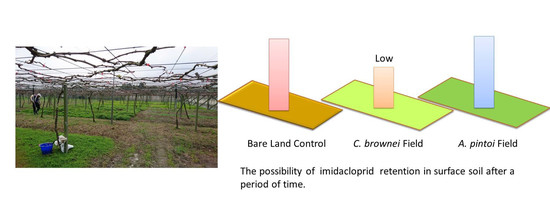
Effect of Growing Groundcover Plants in a Vineyard on Dissipation of Two Neonicotinoid Insecticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Vineyard, Groundcover Plants, and Experimental Design
2.2. Pesticide Application
2.3. Soil and Plant Pesticide Extraction
2.4. Pesticide Analysis and Standard Curve
2.5. Statistical Analysis
3. Results
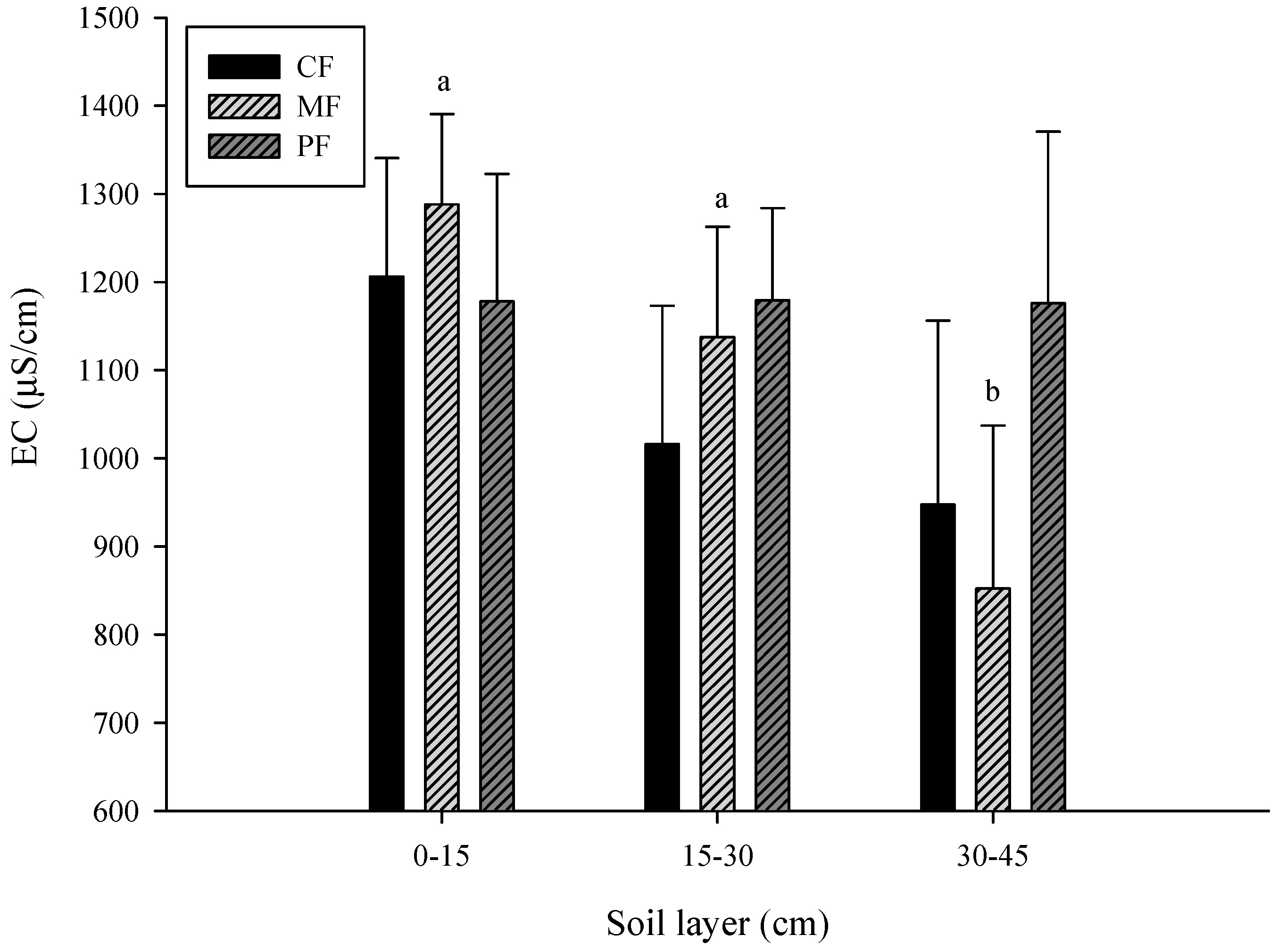
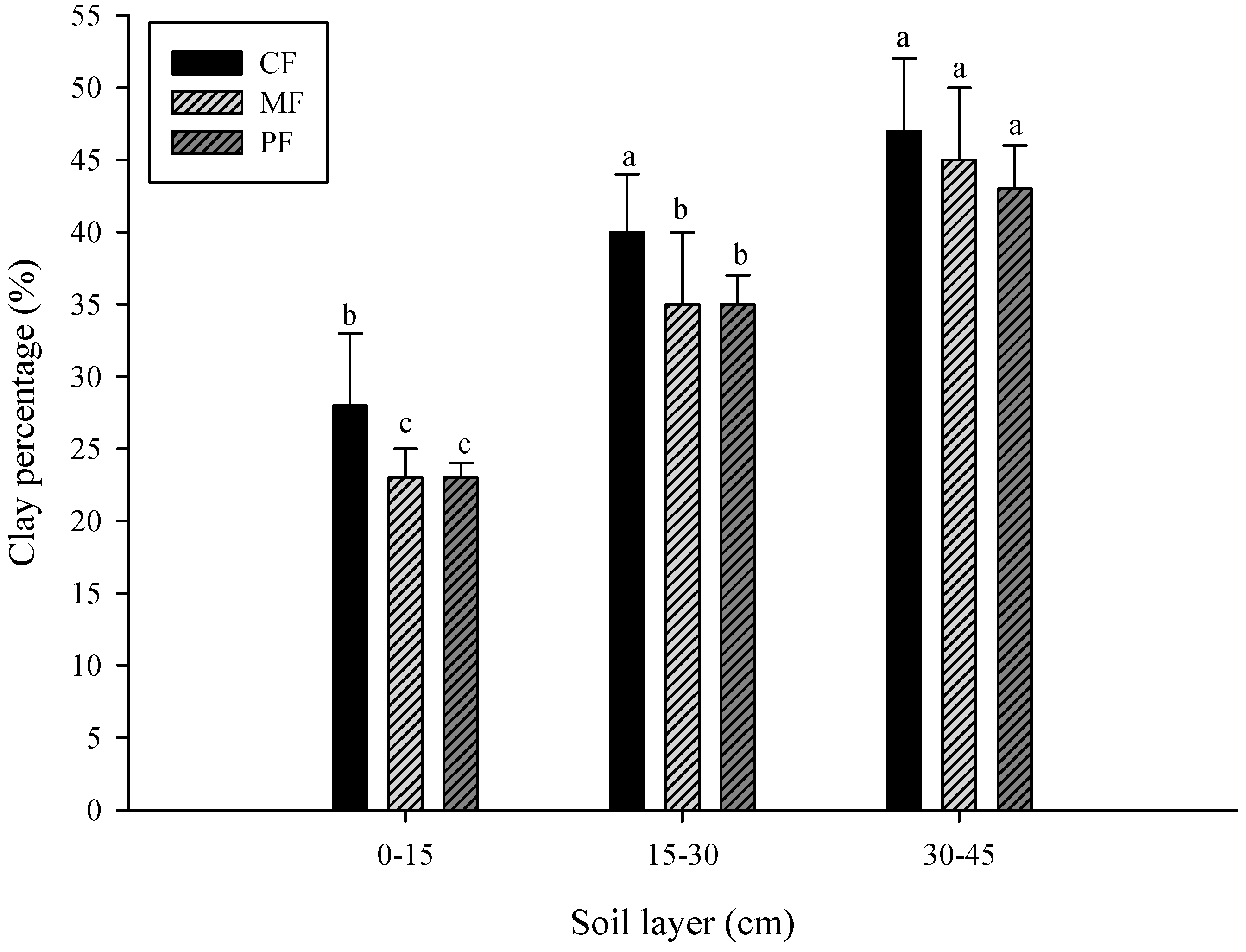
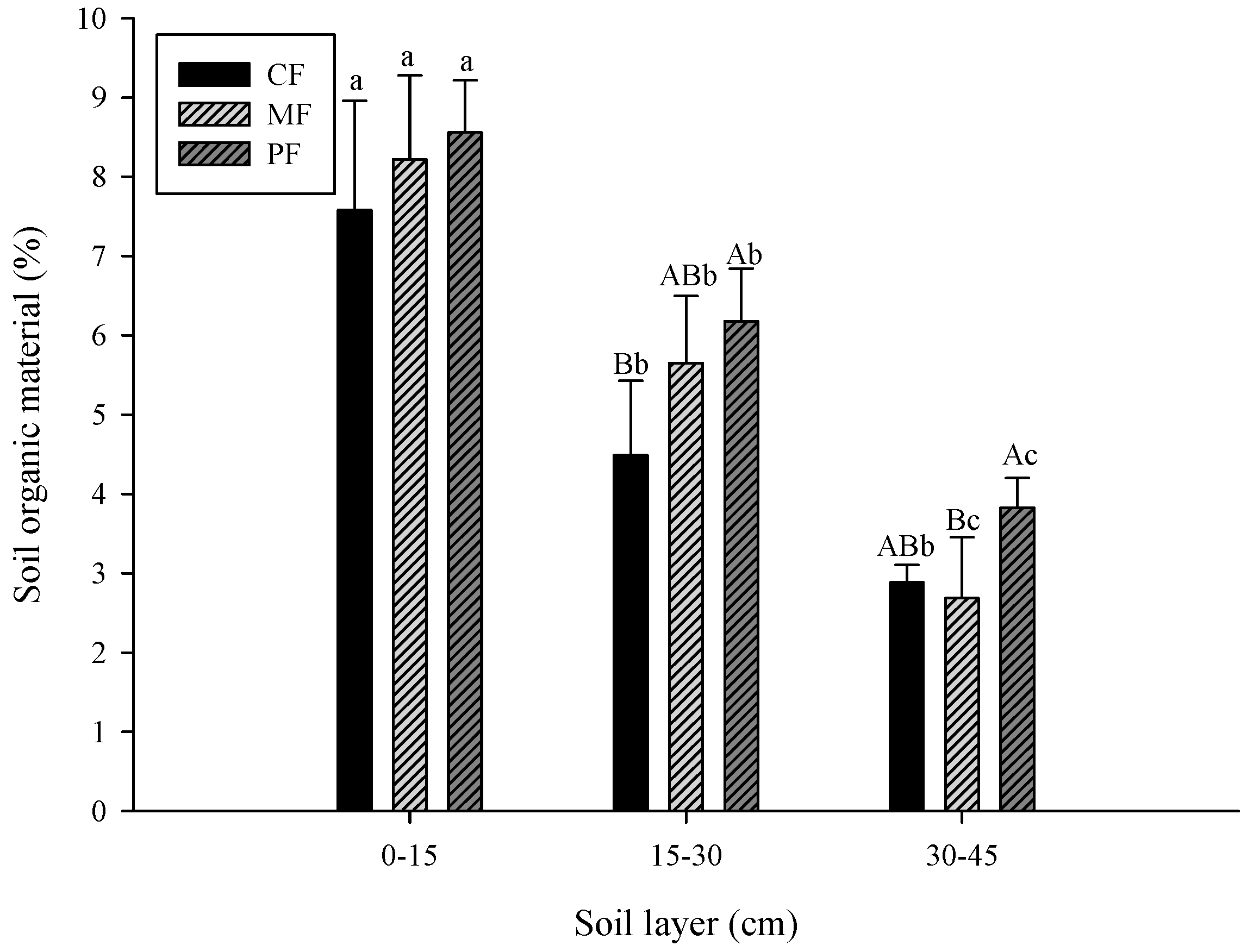
3.1. Change in Soil Properties
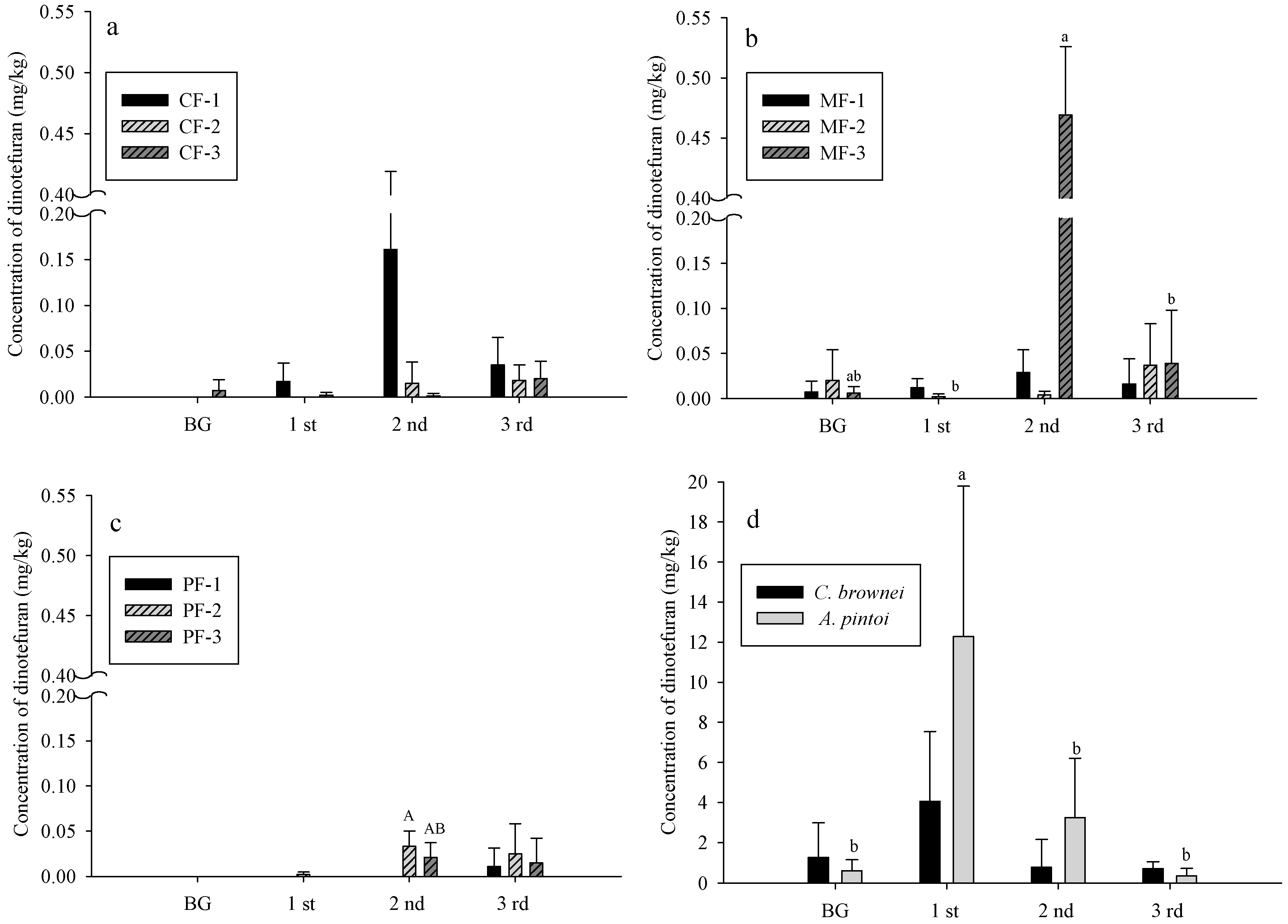
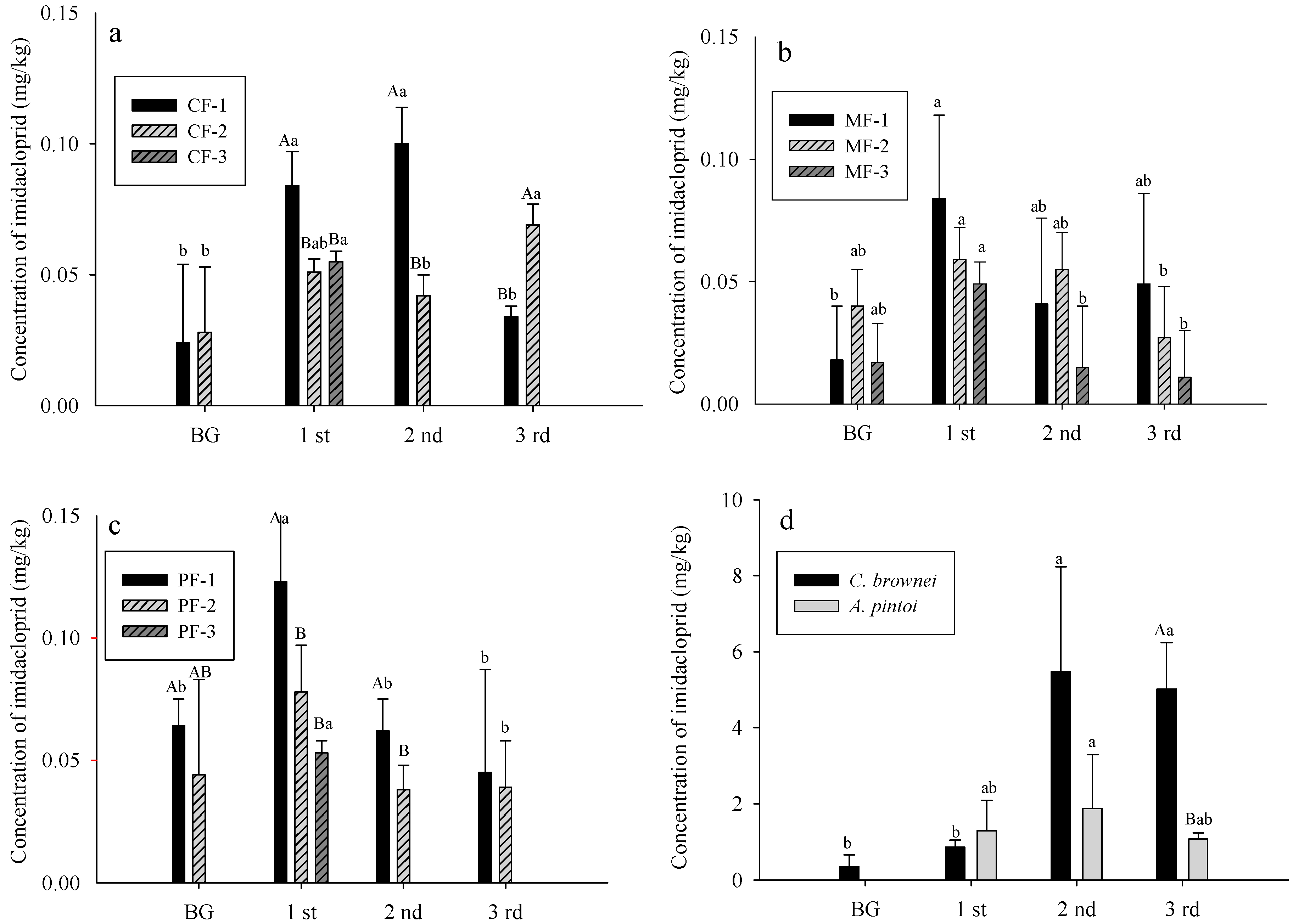
- CF: control field; MF: C. brownei field (mint field); PF: A. pintoi field (peanut field).
- Uppercase letters represent significant differences for different treatments in the same soil layer; lowercase letters represent significant differences for the same treatment in different soil layers.
- Bars without letters indicate no significant differences shown.
3.2. Dinotefuran Level in Soil and Plants
3.3. Imidacloprid Level in Soils and Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paredes, D.; Cayuela, L.; Gurr, G.M.; Campos, M. Is ground cover vegetation an effective biological control enhancement strategy against olive pests? PLoS ONE 2015, 10, e0117265. [Google Scholar] [CrossRef] [PubMed]
- Johns, G.G. Effect of Arachis pintoi groundcover on performance of bananas in northern New South Wales. Aust. J. Exp. Agric. 1994, 34, 1197–1204. [Google Scholar] [CrossRef]
- Zhong, Z.; Huang, X.; Feng, D.; Xing, S.; Weng, B. Long-term effects of legume mulching on soil chemical properties and bacterial community composition and structure. Agric. Ecosyst. Environ. 2018, 268, 24–33. [Google Scholar] [CrossRef]
- Hulugalle, N.R. Effect of cover crop on soil physical and chemical properties of an alfisol in the Sudan savannah of Burkina faso. Arid Soil Res. Rehabil. 1988, 2, 251–267. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Merwin, I.A. Soil physical conditions in a New York orchard after eight years under different groundcover management systems. Plant Soil 2001, 234, 233–237. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, D.; Zou, N.; Yu, X.; Zhang, Z.; Liu, F.; Mu, W. Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere 2018, 201, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Plant Protection Information System. In Council of Agriculture, Executive Yuan, R.O.C. (Taiwan): 2018. Available online: https://otserv2.tactri.gov.tw/ppm/ (accessed on 10 October 2018).
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wang, Y.; Zheng, Y. Enantioselective degradation of chiral insecticide dinotefuran in greenhouse cucumber and soil. Chirality 2015, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, L.; Pulcini, P.; Pochi, D.; Rosati, S.; Lusco, L.; Conte, E. Preliminary study on persistence in soil and residues in maize of imidacloprid. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2011, 46, 469–472. [Google Scholar]
- Kurwadkar, S.T.; Dewinne, D.; Wheat, R.; McGahan, D.G.; Mitchell, F.L. Time dependent sorption behavior of dinotefuran, imidacloprid and thiamethoxam. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kegley, S.E.; Hill, B.R.; Orme, S.; Choi, A.H. PAN Pesticide Database; Pesticide Action Network North America: Berkeley, CA, USA, 2016. [Google Scholar]
- Wood, T.J.; Goulson, D. The Environmental Risks of neonicotinoid pesticides: A review of the evidence post-2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Good Agriculture Practice. In Council of Agriculture, Executive Yuan, R.O.C. (Taiwan): 2013. Available online: https://taft.coa.gov.tw/public/data/531311442871.pdf (accessed on 10 October 2018).
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties, 2nd ed.; Klute, A., Ed.; ASA, SSSA, Inc.: Madison, WI, USA, 1982; Part 2; pp. 199–224. [Google Scholar]
- Rhoades, J.D. Soluble Salts. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; ASA, SSSA, Inc.: Madison, WI, USA, 1982; Part 2; pp. 149–157. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, Part 1 Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; ASA, SSSA, Inc.: Madison, WI, USA, 1986; Part 1; pp. 383–411. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Method of Test for Pesticide Residues in Foods–Multiresidue Analysis (5) in Ministry of Health and Welfare, R.O.C. (Taiwan): 2013. Available online: https://www.fda.gov.tw/upload/133/Content/2013122411585259323.pdf (accessed on 10 October 2018).
- Gardner, W.H. Water content. In Methods of Soil Analysis. Part 1 Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA, ASA, Inc.: Madison, WI, USA, 1986; Part 1; pp. 493–544. [Google Scholar]
- Weed, D.A.J.; Kanwar, R.S.; Stoltenberg, D.E.; Pfeiffer, R.L. Dissipation and distribution of herbicides in the soil profile. J. Environ. Qual. 1995, 24, 68–79. [Google Scholar] [CrossRef]
- Terrestrial field dissipation of residues following application of MTI-446 to bare soil in California, Georgia, and New York: Lab Project Number: 41421A007. Material. Unpublished.
- Sarkar, M.A.; Roy, S.; Kole, R.K.; Chowdhury, A. Persistence and metabolism of imidacloprid in different soils of West Bengal. Pest Manag. Sci. 2001, 57, 598–602. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Cox, L.; Hermosín, M.C.; Celis, R.; Cornejo, J. Sorption of two polar herbicides in soils and soil clays suspensions. Water Res. 1997, 31, 1309–1316. [Google Scholar] [CrossRef]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cespedes, F.; Gonzalez-Pradas, E.; Fernandez-Perez, M.; Villafranca-Sanchez, M.; Socias-Viciana, M.; Urena-Amate, M.D. Effects of dissolved organic carbon on sorption and mobility of imidacloprid in soil. J. Environ. Qual. 2002, 31, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Rouchaud, J.; Gustin, F.; Wauters, A. Soil biodegradation and leaf transfer of insecticide imidacloprid applied in seed dressing in sugar beet crops. Bull. Environ. Contam. Toxicol. 1994, 53, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Stork, A. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 2003, 56, 35–40. [Google Scholar]

| Sampling Dates | Dinotefuran | Imidacloprid | |||
|---|---|---|---|---|---|
| Application Date | Days after Application | Application Date | Days after Application | ||
| Background | 29 August | 4 September | 1 September | ||
| First sampling | 5 September | 1 | 7 September | 4 | |
| Second sampling | 12 September | 8 | 12 September | 5 | |
| Third sampling | 5 October | 31 | 26 September | 9 | |
| Treatment a | Total N (%) | Available P (mg/kg) | Exchangeable K (mg/kg) |
|---|---|---|---|
| CF | 0.49 ± 0.07 | 493.00 ± 58.03 a | 367.67 ± 53.82 |
| MF | 0.49 ± 0.04 | 561.67 ± 19.22 ab | 385.33 ± 87.31 |
| PF | 0.51 ± 0.04 | 565.67 ± 11.59 b | 400.67 ± 62.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, J.-H.; Liao, C.-S.; Kuo, Y.-W.; Chen, W.-C.; Huang, W.-T. Effect of Growing Groundcover Plants in a Vineyard on Dissipation of Two Neonicotinoid Insecticides. Sustainability 2019, 11, 798. https://doi.org/10.3390/su11030798
Yen J-H, Liao C-S, Kuo Y-W, Chen W-C, Huang W-T. Effect of Growing Groundcover Plants in a Vineyard on Dissipation of Two Neonicotinoid Insecticides. Sustainability. 2019; 11(3):798. https://doi.org/10.3390/su11030798
Chicago/Turabian StyleYen, Jui-Hung, Chien-Sen Liao, Ya-Wen Kuo, Wen-Ching Chen, and Wan-Ting Huang. 2019. "Effect of Growing Groundcover Plants in a Vineyard on Dissipation of Two Neonicotinoid Insecticides" Sustainability 11, no. 3: 798. https://doi.org/10.3390/su11030798
APA StyleYen, J.-H., Liao, C.-S., Kuo, Y.-W., Chen, W.-C., & Huang, W.-T. (2019). Effect of Growing Groundcover Plants in a Vineyard on Dissipation of Two Neonicotinoid Insecticides. Sustainability, 11(3), 798. https://doi.org/10.3390/su11030798