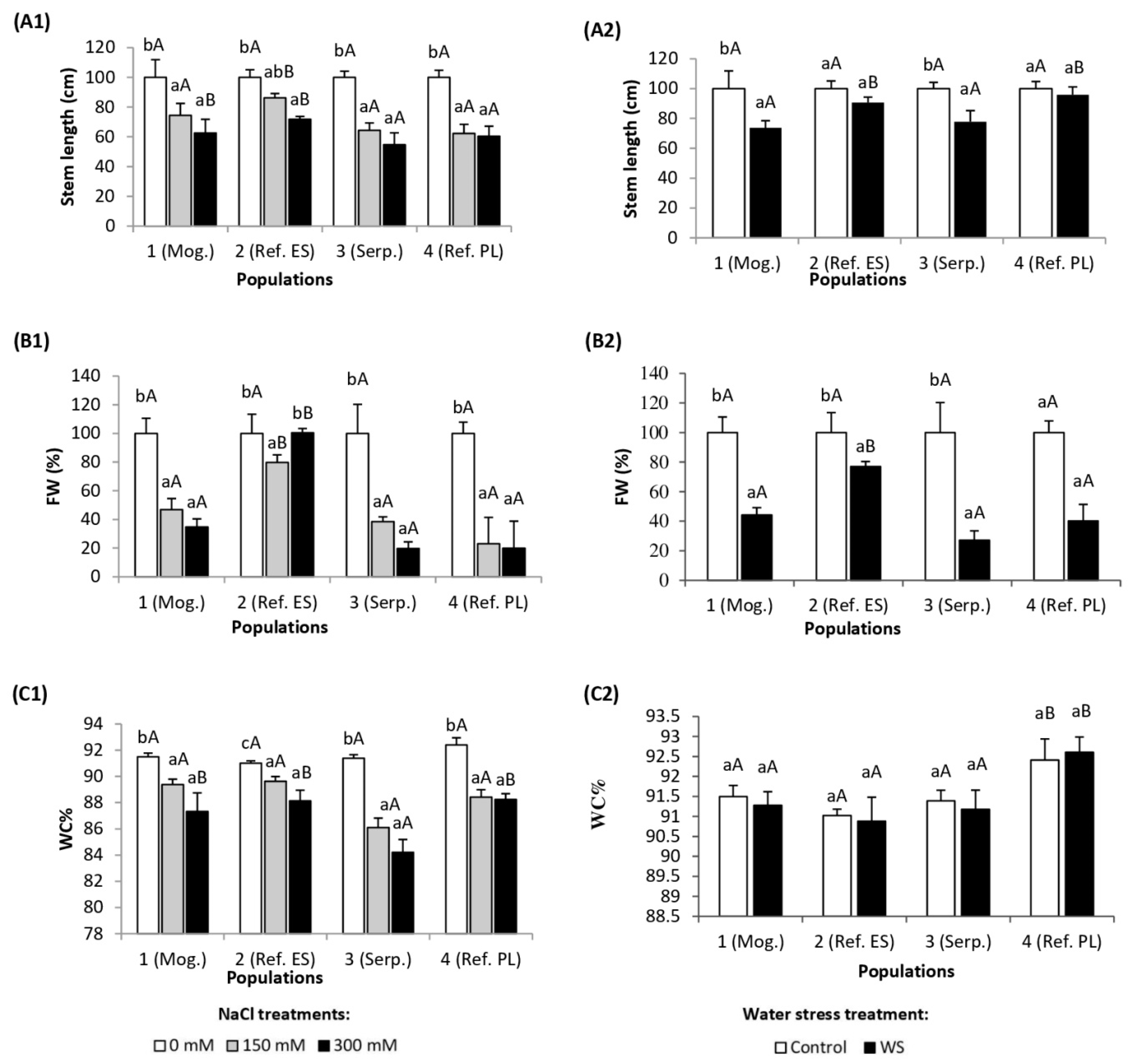
3.1. Growth Parameters
3.2. Ion Content in Leaves
3.3. Photosynthetic Pigments
3.4. Lipid Peroxidation and Non-Enzymatic Antioxidants
3.5. Osmolytes
3.6. Correlations between Growth and Biochemical Parameters in S. vulgaris Populations under Salinity and Drought Stress
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hagare, D.; Maheshwari, B.; Dillon, P. Impact of prolonged drought on salt accumulation in the root zone due to recycled water irrigation. Water Air Soil Pollut. 2015, 226, 90. [Google Scholar] [CrossRef]
- Martínez-Fernández, J.; González-Zamora, A.; Sánchez, N.; Gumuzzio, A.; Herrero-Jimenéz, C.M. Satelite soil moisture for agricultural drought monitoring: Assessment of the SMOS derived Soil Water Deficit Index. Remote Sens. Environ. 2016, 177, 277–286. [Google Scholar] [CrossRef]
- Vicente, O.; Al Hassan, M.; Boscaiu, M. Contribution of osmolyte accumulation to abiotic stress tolerance in wild plants adapted to different stressful environments. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 13–25. [Google Scholar]
- Laiskhanov, S.U.; Otarov, A.; Savin, I.Y.; Tanirbergenoc, S.I.; Mamutov, Z.U.; Diusekov, S.N.; Zhogolev, A. Dynamics of soil salinity in irrigation areas in South Kazakstan. Pol. J. Environ. Stud. 2016, 25, 2469–2475. [Google Scholar] [CrossRef]
- López-Jurado, J.; Baolo, F.; Maetos-Naranjo, E. Deciphering the ecophysiological traits involved during water stress acclimation and recovery of the threatened wild carnation, Dianthus inoxianus. Plant Physiol. Biochem. 2006, 109, 397–405. [Google Scholar] [CrossRef]
- Lopez, R.R.; Winter, J.M.; Elliott, J.; Ruane, A.C.; Porter, Ch.; Hoogenboom, G. Integrating growth stage deficit irrigation into a process based crop model. Agric. For. Meteorol. 2017, 243, 84–92. [Google Scholar] [CrossRef]
- Yeah, C.-H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.-Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. 2012, 195, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2018, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotech. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Gorim, L.Y.; Vanderberg, A. Evaluation of wild lentil species as genetic resources to improve drought tolerance in cultivated lentil. Front. Plant Sci. 2017, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Ariga, H.; Katori, T.; Yoshihara, R.; Hase, S.; Nozawa, S.; Narumi, I.; Iuchi, S.; Kobayashi, M.; Tezuka, K.; Hayashi, T.; et al. Arabidopsis sos1 mutant in a salt-tolerance accession revealed an importance of salt acclimation ability in plant salt tolerance. Plant Signal. Behav. 2013, 8, e24779. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Lefebre-De Vos, D.; Le DIsquet, I.; Leprince, A.S.; Bordeneave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savouré, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Al Hassan, M.; Naranjo, M.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant system in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-induced variation in biochemical markers provides insight into the mechanisms of salt tolerance in common (Phaseolus vulgaris) and runner (P. coccineus) beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, R.; Ortiz, L.T.; García, P. Nutrient and fatty acid composition of wild edible bladder campion populations [Silene vulgaris (Moench.) Garcke. Int. J. Food Sci. Technol. 2006, 41, 1239–1242. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.2006.01187.x (accessed on 16 October 2006). [CrossRef]
- Ranfa, A.; Bodesmo, M. An Ethnobotanical investigation of traditional knowledge and uses of edible wild plants in the Umbria Region, Central Italy. J. Appl. Bot. Food Qual. 2017, 90, 246–258. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Gazioğlu, I.; Sevgi, E.; Sönmez, F. Anticholinesterase, Antioxidant, Antiaflatoxigenic Activities of Ten Edible Wild Plants from Ordu Area, Turkey. Iran. J. Pharm. Res. 2018, 17, 1047–1056. [Google Scholar] [PubMed]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef]
- Boari, F.; Cefola, M.; Di Gioia, F.; Pace, B.; Serio, F.; Cantore, V. Effect of cooking methods on antioxidant activity and nitratecontent of selected wild Mediterranean plants. Int. J. Food Sci. Nutr. 2013, 64, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Disciglio, G.; Tarantino, A.; Frabboni, L.; Gagliardi, A.; Giuliani, M.M.; Tarantino, E.; Gatta, G. Qualitative characterization of cultivated and wild edible plants: Mineral elements, phenols content and antioxidant capacity. Ital. J. Agron. 2017, 12, 1036. [Google Scholar] [CrossRef]
- Friedrich, H.C. Caryophyllaceae. In Illustriete Flora von Mitteleuropa; Heigi, G., Ed.; Parey: Berlin, Germany, 1979; Volume 3. [Google Scholar]
- Bargish, T.A.; Rahmani, F. SRAP markers based genetic analysis of Silene species. J. Trop. Biol. Conserv. 2016, 13, 57–70. [Google Scholar] [CrossRef]
- Sebasky, M.E.; Keller, S.K.; Taylor, D.R. Investigating past range dynamics for a weed of cultivation, Silene vulgaris. Ecol. Evol. 2016, 6, 4800–4811. [Google Scholar] [CrossRef]
- Leuscher, C.; Ellenberg, H. Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats; Springer International Publishing: Cham, Swittzerland, 2018. [Google Scholar]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Identification of salt stress biomarkers in Romanian Carpathian populations of Picea abies (L.) Karst. PLoS ONE 2015, 10, e0135419. [Google Scholar] [CrossRef]
- Bratteler, M.; Lexter, C.; Widmer, A. Genetic architecture of traits associated with serpentine adaptation in Silene vulgaris (Caryophyllaceae). J. Evol. Biol. 2006, 19, 1149–1156. [Google Scholar] [CrossRef]
- Sandner, T.M.; Matthies, D. Fluctuating asymmetry of leaves is a poor indicator of environmental stress and genetic stress by inbreding Silene vulgaris. Ecol. Indic. 2017, 79, 247–253. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Panufnik, D. The adaptation of Silene vulgaris to the growth on a calamine waste heap (S. Poland). Environ. Pollut. 1998, 101, 415–426. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E. Remediation of soil-free grounds contaminated by zinc, lead and cadmium with the use of metallophytes. Pol. J. Environ. Stud. 2008, 17, 707–712. [Google Scholar]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R.; Walasek, K. Effects of Zn, Cd, Pb on physiological response of Silene vulgaris plants from selected populations. Pol. J. Environ. Stud. 2011, 20, 599–604. [Google Scholar]
- Verkleji, J.A.C.; Prast, J.E. Cadmium tolerance and co-tolerance in Silene vulgaris (Moench) Gracke. New. Phytol. 1989, 111, 637–645. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Czura, A.; Grabski, K.; Tukaj, Z. The effect of conditioned medium obtained from Scenedesmus subspicatus on suspension culture of Silene vulgaris. Acta Physiol. Plant. 2009, 31, 881–887. [Google Scholar] [CrossRef]
- Ernst, W.H.O.; Nelissen, H.J.M. Life-cycle phases of a zinc- and cadmium resistant ecotype of Silene vulgaris in risk assessment of polymetallic mine soils. Environ. Pollut. 2000, 107, 329–338. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Ciepał, R. Phytoextraction of zinc, lead and cadmium with Silene vulgaris (Moench) Gracke in the post industrial area. Ecol. Chem. Eng. 2009, 16, 831–837. [Google Scholar]
- Baloun, J.; Nevrtalova, E.; Kovacova, V.; Hudzieczek, V.; Cegan, R.; Vyskot, B.; Hobza, R. Characterization of the HMA7 gene and transcriptomic analysis of candidate genes for copper tolerance in two Silene vulgaris ecotypes. J. Plant Physiol. 2014, 171, 1188–1196. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; García-Gonzalo, P.; Alarcón, R.; González-Rodrígez, A.; Lobo, M.C.; Pèrez-Sanz, A. Effect of genotype, Cr (III) and Cr(VI) on plant growth and micronutrient status in Silene vulgaris Moench). Span. J. Agric. Res. 2013, 11, 685–694. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; García-Gonzalo, P.; Lobo, M.C.; Pèrez-Sanz, A. Chromium speciation modifies root exudation in two genotypes of Silene vulgaris. Environ. Exp. Bot. 2014, 107, 1–6. [Google Scholar] [CrossRef]
- García-Gonzalo, P.; Pradas del Real, A.E.; Lobo, M.C.; Pèrez-Sanz, A. Different genotypes of Silene vulgaris (Moench) Garcke grown on chromium-contaminated soils influence root organic acid composition and rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 25713–25724. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R.; Pease, A.J.; Malaisse, F. Studies on copper and cobalt tolerance in three closely related taxa within genus Silene L. (Carypyhyllaceae). Plant Soil. 1983, 73, 377–385. [Google Scholar] [CrossRef]
- Gonelli, C.; Galardi, F.; Gabbrielli, R. Nickel and copper tolerance and toxicity in three Tuscan populations of Silene paradoxa. Physiol. Plant. 2001, 113, 507–514. [Google Scholar] [CrossRef]
- Paliouris, G.; Hutchinson, T.C. Arsenic, cobalt and nickel tolerances in two populations of Silene vulgaris (Moench) Gracke from Ontario, Canada. New Phytol. 1991, 117, 449–459. [Google Scholar] [CrossRef]
- Krämerm, U. Conceptualizing plant systems evolution. Curr. Opin. Plant Biol. 2018, 42, 66–75. [Google Scholar] [CrossRef]
- Koźmińska, A.; Al Hassan, M.; Hanus-Fajerska, E.; Naranjo, M.A.; Boscaiu, M.; Vicente, O. Comparative analysis of water deficit and salt tolerance mechanisms in Silene. S. Afr. J. Bot. 2018, 117, 193–206. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Saánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; Palazzo de Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Reberd, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Nardini, A.; Lo Gullo, M.A.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Stace, C.A. Plant Taxonomy and Biosystematics; PWN: Warszawa, Poland, 1993; p. 340. [Google Scholar]
- Yang, L.; Fountain, J.C.; Wang, H.; Ni, X.; Ji, P.; Lee, R.D.; Kemerait, R.C.; Scully, B.T.; Guo, B. Stress sensitivity is associated with differential accumulation of reactive oxygen and nitrogen species in maize genotypes with contrasting levels of drought tolerance. Int. J. Mol. Sci. 2015, 16, 24791–24819. [Google Scholar] [CrossRef]
- Ouyang, B.; Yang, T.; Li, H.X.; Zhang, L.; Zhang, Y.; Zhang, J.; Fei, Z.; Ye, Z. Identification of early salt stress response genes in tomato root by suppression subtractive hybridization and microarray analysis. J. Exp. Bot. 2007, 58, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Beritognolo, I.; Harfouche, A.; Brilli, F.; Prosperini, G.; Gaudet, M.; Brosche, M.; Salani, F.; Kuzminsky, E.; Auvinen, P.; Paulin, L.; et al. Comparative study of transcriptional and physiological responses to salinity stress in two contrasting Populus alba L. genotypes. Tree Physiol. 2011, 31, 1335–1355. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Song, L.; Shu, Y.; Wang, S.; Wang, Z.; Yu, T.; Gu, W.; Ma, H. Comparative proteomic analysis of seedling leaves of different salt tolerant soybean genotypes. J. Proteom. 2012, 75, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Wu, D.; Liu, J.; Li, D.; Xu, C.; Li, S.; Li, P.; Zhang, H.; Liu, X.; Jiang, C.; et al. Proteomic analysis of seedling roots of two maize inbred lines that differ significantly in the salt stress response. PLoS ONE 2015, 10, e0116697. [Google Scholar] [CrossRef]
- Widodo, W.; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Wang, W.S.; Zhang, F.; Deng, J.L.; Li, Z.K.; Fu, B.Y. Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLoS ONE 2014, 9, e108020. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Grossi, D.; Rustioni, L.; Di Lorenzo, G.S.; Failla, O.; Brancadoro, L. Water deficit effects on grapevine woody tissue pigmentations. Hort. Sci. Prague 2016, 43, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Rajakaruna, N. Lessons on evolution from the study of edaphic specialization. Bot. Rev. 2018, 84, 39–78. [Google Scholar] [CrossRef]
- Vasseur, F.; Bresson, J.; Wang, G.; Schwab, R.; Weigl, D. Image-based method for phenotyping growth dynamics and fitness components in Arabidopsis thaliana. Plant Methods 2018, 14, 63. [Google Scholar] [CrossRef]
- Andarde, E.R.; Ribeiro, V.N.; Azvedo, C.V.G.; Chiorato, A.F.; Williams, T.C.R.; Carbonell, S.A.M. Biochemical indicators of drought tolerance in the common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 277–289. [Google Scholar] [CrossRef]
- Baha, H.; Tekaya, M.; Drine, S.; Gusami, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. S. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Sen, A.; Ozturk, I.; Yaycili, O.; Alikamanoglu, S. Drought tolerance in irradiated wheat studied by genetic and biochemical markers. J. Plant Growth Regul. 2017, 36, 669–676. [Google Scholar] [CrossRef]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 11. Available online: http://www.thericejournal.com/content/5/1/11 (accessed on 22 June 2012). [CrossRef] [Green Version]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; De Angeli, A. Vacuolar Chloride Fluxes Impact Ion Content and Distribution during Early Salinity Stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptations in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. Plants salt tolerance and Na+ sensing and transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Plett, D.C.; Møller, I.S. Na (+) transport in glycophytic plants: What we know and would like to know. Plant Cell Environ. 2010, 33, 612–626. [Google Scholar] [CrossRef]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Biol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Wyn Jones, R.; Storey, R.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell Membrane Activities in Plants; Marre, E., Ciferri, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 121–136. [Google Scholar]
- Genc, Y.; Oldach, K.; Taylor, J.; Lyons, G.H. Uncoupling of sodium and chloride to assist breeding for salinity tolerance in crops. New Phytol. 2016, 210, 145–156. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Ann Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin; Imas, P. Potassium uptake in relation to drought tolerance of chickpea under rain-fed conditions. J. Plant Nutr. 2004, 37, 1120–1138. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Chen, L.; Liu, L.; Wan, X. Maintenance of mesophyll potassium and regulation of plasma membrane H+-ATPase are associated with physiological responses of tea plants to drought and subsequent rehydration. Crop J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Zaharia, A.; Vicente, O.; Sestras, R. Comparative analysis of osmotic and ionic stress effects on seed germination in Tagetes (Asteraceae) cultivars. Prop. Ornam. Plants 2015, 15, 63–72. [Google Scholar]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Koźmińska, A.; Al Hassan, M.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Vincente, O. Responses of succulents to drought: Comparative analysis of four Sedum (Crassulaceae) species. Sci. Hort. 2019, 243, 235–242. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Ozgur, M.; Oscan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Functional compounds and antioxidant properties of dried green and red peppers. Afr. J. Agric. Res. 2011, 6, 5638–5644. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavourable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Chandna, R.; Azooz, M.M.; Ahmad, P. Recent advances of metabolomics to reveal plant response during salt stress. In Salt Stress in Plants. Signalling, Omics and Adaptations; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; pp. 1–14. [Google Scholar]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica napus var oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M.R. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, A.; Zhy, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Phys. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. AoB Plants 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2013, 13, 309–317. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Cvikrová, M.; Gemperlová, L.; Martincová, O.; Vanková, R. Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Vera Hernandez, F.P.; Ortega-Ramirez, M.A.; Martinez Nunez, M.; Ruiz-Rivas, M.; Rosas Cardenas, F. Proline as a probable biomarker of cold stress tolerance in sorgum (Sorghum bicolor). Mex. J. Biotechnol. 2018, 3, 77–86. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gres, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Tasdighi, A.; Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pac. J. Trop. Biomed. 2016, 6, 886–891. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Guglielminetti, L.; Angelini, L.G. The effect of soil moisture depletion on Stevia (Stevia rebaudiana Bertoni) grown in greenhouse conditions: Growth, steviol glycosides content, soluble sugars and total antioxidant capacity. Sci. Hortic. 2015, 183, 93–99. [Google Scholar] [CrossRef]






| Population Code | Origin | Latitude | Longitude | Altitude (m a.s.l.) | Annual Rainfall (mm) | Mean Annual Day Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 Mog. | Mogente stand (SE Spain) | N38°52′ | W0°48′ | 680 | 516 | 14.6 |
| 2 Ref. ES | Valencia—reference stand (SE Spain) | N39°28′ | W0°23′ | 13 | 468 | 17.4 |
| 3 Serp. | Serpentine stand (SW Poland) | N50°84′ | E16°48′ | 216 | 461 | 15.4 |
| 4 Ref. PL | Poznań—reference stand (SW Poland) | N52°41′ | E16°93′ | 69 | 511 | 15.7 |
| R2 Populations | |||||
|---|---|---|---|---|---|
| Biomarker | 1 (Mog.) | 2 (Ref. ES) | 3 (Serp.) | 4 (Ref. PL) | p-Value |
| FW% | 0.31 | 0.49 | 0.45 | 0.53 | 0.12 |
| SL (%) | 0.29 | 0.67 | 0.76 | 0.36 | 0.12 |
| Na+ | 0.34 | 0.92 | 0.87 | 0.75 | 0.15 |
| Cl− | 0.29 | 0.67 | 0.18 | 0.89 | 0.30 |
| K+ | 0.21 | 0.87 | 0.84 | 0.57 | 0.13 |
| K+/Na+ | 0.31 | 0.62 | 0.14 | 0.81 | 0.29 |
| Chl. a | 0.13 | 0.57 | 0.17 | 0.75 | 0.13 |
| Chl. b | 0.18 | 0.62 | 0.38 | 0.58 | 0.11 |
| Caro. | 0.63 | 0.78 | 0.65 | 0.76 | 0.17 |
| MDA | 0.24 | 0.79 | 0.19 | 0.34 | 0.16 |
| TPC | 0.16 | 0.54 | 0.87 | 0.47 | 0.49 |
| TF | 0.15 | 0.25 | 0.15 | 0.28 | 0.33 |
| Pro | 0.33 | 0.62 | 0.84 | 0.37 | 0.35 |
| TSS | 0.34 | 0.46 | 0.67 | 0.46 | 0.37 |
| R2 Populations | |||||
|---|---|---|---|---|---|
| Biomarker | 1 (Mog.) | 2 (Ref. ES) | 3 (Serp.) | 4 (Ref. PL) | p-Value |
| FW% | 0.52 | 0.38 | 0.58 | 0.42 | 0.35 |
| SL (%) | 0.41 | 0.51 | 0.12 | 0.72 | 0.21 |
| Na+ | 0.45 | 0.46 | 0.23 | 0.94 | 0.71 |
| Cl− | 0.42 | 0.13 | 0.63 | 0.84 | 0.13 |
| K+ | 0.42 | 0.18 | 0.74 | 0.79 | 0.52 |
| K+/Na+ | 0.21 | 0.11 | 0.78 | 0.91 | 0.17 |
| Chl. a | 0.33 | 0.65 | 0.76 | 0.56 | 0.3 |
| Chl. b | 0.23 | 0.29 | 0.45 | 0.69 | 0.78 |
| Caro. | 0.32 | 0.55 | 0.17 | 0.72 | 0.73 |
| MDA | 0.21 | 0.28 | 0.33 | 0.59 | 0.93 |
| TPC | 0.21 | 0.43 | 0.32 | 0.66 | 0.15 |
| TF | 0.43 | 0.86 | 0.63 | 0.74 | 0.94 |
| Pro | 0.29 | 0.15 | 0.83 | 0.59 | 0.54 |
| TSS | 0.31 | 0.57 | 0.78 | 0.56 | 0.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Al Hassan, M.; Halecki, W.; Vicente, O. Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations. Sustainability 2019, 11, 800. https://doi.org/10.3390/su11030800
Koźmińska A, Wiszniewska A, Hanus-Fajerska E, Boscaiu M, Al Hassan M, Halecki W, Vicente O. Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations. Sustainability. 2019; 11(3):800. https://doi.org/10.3390/su11030800
Chicago/Turabian StyleKoźmińska, Aleksandra, Alina Wiszniewska, Ewa Hanus-Fajerska, Monica Boscaiu, Mohamad Al Hassan, Wiktor Halecki, and Oscar Vicente. 2019. "Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations" Sustainability 11, no. 3: 800. https://doi.org/10.3390/su11030800
APA StyleKoźmińska, A., Wiszniewska, A., Hanus-Fajerska, E., Boscaiu, M., Al Hassan, M., Halecki, W., & Vicente, O. (2019). Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations. Sustainability, 11(3), 800. https://doi.org/10.3390/su11030800










