The Impact of Selected Pretreatment Procedures on Iron Dissolution from Metallic Iron Specimens Used in Water Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Fe0 materials and sand
2.3. Experimental Procedure
2.3.1. Fe0 Pretreatment
2.3.2. Iron Dissolution Studies
2.4. Analytical Method
3. Results and Discussion
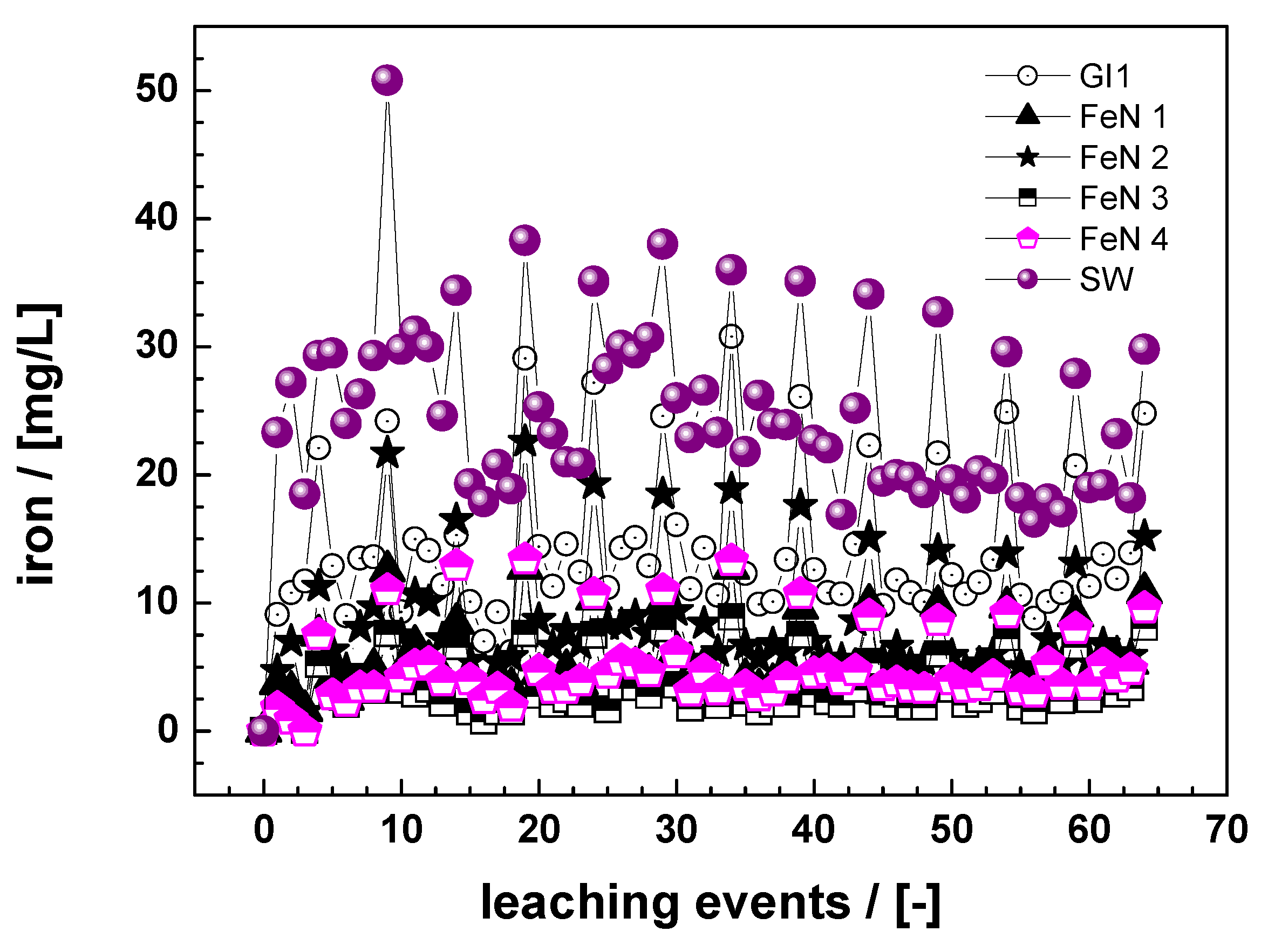
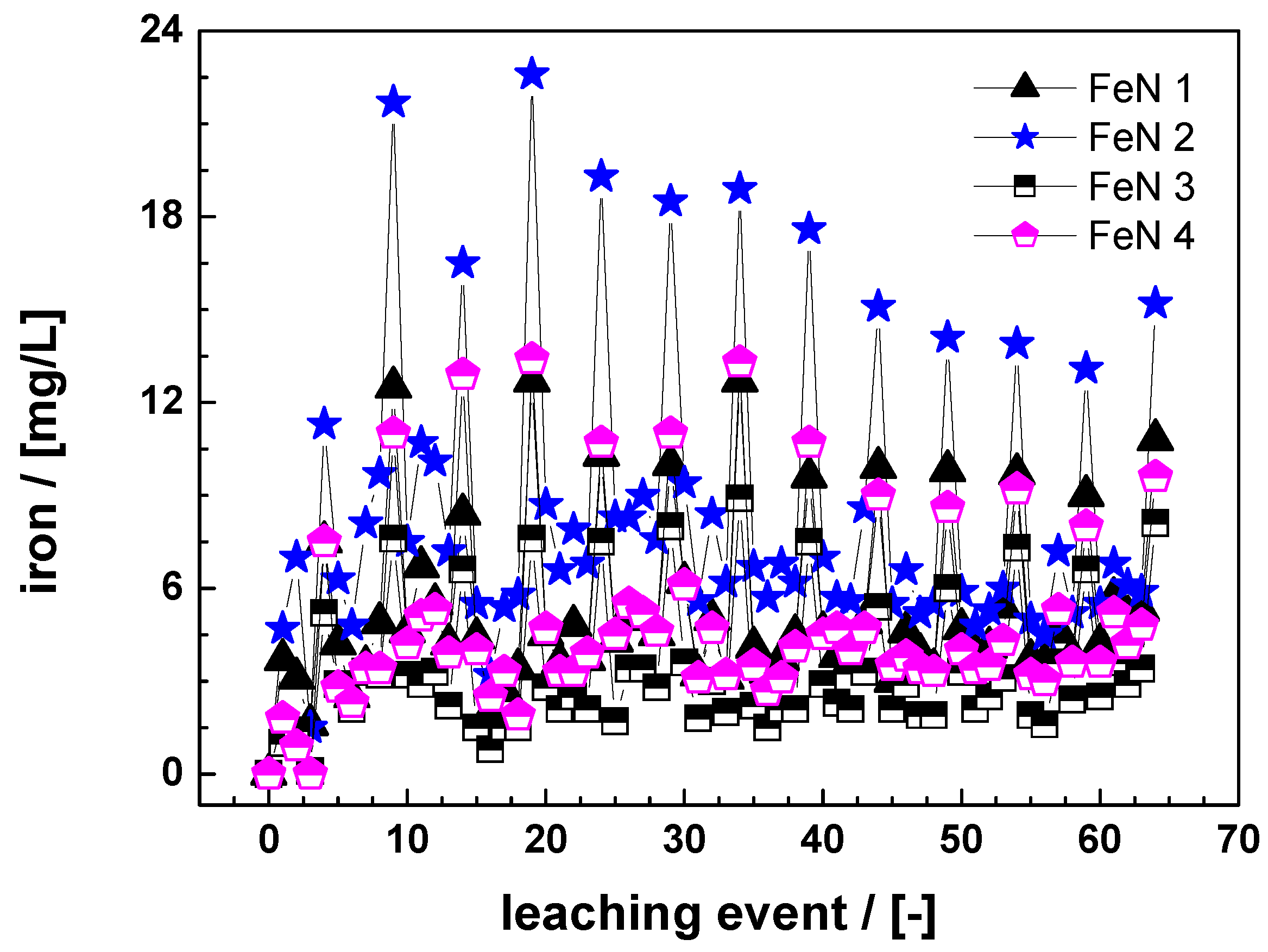
3.1. Reactivity of Fe0 Materials
3.1.1. Batch Experiments
3.1.2. Column Experiments
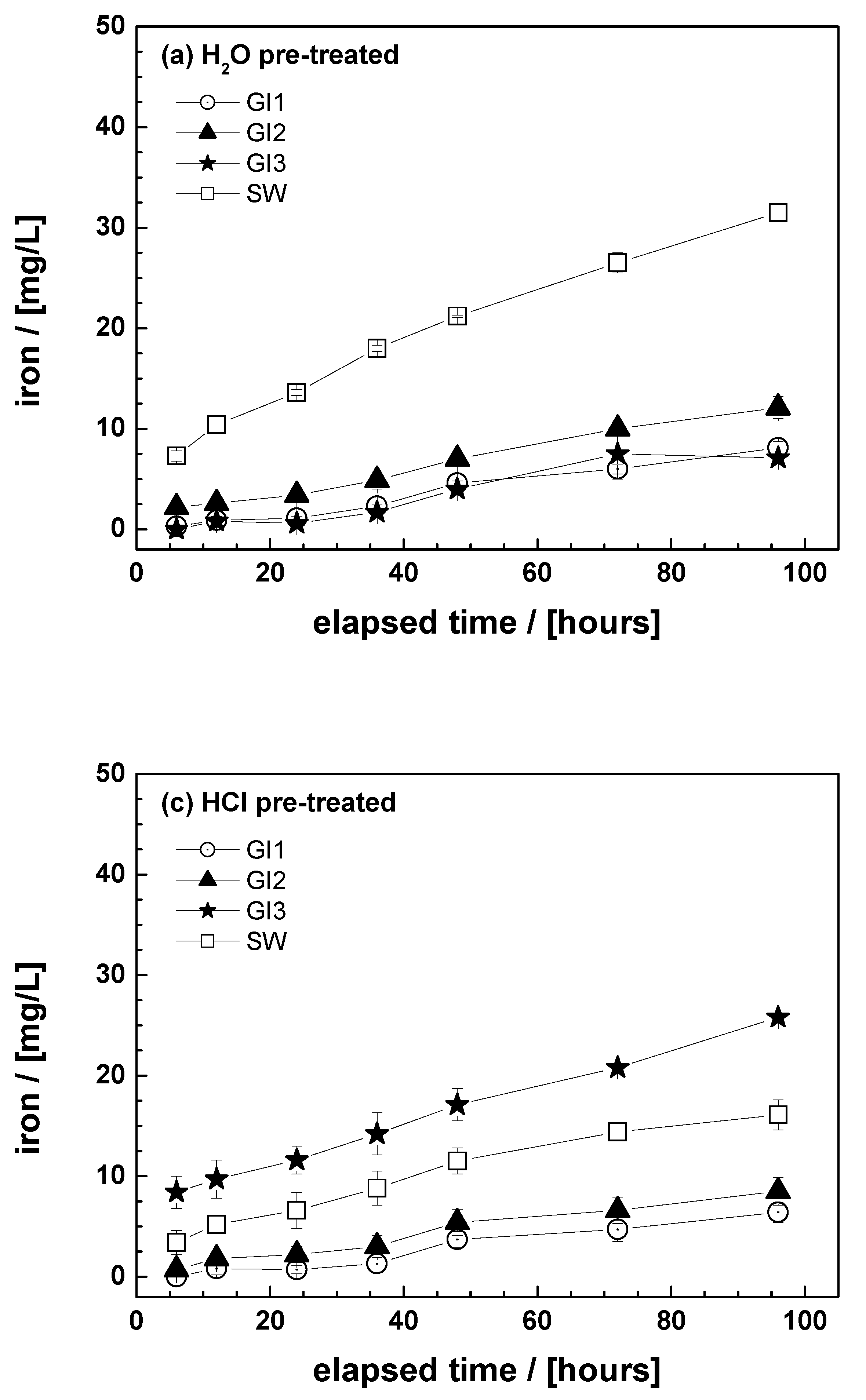
3.2. Effect of Material Pretreatment
3.3. Discussion
3.4. Time for a Critical Discussion
3.5. A Framework for Future Research on the Fe0/H2O System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bischof, G. On putrescent organic matter in potable water. I. Proc. R. Soc. Lond. 1877, 26, 179–184. [Google Scholar]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- van der Taelen, I. The Antwerp water works during the war period. J. Am. Water Works Assoc. 1919, 6, 486–489. [Google Scholar] [CrossRef]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Works Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Gould, J.P. The kinetics of hexavalent chromium reduction by metallic iron. Water Res. 1982, 16, 871–877. [Google Scholar] [CrossRef]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- O´Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ "iron wall" for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- van Craenenbroeck, W. Easton & Anderson and the water supply of Antwerp (Belgium). Ind. Archaeol. Rev. 1998, 20, 105–116. [Google Scholar]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Cong, X.; Xue, N.; Wang, S.; Li, K.; Li, F. Reductive dechlorination of organochlorine pesticides in soils from an abandoned manufacturing facility by zero-valent iron. Sci. Total Environ. 2010, 408, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wu, J.; Huang, W.; Li, Y.; Jiang, G. The effects of flow rate and concentration on nitrobenzene removal in abiotic and biotic zero-valent iron columns. Sci. Total Environ. 2016, 560–561, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gernjak, W.; Keller, J. Long-term performance of enhanced-zero valent iron for drinking water treatment: A lab-scale study. Chem. Eng. J. 2017, 315, 124–131. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Rosa Greco, R.; Moraci, N. Selective removal of heavy metals from landfill leachate by reactive granular filters. Sci. Total Environ. 2018, 644, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Vollprecht, D.; Krois, L.-M.; Sedlazeck, K.P.; Müller, P.; Mischitz, R.; Olbrich, T.; Pomberger, R. Removal of critical metals from waste water by zero-valent iron. J. Clean. Prod. 2018, 208, 1409–1420. [Google Scholar] [CrossRef]
- Xin, J.; Tang, F.; Yan, J.; La, C.; Liu, W. Investigating the efficiency of microscale zero valent iron-based in situ reactive zone (mZVI-IRZ) for TCE removal in fresh and saline groundwater. Sci. Total Environ. 2018, 626, 638–649. [Google Scholar] [CrossRef]
- Zou, H.; Hu, E.; Yang, S.; Gong, L.; He, F. Chromium(VI) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci. Total Environ. 2019, 650, 419–426. [Google Scholar] [CrossRef]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef]
- Nichols, W.R. Water Supply, Considered Mainly from a Chemical and Sanitary Standpoint; John Wiley & Sons: New York, NY, USA, 1883; 260p. [Google Scholar]
- Baker, M. Sketch of the history of water treatment. Am. Water Works Assoc. 1934, 26, 902–938. [Google Scholar] [CrossRef]
- Anderson, W. On the purification of water by agitation with iron and by sand filtration. J. Soc. Arts 1886, 35, 29–38. [Google Scholar] [CrossRef]
- Leffmann, H. Direct and indirect methods of electrical purification of water. J. Frankl. Inst. 1907, 164, 205–216. [Google Scholar] [CrossRef]
- Bojic, A.Lj.; Bojic, D.; Andjelkovic, T. Removal of Cu2+ and Zn2+ from model wastewaters by spontaneous reduction–coagulation process in flow conditions. J. Hazard. Mater. 2009, 168, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Schöner, A. Metallic iron for environmental remediation: Learning from electrocoagulation. J. Hazard. Mater. 2010, 175, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Samarghandi, M.R.; Zarrabi, M.; Amrane, A.; Sepehr, M.N.; Noroozi, M.; Saied Namdari, S.; Zarei, A. Kinetic of degradation of two azo dyes from aqueous solutions by zero iron powder: Determination of the optimal conditions. Desalin. Water Treat. 2012, 40, 137–143. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freiberg Online Geosci. 2015, 32, 1–80. [Google Scholar]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Heimann, S. Testing granular iron for fluoride for aqueous fluoride removal. Freiberg Online Geosci. 2018, 52, 1–80. [Google Scholar]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Nanseu-Njiki, C.P.; Hu, R.; Nassi, A.; Noubactep, C.; Licha, T. Characterizing the reactivity of metallic iron for water defluoridation in batch studies. Chemosphere 2019, 219, 855–863. [Google Scholar] [CrossRef]
- Qiu, S.R.; Lai, H.-F.; Roberson, M.J.; Hunt, M.L.; Amrhein, C.; Giancarlo, L.C.; Flynn, G.W.; Yarmoff, J.A. Removal of contaminants from aqueous solution by reaction with iron surfaces. Langmuir 2000, 16, 2230–2236. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I. Sustaining the efficiency of the Fe(0)/H2O system for Cr(VI) removal by MnO2 amendment. Chemosphere 2019, 214, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Oh, J.; Sohn, K. Mechanistic aspects of nitrate reduction by Fe(0) in water. J. Korean Chem. Soc. 2001, 45, 395–398. [Google Scholar]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wei, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Sizirici, B.; Yildiz, I.; Al Ali, A.; Alkhemeiri, A.; Alawadi, R. Modified biosand filters enriched with iron oxide coated gravel to remove chemical, organic and bacteriological contaminants. J. Water Process Eng. 2019, 27, 110–119. [Google Scholar] [CrossRef]
- Gu, B.; Liang, L.; Dickey, M.J.; Yin, X.; Dai, S. Reductive precipitation of uranium (VI) by zero-valent iron. Environ. Sci. Technol. 1998, 32, 3366–3373. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of groundwater and impoundments using nano-zero valent iron, Fe0. Meteor. Hydrol. Water Manag. 2015, 3, 21–38. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of water using ZVI, Fe0. Water 2015, 7, 3671–3831. [Google Scholar] [CrossRef]
- Antia, D.D.J. ZVI (Fe0) Desalination: Stability of product water. Resources 2016, 5, 15. [Google Scholar] [CrossRef]
- Antia, D.D.J. Provision of desalinated irrigation water by the desalination of groundwater within a saline aquifer. Hydrology 2017, 4, 45. [Google Scholar] [CrossRef]
- Richardson, J.P.; Nicklow, J.W. In situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Crane, R.A.; Mwakabona, H.T.; Noubactep, C.; Njau, K.N. Technologies for decentralized fluoride removal: Testing metallic iron based filters. Water 2015, 7, 6750–6774. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.H.; Van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Puls, R.W.; Lee, T.R.; Woods, L.L. Fifteen-year assessment of a permeable reactive barrier for treatment of chromate and trichloroethylene in groundwater. Sci. Total Environ. 2014, 468–469, 186–194. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Sexton, M.R.; Acree, S.D.; Puls, R.W.; Blowes, D.W.; Kalinowski, C.; Tilton, J.M.; Woods, L.L. Geochemical and isotope study of trichloroethene degradation in a zero-valent iron permeable reactive barrier: A twenty-two-year performance evaluation. Environ. Sci. Technol. 2019, 53, 296–306. [Google Scholar] [CrossRef]
- Wilson, E.K. Zero-valent metals provide possible solution to groundwater problems. Chem. Eng. News 1995, 73, 19–22. [Google Scholar] [CrossRef]
- Fairweather, V. When toxics meet metal. Civil Eng. 1996, 66, 44–48. [Google Scholar]
- Tratneyk, P.G. Putting corrosion to use: Remediating contaminated groundwater with zero-valent metals. Chem. Ind. 1996, 13, 499–503. [Google Scholar]
- Henderson, A.D.; Demond, A.H. Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Makota, S.; Nde-Tchoupé, A.I.; Mwakabona, H.T.; Tepong-Tsindé, R.; Noubactep, C.; Nassi, A.; Njau, K.N. Metallic iron for water treatment: Leaving the valley of confusion. Appl. Water Sci. 2017. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Gwenzi, W.; Wu, S.; Noubactep, C. Fe0/H2O systems for environmental remediation: The scientific history and future research directions. Water 2018, 10, 1739. [Google Scholar] [CrossRef]
- Hu, R.; Noubactep, C. Iron corrosion: Scientific heritage in jeopardy. Sustainability 2018, 10, 4138. [Google Scholar] [CrossRef]
- Li, J.; Dou, X.; Qin, H.; Sun, Y.; Yin, D.; Guan, X. Characterization methods of zerovalent iron for water treatment and remediation. Water Res. 2018, 148, 70–85. [Google Scholar] [CrossRef]
- Miehr, R.; Tratnyek, G.P.; Bandstra, Z.J.; Scherer, M.M.; Alowitz, J.M.; Bylaska, J.E. Diversity of contaminant reduction reactions by zerovalent iron: Role of the reductate. Environ. Sci. Technol. 2004, 38, 139–147. [Google Scholar] [CrossRef]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef]
- Btatkeu, K.B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Jekel, M. Microstructural characterization of granular cast iron for permeable reactive barriers. Groundw. Monit. Remed. 2014, 34, 117–122. [Google Scholar] [CrossRef]
- Kim, H.; Yang, H.; Kim, J. Standardization of the reducing power of zero-valent iron using iodine. J. Environ. Sci. Health A 2014, 49, 514–523. [Google Scholar] [CrossRef]
- Li, S.; Ding, Y.; Wang, W.; Lei, H. A facile method for determining the Fe(0) content and reactivity of zero valent iron. Anal. Methods 2016, 8, 1239–1248. [Google Scholar] [CrossRef]
- Piwowarsky, E. Gußeisen; Springer Verlag: Berlin, Germany, 1951; 1070p. (In German) [Google Scholar]
- Dickerson, R.E.; Gray, H.B.; Haight, G.P., Jr. Chemical Principles, 3rd ed.; Benjamin/Cummings Inc.: London, UK; Amsterdam, The Netherlands, 1979. [Google Scholar]
- Nesic, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Lazzari, L. General Aspects of Corrosion, Chapter 9.1, Vol. V; Encyclopedia of Hydrocarbons, Istituto Enciclopedia Italiana: Rome, Italy, 2008. [Google Scholar]
- Noubactep, C. The suitability of metallic iron for environmental remediation. Environ. Progr. Sust. Energy 2010, 29, 286–291. [Google Scholar] [CrossRef]
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited. The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Loura Mbenguela, B. Mitigation of contamination in effluents by metallic iron: The role of iron corrosion products. Environ. Technol. Innov. 2017, 8, 71–83. [Google Scholar] [CrossRef]
- Touomo-Wouafo, M.; Donkeng-Dazie, J.; Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Ludvík, J. Role of pre-corrosion of Fe0 on its efficiency in remediation systems: An electrochemical study. Chemosphere 2018, 209, 617–622. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior. J. Electrochem. Soc. 2000, 147, 4087–4092. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Sauter, M.; Merkel, B. Testing the suitability of zerovalent iron materials for reactive walls. Environ. Chem. 2005, 2, 71–76. [Google Scholar] [CrossRef]
- Pierce, E.M.; Wellman, D.M.; Lodge, A.M.; Rodriguez, E.A. Experimental determination of the dissolution kinetics of zero-valent iron in the presence of organic complexants. Environ. Chem. 2007, 4, 260–270. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the reactivity of metallic iron in Fe0/EDTA/H2O systems with column experiments. Chem. Eng. J. 2010, 162, 656–661. [Google Scholar] [CrossRef]
- Hildebrant, B. Characterizing the reactivity of commercial steel wool for water treatment. Freiberg Online Geosci. 2018, 53, 1–80. [Google Scholar]
- Saywell, L.G.; Cunningham, B.B. Determination of iron: Colorimetric o-phenanthroline method. Ind. Eng. Chem. Anal. Ed. 1937, 9, 67–69. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. B 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef]
- Allred, B.J. Batch test screening of industrial product/byproduct filter materials for agricultural drainage water treatment. Water 2017, 9, 791. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672. [Google Scholar] [CrossRef]
- Moraci, N.; Ielo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotechn. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef]
- Morrison, S.J. Comparison of oxidized to reduced forms of iron for in situ remediation of uranium-contaminated groundwater, Fry Canyon, Utah. In Proceedings of the 1997 GSA Annual Meeting, Salt Lake City, UT, USA, 20–23 October 1997. [Google Scholar]
- Indelicato, B.M. Comparision of Zero-Valent Iron and Activated Carbon for Treating Chlorinated Contaminants in Groundwater. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1998. [Google Scholar]
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628. [Google Scholar] [CrossRef]
- Mueller, N.C.; Braun, J.; Bruns, J.; Cerník, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2011, 19, 550–558. [Google Scholar] [CrossRef]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based IIT Bombay arsenic filter in West Bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017. [Google Scholar]
- Qin, H.; Guan, X.; Bandstra, J.Z.; Johnson, R.L.; Tratnyek, P.G. Modeling the kinetics of hydrogen formation by zerovalent iron: Effects of sulfidation on micro- and nano-scale particles. Environ. Sci. Technol. 2018, 52, 13887–13896. [Google Scholar] [CrossRef]
- Marwa, J.; Lufingo, M.; Noubactep, C.; Machunda, R. Defeating fluorosis in the East African Rift Valley: Transforming the Kilimanjaro into a rainwater harvesting park. Sustainability 2018, 10, 4194. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Tepong-Tsindé, R.; Lufingo, M.; Pembe-Ali, Z.; Lugodisha, I.; Mureth, R.I.; Nkinda, M.; Marwa, J.; Gwenzi, W.; Mwamila, T.B.; et al. White teeth and healthy skeletons for all: The path to universal fluoride-free drinking water in Tanzania. Water 2019, 11, 131. [Google Scholar] [CrossRef]
- Johnson, T.L.; Scherer, M.M.; Tratnyek, P.G. Kinetics of halogenated organic compound degradation by iron metal. Environ. Sci. Technol. 1996, 30, 2634–2640. [Google Scholar] [CrossRef]
- Reardon, J.E. Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ. Sci. Technol. 1995, 29, 2936–2945. [Google Scholar] [CrossRef]
- Reardon, E.J.; Fagan, R.; Vogan, J.L.; Przepiora, A. Anaerobic corrosion reactionkinetics of nano-sized iron. Environ. Sci. Technol. 2008, 42, 2420–2426. [Google Scholar] [CrossRef]
- Velimirovic, M.; Carniatoc, L.; Simons, Q.; Schoups, G.; Seuntjens, P.; Bastiaens, L. Corrosion rate estimations of microscale zerovalent iron particles via direct hydrogen production measurements. J. Hazard. Mater. 2014, 270, 18–26. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. CLEAN–Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Macé, C.; Desrocher, S.; Gheorghiu, F.; Kane, A.; Pupeza, M.; Cernik, M.; Kvapil, P.; Venkatakrishnan, R.; Zhang, W.-X. Nanotechnology and groundwater remediation: A step forward in technology understanding. Remed. J. 2006, 16, 23–33. [Google Scholar] [CrossRef]
- Avilés, M.; Garrido, S.E.; Esteller, M.V.; De La Paz, J.S.; Najera, C.; Cortés, J. Removal of groundwater arsenic using a household filter with iron spikes and stainless steel. J. Environ. Manag. 2013, 131, 103–109. [Google Scholar] [CrossRef]
- Wenk, C.B.; Kaegi, R.; Hug, S.J. Factors affecting arsenic and uranium removal with zero-valent iron: Laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ. Chem. 2014, 11, 547–557. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2016, 166, 567–573. [Google Scholar] [CrossRef]
- Trois, C.; Cibati, C. South African sands as an alternative to zero valent iron for arsenic removal from an industrial effluent: Batch experiments. J. Environ. Chem. Eng. 2015, 3, 488–498. [Google Scholar] [CrossRef]
- Ebelle, T.C.; Makota, S.; Tepong-Tsindé, R.; Nassi, A.; Noubactep, C. Metallic iron and the dialogue of the deaf. Fresenius Environ. Bull 2019, in press. [Google Scholar]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef]
- Noubactep, C.; Makota, S.; Bandyopadhyay, A. Rescuing Fe0 remediation research from its systemic flaws. Res. Rev. Insights 2017. [Google Scholar] [CrossRef]
- Noubactep, C. An analysis of the evolution of reactive species in Fe0/H2O systems. J. Hazard. Mater. 2009, 168, 1626–1631. [Google Scholar] [CrossRef]
- Noubactep, C. On the validity of specific rate constants (kSA) in Fe0/H2O systems. J. Hazard. Mater. 2009, 164, 835–837. [Google Scholar] [CrossRef]
- Gheju, M. Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water 2018, 10, 651. [Google Scholar] [CrossRef]
- Cohen, M. Thin oxide films on iron. J. Electrochem. Soc. 1974, 121, 191C–197C. [Google Scholar] [CrossRef]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust-covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, C.; Khan, E.; Chen, S.S.; Li, X.-d. Aging effects on chemical transformation and metal(loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment. Sci. Total Environ. 2018, 615, 498–507. [Google Scholar] [CrossRef]
- Hlongwane, N.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci. Total Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497. [Google Scholar] [CrossRef]
- Noubactep, C.; Meinrath, G.; Dietrich, P.; Merkel, B. Mitigating uranium in groundwater: Prospects and limitations. Environ. Sci. Technol. 2003, 37, 4304–4308. [Google Scholar] [CrossRef]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Khudenko, B.M. Electrochemical treatment of materials. U.S. Patent US08804355, 1999. [Google Scholar]
- Reynolds, G.W.; Hoff, J.T.; Gillham, R.W. Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ. Sci. Technol. 1990, 24, 135–142. [Google Scholar] [CrossRef]
- White, A.F.; Peterson, M.L. Reduction of aqueous transition metal species on the surfaces of Fe(II)-containing oxides. Geochim. Cosmochim. Acta 1996, 60, 3799–3814. [Google Scholar] [CrossRef]
- Shifrin, S.M.; Spivakova, O.M.; Krasnoborodko, I.G. On Color Removal from Textile Wastewater; Selected Parers of the Leningrad Civil Engineering Institute: Sankt-Peterburg, Russia, 1971; Issue No. 69. [Google Scholar]
- Noubactep, C. On the operating mode of bimetallic systems for environmental remediation. J. Hazard. Mater. 2009, 164, 394–395. [Google Scholar] [CrossRef]
- Bayer, P.; Finkel, M. Life cycle assessment of active and passive groundwater remediation technologies. J. Contam. Hydrol. 2006, 83, 171–199. [Google Scholar] [CrossRef]
- Lemming, G.; Hauschild, M.Z.; Bjerg, P.L. Life cycle assessment of soil and groundwater remediation technologies: Literature review. Int. J. Life Cycle Assess. 2010, 15, 115–127. [Google Scholar] [CrossRef]
- Mak, M.S.H.; Lo, I.M.C. Environmental life cycle assessment of permeable reactive barriers: Effects of construction methods, reactive materials and groundwater constituents. Environ. Sci. Technol. 2011, 45, 10148–10154. [Google Scholar] [CrossRef]
- Horbach, J. Determinants of environmental innovations-new evidence from German panel data sources. Res. Policy 2008, 37, 163–173. [Google Scholar] [CrossRef]
- Paredis, E. Sustainability transitions and the nature of technology. Found. Sci. 2011, 16, 195–225. [Google Scholar] [CrossRef]
- Bloom, N.; Schankerman, M.; Van Reenen, J. Identifying technology spillovers and product market rivalry. Econometrica 2013, 81, 1347–1393. [Google Scholar]
- Aldieri, L.; Vinci, C.P. The role of technology spillovers in the process of water pollution abatement for large international firms. Sustainability 2017, 9, 868. [Google Scholar] [CrossRef]
- Hayek, P.; Stejskal, J. R&D Cooperation and knowledge spillover effects for sustainable business innovation in the chemical industry. Sustainability 2018, 10, 1064. [Google Scholar]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef]
- Noubactep, C. Aqueous contaminant removal by metallic iron: Is the paradigm shifting? Water SA 2011, 37, 419–426. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3057–3062. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]


| Origin | Original Denotation | Trade Mark | Type | Code | m | N | P |
|---|---|---|---|---|---|---|---|
| (g) | (-) | (%) | |||||
| Germany | IpuTech GmbH | FG 1000/3000 | filings | GI1 | 2.19 | >232 | 22.8 |
| Germany | MAZ mbH | Sorte 69 | scrap iron | GI2 | 2.00 | >232 | n.a. |
| Germany | Hartgusstrahlmittel | Würth | filings | GI3 | 2.00 | 232 | n.a. |
| Kenya | Shivkrupa Investements | Champion | steel wool | SW | 2.22 | n.a. | 37.5 |
| China | Qingdao | Three stars | nails | FeN1 | 2.21 | 6 | 6.1 |
| Unknown | n.s | Santoplus | nails | FeN2 | 2.28 | 4 | 9.3 |
| Unknown | n.s | Caserio | nails | FeN3 | 2.06 | 2 | 4.0 |
| Cameroon | Prometal | Tik | nails | FeN4 | 2.32 | 3 | 5.3 |
| Material | Treatment | [Fe] | [Fe] | P | Order | Factor |
|---|---|---|---|---|---|---|
| (mg L−1) | (mg L−1) | (%) | (-) | (-) | ||
| None | 8.8 | 0.7 | 100 | 1 | 1.0 | |
| H2O | 8.1 | 0.6 | 91 | 3 | 0.9 | |
| GI1 | NaCl | 7.2 | 0.4 | 81 | 4 | 0.8 |
| HCl | 8.5 | 1.4 | 96 | 2 | 1.0 | |
| Acetone | 5.8 | 0.3 | 66 | 6 | 0.7 | |
| EDTA | 6.1 | 0.4 | 69 | 5 | 0.7 | |
| None | 14.2 | 1.3 | 100 | 2 | 1.6 | |
| H2O | 12.1 | 1.1 | 86 | 3 | 1.4 | |
| GI2 | NaCl | 9.6 | 1.1 | 68 | 4 | 1.1 |
| HCl | 16.1 | 1.5 | 114 | 1 | 1.8 | |
| Acetone | 9.2 | 1.0 | 65 | 5 | 1.0 | |
| EDTA | 8.1 | 0.7 | 57 | 6 | 0.9 | |
| None | 8.2 | 0.2 | 100 | 1 | 0.9 | |
| H2O | 7.1 | 0.1 | 87 | 2 | 0.8 | |
| GI3 | NaCl | 6.0 | 0.5 | 73 | 4 | 0.7 |
| HCl | 6.4 | 1.0 | 78 | 3 | 0.7 | |
| Acetone | 5.4 | 0.4 | 65 | 6 | 0.6 | |
| EDTA | 5.4 | 0.4 | 66 | 5 | 0.6 | |
| None | 42.8 | 3.0 | 100 | 1 | 4.8 | |
| H2O | 31.5 | 0.8 | 73 | 4 | 3.6 | |
| SW | NaCl | 33.3 | 5.1 | 78 | 2 | 3.8 |
| HCl | 25.8 | 0.3 | 60 | 6 | 2.9 | |
| Acetone | 30.3 | 1.3 | 71 | 5 | 3.4 | |
| EDTA | 32.2 | 4.6 | 75 | 3 | 3.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Ndé-Tchoupé, A.I.; Lufingo, M.; Xiao, M.; Nassi, A.; Noubactep, C.; Njau, K.N. The Impact of Selected Pretreatment Procedures on Iron Dissolution from Metallic Iron Specimens Used in Water Treatment. Sustainability 2019, 11, 671. https://doi.org/10.3390/su11030671
Hu R, Ndé-Tchoupé AI, Lufingo M, Xiao M, Nassi A, Noubactep C, Njau KN. The Impact of Selected Pretreatment Procedures on Iron Dissolution from Metallic Iron Specimens Used in Water Treatment. Sustainability. 2019; 11(3):671. https://doi.org/10.3390/su11030671
Chicago/Turabian StyleHu, Rui, Arnaud Igor Ndé-Tchoupé, Mesia Lufingo, Minhui Xiao, Achille Nassi, Chicgoua Noubactep, and Karoli N. Njau. 2019. "The Impact of Selected Pretreatment Procedures on Iron Dissolution from Metallic Iron Specimens Used in Water Treatment" Sustainability 11, no. 3: 671. https://doi.org/10.3390/su11030671
APA StyleHu, R., Ndé-Tchoupé, A. I., Lufingo, M., Xiao, M., Nassi, A., Noubactep, C., & Njau, K. N. (2019). The Impact of Selected Pretreatment Procedures on Iron Dissolution from Metallic Iron Specimens Used in Water Treatment. Sustainability, 11(3), 671. https://doi.org/10.3390/su11030671







