Abstract
In this paper, the anaerobic co-digestion of three different organic wastes, including livestock manure, slaughterhouse waste, and agricultural by-products (ABs), was carried out to enhance the efficiency of mono-digestion of livestock manure. The characteristics of co-digestion were evaluated at different mixing ratios. The experiment was performed using the Batch test and was divided into two parts. The first experimental section (EXP. 1) was designed to evaluate the seasonal characteristics of ABs, which are the different ratios of fruits and vegetables, where the mixing ratio of spring (fruits:vegetables = 3:7) showed the highest biogas yield (0.24 m3/kg volatile solids). The second experiment (EXP. 2) was conducted by using ABs in the ratio that gave the highest biogas yield in EXP. 1 in combinations of three wastes livestock manure, slaughterhouse waste, and ABs. The highest CH4 yield was 0.84 m3/kg volatile solids (VS), which was obtained with a mixing ratio that had even amounts of the three feedstocks. In addition, the results of the second biochemical methane potential test, which assessed the digestive efficiency according to the mixing ratio of the three types of organic waste, showed that the CH4 production rate of the merged digestion was approximately 1.03–1.29 times higher than that of the mono-digestion of livestock manure. The results of our experiment were verified using the modified Gompertz model, the results of which were relatively similar to the experimental results.
1. Introduction
The significant increase in energy consumption in recent times due to the increase in population has become a global issue. This increase in energy consumption has, in turn, led to an increase in the demand for fossil fuel, such as coal, oil, and natural gas, which are used as primary energy sources, thereby resulting in higher costs of fossil fuels [1]. Since fossil fuels are known to be one of the sources of environmental pollution, such as greenhouse gas emissions, it is necessary to develop environmentally friendly and renewable energy sources that can replace fossil fuels worldwide [2,3]. Accordingly, research has been conducted for the production of new and renewable energy using biomass that is low in cost [4]. Anaerobic digestion is one of the most promising energy production technologies as it uses organic wastes, such as sludge, food waste, manure, and agricultural byproducts (ABs), and prevents environmental pollution through its high treatment efficiency and the production of biogas as an energy source [5,6,7]. Anaerobic digestion is a biochemical reaction that employs microorganisms. Under anaerobic conditions, organic wastes are degraded by microorganisms to produce biogas that contains 50–80% methane (CH4) and 30–50% carbon dioxide (CO2); these can be used as energy sources in various fields [8]. The main stages of the process of anaerobic digestion are hydrolysis, acidogenesis, acetogenesis, and methanogenesis [9]. At the hydrolysis stage, the extracellular enzymes disassemble complex, undissolved material into its respective monomers. In the second stage, dissolved compounds, which are now present within the fermentative bacteria, are converted into volatile fatty acids (VFAs). During acetogenesis, the VFAs are transformed into acetate, H2, and CO2, as well as new cellular material. At the stage of methanogenesis, which is the last stage, the acetate, H2, and CO2, formate, or methanol are converted into methane, CO2, and new cellular material [10].
Regardless of these benefits, it is difficult to operate anaerobic digestion at optimum conditions, owing to the characteristics of the processes that use microorganisms [11]. In particular, the efficiency of the process is affected by the microbial composition, type of feedstock, and operating conditions, such as the temperature, pH, and organic loading rate. Moreover, an imbalance of the influencing factors can lead to a decline in the process efficiency or complete operation failure [12]. Among the various influencing factors, the components of feedstock that are capable of biodegradation and conversion into biogas are the factors that mainly affect the efficiency of operation [13]. In addition, the characteristics of feedstock are must be considered in determining the design of the process and operating conditions, which are the influencing factors of digestion efficiency [14]. However, it is difficult to run the anaerobic digestion process with a single feedstock because of the inhibitory factors, depending on the characteristics of raw materials. For example, animal manure that is mainly used for anaerobic digestion has a low organic loading rate (OLR) and contains high N content, which can be converted to toxic substances, such as ammonia, during the process; ammonia can then lead to the extinction of microorganisms. In particular, some studies have reported that swine or cattle manure occasionally contain more than 4 g N/L of ammonia and show low digestion efficiency [15,16]. Niu et al. [17] reported that biogas production from anaerobic digestion of chicken manure was stopped at a total ammonia nitrogen (TAN) concentration of more than 8000 mg/L and volatile fatty acids (VFAs) concentration of more than 25,000 mg/L. Moreover, owing to the low OLR, animal manure requires high feedstock input for anaerobic digestion. However, the high input of feedstock could cause an accumulation of inhibition factors, which can lead to process failure. Li et al. [18] confirmed that the CH4 yield from chicken manure decreased to 126.6, 91.5, 76.0, and 66.2 mL/g as the OLR increased to 8, 16, 32, and 64 g volatile solids (VS)/L, respectively. In addition, the yield from swine manure decreased to 124.4, 109.9, 88.9, and 79.6 mL/g as the OLR increased to the same concentration as that of chicken manure, respectively. The authors claimed that the reason for this decrease was an increase in soluble material, such as TAN, with an increase in the OLR.
Lately, anaerobic co-digestion that involves the mixing of more than two different feedstocks has been suggested to solve the limitation of the mono-digestion of animal manure. Anaerobic co-digestion is a method in which feedstocks with different characteristics are simultaneously injected into one reaction tank and digested. By mixing the raw materials, the following advantages can be secured: (1) equalization of nutrients, (2) dilution of the inhibition factor, and (3) supplementation of other ingredients [19]. In particular, ABs (such as fruits or vegetables) are suitable feedstocks, owing to their high contents of C and other nutrients [20]. Slaughterhouse waste is also a recommended substrate, owing to its high organic content, including proteins and lipids, which are expected to increase the OLR of animal manure [21]. Alvarez and Liden [22] reported that the co-digestion of animal manure, slaughterhouse waste, and fruit-vegetable waste (FV) shows better results than the mono-digestion of each substrate, with CH4 yield being in the range of 0.27–0.35 m3/kg VS. However, the co-digestion of slaughterhouse waste and FV (mixing ratio of 5:5) showed low efficiency with 0.04 ± 0.01 m3/kg VS, which was due to a decrease in pH with an increase in VFAs. In addition, the CH4 yield of each mono-digestion process was 0.06 ± 0.01 m3/kg VS for slaughterhouse waste, 0.21 ± 0.01 m3/kg VS for animal manure, and a negligible amount for the FV. However, owing to the complex operating conditions, co-digestion is more difficult to apply than mono-digestion [23]. For example, the range of C/N, which is an important factor in co-digestion, is wide, i.e., from 6:1 to 90:1, depending on the result of the research, and it changes with the mixing ratio of feedstock [24]. Therefore, more studies that evaluate various mixtures of feedstocks for anaerobic digestion are necessary. Moreover, the characteristics of feedstock, the effect of mixing ratio, and the change in characteristics through mixing feedstock need to be explored for this purpose as well.
In this study, we aimed to (i) evaluate the efficiency of anaerobic co-digestion using three different substrates, including swine manure, slaughterhouse waste, and ABs, to enhance the efficiency of anaerobic digestion and biogas production; in addition, elemental analysis was conducted on each feedstock to confirm the characteristics of the substrates; (ii) perform biochemical methane potential test (BMP test) on ABs to determine the effect of the mixing ratio of fruits and vegetables to evaluate biogas production potential from each substrate; (iii) apply a modified Gompertz model to investigate the practical result of the BMP test.
2. Materials and Methods
2.1. Materials
In this study, organic waste, which usually originates from rural areas, was collected, including filtered swine manure (FSM) and carcasses (CC) from the O farm, Yongin-si, and ABs from the S agricultural and fisheries market, Suwon-si, Korea. The samples were stored in a refrigerator at 4 °C until elemental analysis (Flash EA-1112, Thermo Finnigan) was performed.
2.2. Mixing Ratio for the BMP Test
In this study, a BMP test was performed to evaluate the biomass production of the target waste. The experiment was conducted by dividing it into a first and second phase. The first experiment (EXP. 1) was conducted on ABs in consideration of the seasonal rate of vegetable and fruit emissions in the S agricultural and fisheries market in Suwon, which was the source of raw materials. EXP. 1 was conducted to identify the influence of seasonal rate between fruits and vegetables, and to find the optimal ratio for mixing that could then be used in EXP. 2. The ratio of fruits and vegetables was 3:7 in spring, 1:9 in summer, 5:5 in autumn, and 9:1 in winter. Only fruits and vegetables were included (Table 1). The second experiment (EXP. 2) was conducted by selecting the ratio of ABs that gave the highest biogas production in the first experiment; EXP. 2 used a total of three types of waste, including FSM, CC, and ABs. The waste ratio was tested with only FSM, and the mixing ratio is summarized in Table 2.

Table 1.
The mixing ratio of EXP. 1.

Table 2.
The mixing ratio of EXP. 2.
2.3. Medium for the BMP Test
The experiment was performed by referring to the BMP test method suggested by Owen et al. [25] and Jeong et al. [26]. The nutrient medium for anaerobic microorganisms in the BMP test was created according to the method described by Shelton and Tiedje [27]. The composition of the growth medium is summarized in Table 3. After sterilizing the nutrient medium in a high-pressure sterilizer for approximately 15 min, the oxygen contained in the nutrient medium was removed using N2 gas. The cap was then closed and sealed to shut off oxygen inflow, and the medium was cooled to 25–30 °C. The culture medium was mixed at a ratio of about 10% of the capacity of the nutrient medium. Oxygen in the headspace was released by N2 gas, and the medium was stored in 35 ± 2 °C after it was sealed. Each BMP test was duplicated two times to ensure the accuracy of the experiment.

Table 3.
Composition of basic anaerobic medium for the biochemical methane potential (BMP) test.
Moving on, 300 mL of the nutrient medium and 30 mL of the planting sludge were sequentially injected into a 500-mL Duran bottle and 2 g VS/L of each feedstock was injected. The pH of each bottle was then adjusted to 7.0 to 7.2 using 2 N HCl and 2 N NaOH; 2.6 g/L of NaHCO3 was injected to prevent a rapid decrease in pH due to the scattering effects in the early stages of the reaction. Finally, N2 gas was injected to create an anaerobic atmosphere in the bottle, which was then sealed for 60 d under 35 °C (Figure 1). The biogas composition was analyzed using a gas chromatograph (GC-TCD, Agilent 7890A, Agilent Technologies, Inc., USA). The gas chromatography conditions are shown in Table 4.

Figure 1.
Schematic of the BMP test.

Table 4.
Gas chromatography analysis conditions for CH4.
2.4. Evaluation Method for the BMP Test
Biogas production was measured periodically using 50-mL gastight syringes after conversion to standard conditions (0 °C and 1 atm), excluding 42.2 mmHg of saturated water vapor pressure at 35 °C for consideration of volume expansion and saturated water vapor pressure, depending on the temperature. Biogas and CH4 yields were calculated from the total amount of biogas/CH4 generated per kilogram of VS after the reaction (m3 biogas or CH4/kg VSadded). EXP. 1 was conducted to measure the biogas yield at different seasonal mixing ratios of ABs, which could then be applied in EXP. 2. EXP. 2 was conducted to evaluate biogas production and CH4 yield to confirm the efficiency of each mixing ratio.
2.5. Kinetic Modeling
In this study, for the precise verification of CH4 yield and the complex metabolic process of anaerobic digestion, a modified Gompertz model was used to compare the cumulative CH4 generation obtained through the results of the BMP test. The modified Gompertz model is primarily used to validate cumulative CH4 generation, which is identified through experimental results. It is the most common model used to model the growth curve of a target [28,29]. The formula used in the modified Gompertz model is as follows (Equation (1)):
where Mt is the CH4 production volume (m3 CH4/kg VSadded) over time (t), and Mm is the potential maximum CH4 generation of raw materials (m3 CH4/kg VSadded); t represents the time (d), Rm represents the maximum CH4 generation per day (m3 CH4/kg VSadded/day), and λ represents the days required for organic decomposition.
3. Results and Discussion
3.1. Feedstock Characteristics
To identify the characteristics of the various raw materials used in this study, elemental analyses, as well as the measurement of total solids (TS) and volatile solids (VS), were performed. The ratios of C and N in the raw materials were verified through the elemental analysis results. The proportion of each raw material was analyzed, and it was found that FSM contained 39.5% of C and 3.4% of N, CC contained 58.1% of C and 8.7% of N, and ABs contained 47.6% of C with 2.6% of N. The proportion of C was observed in the order of CC > ABs > FSM and that of N was observed in the order of CC > FSM > ABs. Furthermore, CC had a higher ratio of C and N, especially N, compared to the other raw materials. The ABs had low N content, which led to a high C/N ratio of 18.3. In addition, the C/N ratio of FSM, i.e., the main feedstock, was found to be 11.6.
Carbon and N are important nutrients to support life, in general, and microorganisms. Studies have shown that the optimal C/N ratio for sludge-based anaerobic co-digestion is about 15–20. However, it was difficult to compare this with co-digestion in this study, owing to the characteristics of high N in the FSM and CC.
Three types of feedstock were merged through a constant ratio (Table 5) and the C/N ratio through mixing was calculated as 7.2–8.2. The highest C/N ratio of mixed feed was found to be Mix 4 with 8.4, which contained the highest proportion of ABs. The C/N ratio of Mix 1, which contained the same ratio of each of the three feedstocks, was 7.6. The decrease in the C/N ratio as compared to that of the FSM in all raw materials was believed to be caused by the CC.

Table 5.
Feedstock characteristics.
The VS/TS of the FSM was 68.5%, and the VS/TS ratio of Mix 1–Mix 4 increased to 90% or greater by mixing the CC and ABs with a high VS ratio. In addition to the chemical oxygen demand (COD), the VS was used as a measure of the organic content in anaerobic materials and its yield was used as an indicator of the efficiency of digestion (m3/kg VS). The elimination rate of organic matter can be determined by the VS removal ratio (%), which is an important characteristic of anaerobic digestion [30]. Therefore, the high VS content indicated that the raw material contained a large amount of organic matter; the increase in the VS/TS ratio of the FSM through the mixing of other feedstocks was expected to have a positive effect on the anaerobic digestion efficiency.
3.2. Results of the BMP Test
In this study, digestion characteristics, according to the mixing ratio of livestock manure, slaughter waste, and ABs, and the digestion characteristics, according to the ratio of vegetables and fruits in ABs, according to the season, were evaluated. The ratio of fruits and vegetables according to the season was adjusted based on the emissions from the S agricultural and fisheries market in Suwon. The BMP test results are summarized in Table 6.

Table 6.
Results of EXP. 1.
Fruits contain sugar and are rapidly converted into VFAs by acid-fermenting bacteria. However, this can cause the accumulation of VFAs, which can cause a rapid decrease in the pH inside the reactor [31]. In particular, methanogens have a slower growth rate and are not as adaptable to a rapid decrease in pH compared to acid-fermenting bacteria; therefore, a sudden decrease in pH can reduce the efficiency of anaerobic digestion [32]. This shortcoming of the decrease in pH caused by the accumulation of VFAs is expected to be mitigated through the role of ammonia nitrogen as an alkalinity buffer in the merging of feedstock, such as FSM and CC, used in this study [33].
The results showed that the highest rate of biogas yield was 0.24 m3/kg VS, which was the ratio in spring (fruits:vegetables = 3:7). In addition, for the mono-digestion of fruits and vegetables, the yield of biogas was relatively similar, i.e., 0.18 m3/kg VS and 0.17 m3/kg VS, respectively. The amount of gas generated was 64.8 mL from fruits as compared to 47.4 mL from vegetables. Biogas production tended to increase as the content of fruits increased. These results suggest that sugars contained in fruits increase the yield of biogas.
The results of the second BMP test, which was intended to assess digestive efficiency, according to the mixing ratio of the three selected types of organic wastes, showed that the CH4 production rate of merged digestion was approximately 1.03–1.29 times higher than that of the mono-digestion of FSM (Table 7 and Figure 2). The highest CH4 yield was 0.84 m3/kg VS, which was identified to be from Mix 1 that had an evenly mixed feed. On the other hand, in the case of Mix 3 having a high CC ratio, the CH4 yield was 0.67 m3/kg VS, which was the lowest yield.

Table 7.
Results of EXP. 2.
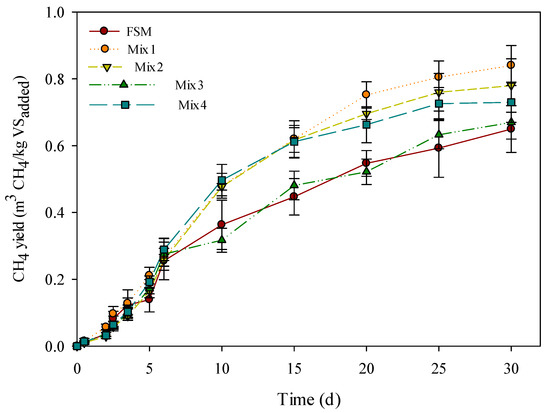
Figure 2.
Daily accumulation of methane yield (error bars indicate the standard deviation).
The gas generation yield was the highest with 17.2 mL of FSM, which had a high content of soluble substance that was easily decomposed; however, the CH4 yield was found to be very low at 0.65 m3/kg VS.
In the case of Mix 2, which had a high proportion of FSM, the amount of gas generated decreased to 9.2 mL, owing to the mixing of solids that were difficult to decompose. However, the CH4 yield was the second-highest at 0.78 m3/kg VSadded. Low biogas production could be attributed to the percentage of VS in the TS content. The VS/TS value in Mix 2 was relatively low at 94.8% as compared with other mixtures. This indicated that the organic fraction was also lower than in the other mixture. Liao and Li [34] found that the specific biogas production increased from 185.3 mL/g VSadded to 332.5 mL/g VSadded as the VS/TS ratio increased from 43.1% to 50.4% in a continuous anaerobic digestion process using wastewater sludge. This result also indicated that the system tended to fail, when the organic content of feed sludge was less than 38%. On the other hand, the CH4 yield was relatively high (0.78 m3/kg VSadded), which is attributed to an increase in carbon sources that are usable for the formation of CH4. The result of elemental analysis in Mix 2 shows that the carbon source in FSM (39.5%) increased to 53.5% by mixing CC and ABs, which contained high carbon sources. Samson and LeDuy [35] suggested that a mixture of carbon-rich substrates with algal biomass may produce interesting results. They reported that a half-and-half mixture of algae with sewage sludge produced a 2.1-fold increase in the CH4 yield, corresponding to 0.36 m3 CH4/kg VS, and a 2.3-fold increase in CH4 productivity, corresponding to 1.41 m3 CH4/m3d. Possibly for the same reasons, the results for Mix 2 seemed to show a high CH4 yield compared to low biogas production.
Mix 3, which contained a large portion of CC, showed 10.7 mL of biogas production and 0.67 m3 CH4/kg VSadded. Mix 3 had the highest concentration of TS (178,679 mg/L) as compared to other mixtures; the raw material of CC also had the highest concentration of TS (318,493 mg/L). Low CH4 yield may have been a consequence of these characteristics. Forster-Carneiro et al. [36] reported that biogas production from food waste increased with a decrease in the percentage of TS, where the maximum cumulative biogas of 7136 mL was obtained from 20% TS. At the same time, the biogas production decreased with an increase in TS percentage, with the maximum cumulative biogas being 6308 mL from 25% TS and 6135 mL from 30% TS. Furthermore, a reactor with lesser TS content showed higher CH4 percentages in the total volume of biogas with 2820, 2089, and 1120 mL from 20% TS, 25% TS, and 30% TS, respectively. Itodo and Awulu [37] found a similar relationship between the TS concentration and biogas yield from poultry, cattle, and piggery slurries. Their results showed that biogas yield decreased from 4.58 to 0.94 L CH4/kg TS, 1.27 to 0.33 L CH4/kg TS, and 1.05 to 0.44 L CH4/kg TS for poultry, cattle, and piggery slurries, respectively, as the TS concentration increased from 5 to 20%. Therefore, it is concluded that the high TS concentration in Mix 3 led to a reduction in the efficiency of both biogas production and CH4 yield.
In the case of Mix 4, gas production was found to be the lowest (7.2 mL), but the CH4 yield was relatively high (0.73 m3/kg VS). This result seemed to be similar to the observations for Mix 3, while the low biogas seemed to be attributable to the lignin content in ABs, regardless of the concentration of TS. Zhu et al. [38] studied the alkaline pretreatment of corn stover anaerobic digestion and found that pretreatment with 5% NaOH removed 31.4% lignin from corn stover, leading to substantially higher biogas production of 372.4 L/kg VS, which was 37.0% higher than that of the untreated samples. Zhang et al. [39] also argued that the cumulative biogas production from co-digestion using banana stem and swine manure increased by 12.1% compared to mixtures not given NaOH pretreatment. The authors suggested that the lignin content decreased to 16.59% from 18.36% by pretreatment with 10% NaOH. Thus, it is speculated that the high proportion of ABs in Mix 4 led to low biogas production.
Among the results of the BMP test, the efficiency of Mix 1 was found to be the highest, where 16.2 mL of gas production occurred, along with 0.84 m3/kg VSadded as methane yield. This result indicated that the difference between the three feedstocks is important to improve the efficiency of anaerobic co-digestion. Kim and Kang [40] similarly reported the co-digestion of sludge (S), microalgal biomass (A), and food waste leachate (F). The best results of the co-digestion (176 L CH4/g VS) were recorded with an equal amount of all three substrates (1S:1F:1A) as compared to those at other mixing ratios: 108.3 L CH4/g VS (1S:1F:4A), 0.7 L CH4/g VS (1S:4F:1A), and 101.2 L CH4/g VS (4S:1F:1A). The authors attributed the results to the different biodegradability of the substrates. The result of Zarkadas and Pilidis [41] were similar to the current study as well. Their study was carried by anaerobic co-digestion using table olive debittering % washing effluent (DWE), cattle manure (CM), and pig manure (PM), where the actual CH4 production was found to vary between 250–300 mL/g VSadded. The higher CH4 production was observed in the mixing ratio of 35 CM:35 PM:30 DWE, which was relatively mixed in an even ratio. Comprehensively, Mix 1 seems to be the optimum mixing ratio among all the mixing ratios tested in the current study.
Conclusively, as the raw materials were mixed, the yield of CH4 increased as compared to that from mono-digestion of manure. In addition, it was confirmed that the characteristics of each raw material were influenced by the mixing ratio. Furthermore, in the case of Mix 1, a combination of three evenly distributed raw materials, the best results were obtained through buffering of the feedstock. On the contrary, some studies have reported opposing results. Vivekanand et al. [42] had mixed three different substrates, including whey (W), manure (M), and fish ensilage (FE); however, they did not find any synergistic effects on methane yield, as compared to the mixture of two substrates. The authors observed the best results (566 mL CH4/g VS) with a mixing ratio of W 10:M 10:FE 80, while the best results with all mixture were found to be at M 15:FE 85 (729 mL CH4/g VS). Despite these results, the authors still suggested the possibility of triple co-digestion, although it may require a wider mapping of optimal feedstock combinations.
3.3. Kinetic Modeling
Experimental data and model-predicted curves for the cumulative biomethane yield from batch thermophilic anaerobic digestion of mixed substrates are shown in Table 8 and Figure 3. The relationship between the cumulative CH4 yield as a function of anaerobic digestion was described by a polynomial regression model. The relationship was characterized by three main phases: (1) a lag phase in which CH4 production was detected for around 1–2 d, (2) a logarithmic phase during which the cumulative CH4 yield increased rapidly after the lag phase until 15–20 d, owing to the significant growth in the anaerobic bacterial population, and (3) the steady-state and death phase after the significant growth period, where the cumulative CH4 yield tended to slowly increase until the curve reached a plateau.

Table 8.
Results of modified Gompertz kinetic modeling.
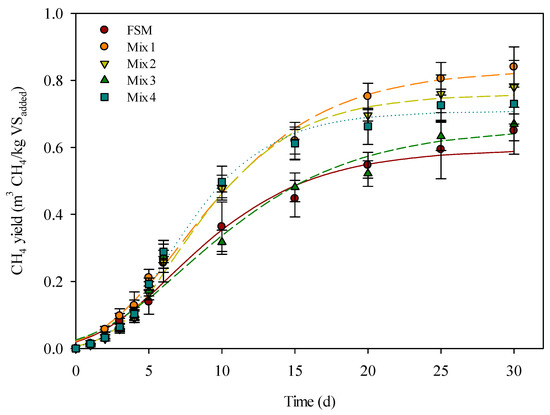
Figure 3.
Experimental data (symbols, error bars indicate the standard deviation) and model simulation/prediction (lines) of cumulative biomethane yield from different mixture ratios of organic substrates.
The results of the kinetic prediction were similar to the experimental results. These results indicated that the experimental data could be acceptable as actual results. As summarized in Table 6, most of the lag phases of mixed substrates were less than 1.5 d, except for those for Mix 2 and Mix 4. The longer lag time of 2.439 d for Mix 2 could have been the result of inhibition [43]. As Mix 2 and Mix 4 have a higher proportion of FSM and Abs, respectively, they also have high toxin and lignin content, along with cellulose concentrations, which are all non-biodegradable substrates, in combination with the inoculum. However, although the cumulative CH4 production could be simulated with the Gompertz model with high R2 values, it could not reflect the two-stage stabilization processes and could lead to a negative lag phase [44].
4. Conclusions
In this study, digestion characteristics were evaluated with respect to mixed feedstock and the suitability of mixing ratios of raw materials was investigated to increase anaerobic digestion efficiency of livestock manure. As a result of the combined anaerobic digestion characteristics of fruits and vegetables, according to the season, the spring ratio (fruits:vegetables = 3:7) was found to be the most efficient with a biogas yield of 0.24 m3/kg VS. In addition, as the ratio of fruits increased, the amount of gas generated tended to increase as well. This trend was confirmed in the mono-digestion of each fruit and vegetable as well.
The CH4 yield from the mixed raw materials was 1.03–1.29 times higher compared to that from mono-digestion of livestock manure. The highest CH4 yield was obtained from an even mixture of the three raw materials (0.84 m3/kg VS) with relatively high gas emissions of 16.2 mL. In the case of mono-digestion of livestock manure, the ratio of VS/TS was found to be about 68.5%, with more soluble substances compared to other raw materials. Gas generation was the highest at 17.2 mL. However, the CH4 yield was found to be 0.65 m3/kg VS, which was lower than that of the mixed feedstock. In addition, verification of results using a modified Gompertz model yielded results similar to those from the actual experimentation. Moreover, this study can be treated as basic research for further studies and testing.
Author Contributions
Conceptualization—S.W.C.; methodology—J.H.J. and W.J.C.; software—J.H.J.; investigation—S.W.C., J.H.J., and W.J.C.; data curation—S.W.C. and J.H.J.; writing—original draft—S.W.C. and J.H.J; writing—review and editing—S.W.C.
Funding
This work was supported by Kyonggi University’s Graduate Research Assistantship 2017.
Acknowledgments
The authors appreciate D.D.N for providing assistance and acknowledge the reviewers of this article. We also acknowledge Kyonggi University for providing Graduate Research Assistantship 2017.
Conflicts of Interest
The authors declare no conflict of interest exists in the submission of this manuscript.
References
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Saidi, R.; Liebgott, P.P.; Gannoun, H.; Gaïda, L.B.; Miladi, B.; Hamdi, M.; Bouallagui, H.; Auria, R. Biohydrogen production from hyperthermophilic anaerobic digestion of fruit and vegetable wastes in seawater: Simplification of the culture medium of Thermotoga maritima. Waste Manag. 2018, 71, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Chang, S.W.; Cha, J.H.; Jeong, S.Y.; Yoon, Y.S.; Lee, S.J.; Tran, M.C.; Ngo, H.H. Dry semi-continuous anaerobic digestion of food waste in the mesophilic and thermophilic modes: New aspects of sustainable management and energy recovery in South Korea. Energy Convers. Manag. 2017, 135, 445–452. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.E.; Al-Shannag, M. Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314. [Google Scholar] [CrossRef]
- Horváth, I.S.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent updates on biogas production-a review. Biofuel. Res. J. 2016, 10, 394–402. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yeop, J.S.; Choi, J.; Kim, S.; Chang, S.W.; Jeon, B.-H.; Guo, W.; Ngo, H.H. A new approach for concurrently improving performance of South Korean food waste valorization and renewable energy recovery via dry anaerobic digestion under mesophilic and thermophilic conditions. Waste Manag. 2017, 66, 161–168. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2018, 1–22. [Google Scholar] [CrossRef]
- Grando, R.L.; de Souza Antune, A.M.; Da Fonseca, F.V.; Sánchez, A.; Barrena, R.; Font, X. Technology overview of biogas production in anaerobic digestion plants: A European evaluation of research and development. Renew. Sustain. Energy Rev. 2017, 80, 44–53. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Fitamo, T.; Boldrin, A.; Dorini, G.; Boe, K.; Angelidaki, I.; Scheutz, C. Optimising the anaerobic co-digestion of urban organic waste using dynamic bioconversion mathematical modelling. Water Res. 2016, 106, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Liao, Q.; Chang, J.-S.; Herrmann, C.; Xia, A. Bioreactors for Microbial Biomass and Energy Conversion; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: Focusing on ammonia inhibition. Biotechnol. Bioeng. 1993, 42, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Qiao, W.; Qiang, H.; Li, Y.-Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Cui, S.; Yu, Q.; Ma, R. Anaerobic co-digestion of animal manures with corn stover or apple pulp for enhanced biogas production. Renew. Energy 2018, 118, 335–342. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef]
- Mokomele, T.; da Costa Sousa, L.; Balan, V.; van Rensburg, E.; Dale, B.E.; Görgens, J.F. Incorporating anaerobic co-digestion of steam exploded or ammonia fiber expansion pretreated sugarcane residues with manure into a sugarcane-based bioenergy-livestock nexus. Bioresour. Technol. 2019, 272, 326–336. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Alvarez, R.; Liden, G. Semi-continuous co-digestion of solid slaughterhouse waste, manure, and fruit and vegetable waste. Renew. Energy 2008, 33, 726–734. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Cook, S.M.; Skerlos, S.J.; Raskin, L.; Love, N.G. A stability assessment tool for anaerobic codigestion. Water Res. 2017, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.; Stuckey, D.; Healy, J., Jr.; Young, L.; McCarty, P. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Chang, S.W.; Ngo, H.H.; Guo, W.; Nghiem, L.D.; Banu, J.R.; Jeon, B.-H.; Nguyen, D.D. Influence of thermal hydrolysis pretreatment on physicochemical properties and anaerobic biodegradability of waste activated sludge with different solids content. Waste Manag. 2019, 85, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.R.; Tiedje, J.M. General method for determining anaerobic biodegradation potential. Appl. Environ. Microbiol. 1984, 47, 850–857. [Google Scholar] [PubMed]
- Nguyen, D.D.; Jeon, B.-H.; Jeung, J.H.; Rene, E.R.; Banu, J.R.; Ravindran, B.; Vu, C.M.; Ngo, H.H.; Guo, W.; Chang, S.W. Thermophilic anaerobic digestion of model organic wastes: Evaluation of biomethane production and multiple kinetic models analysis. Bioresour. Technol. 2019, 280, 269–276. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Chang, S.W.; Jeong, S.Y.; Jeung, J.; Kim, S.; Guo, W.; Ngo, H.H. Dry thermophilic semi-continuous anaerobic digestion of food waste: Performance evaluation, modified Gompertz model analysis, and energy balance. Energy Convers. Manag. 2016, 128, 203–210. [Google Scholar] [CrossRef]
- Parkin, G.F.; Owen, W.F. Fundamentals of anaerobic digestion of wastewater sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Liu, X.; Mei, Z.; Ren, H.; Cao, Q.; Yan, Z. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste. Bioresour. Technol. 2017, 245, 90–97. [Google Scholar] [CrossRef]
- Sanjaya, A.P.; Cahyanto, M.N.; Millati, R. Mesophilic batch anaerobic digestion from fruit fragments. Renew. Energy 2016, 98, 135–141. [Google Scholar] [CrossRef]
- Moukazis, I.; Pellera, F.-M.; Gidarakos, E. Slaughterhouse by-products treatment using anaerobic digestion. Waste Manag. 2018, 71, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, H. Biogas production from low-organic-content sludge using a high-solids anaerobic digester with improved agitation. Appl. Energy 2015, 148, 252–259. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Improved performance of anaerobic digestion ofSpirulinamaxima algal biomass by addition of carbon-rich wastes. Biotechnol. Lett. 1983, 5, 677–682. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Pérez, M.; Romero, L. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour. Technol. 2008, 99, 6994–7002. [Google Scholar] [CrossRef]
- Itodo, I.; Awulu, J. Effects of total solids concentrations of poultry, cattle, and piggerywaste slurries on biogas yield. Trans. ASAE 1999, 42, 1853. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, C.; Liu, X.; Wang, J.; Li, S.; Fan, G.; Zhang, L. Alkaline pretreatment for enhancement of biogas production from banana stem and swine manure by anaerobic codigestion. Bioresour. Technol. 2013, 149, 353–358. [Google Scholar] [CrossRef]
- Kim, J.; Kang, C.-M. Increased anaerobic production of methane by co-digestion of sludge with microalgal biomass and food waste leachate. Bioresour. Technol. 2015, 189, 409–412. [Google Scholar] [CrossRef]
- Zarkadas, I.S.; Pilidis, G.A. Anaerobic Co-Digestion of table olive debittering & washing Effluent, cattle manure and pig manure in batch and high volume laboratory anaerobic digesters: Effect of temperature. Bioresour. Technol. 2011, 102, 4995–5003. [Google Scholar] [CrossRef]
- Vivekanand, V.; Mulat, D.G.; Eijsink, V.G.H.; Horn, S.J. Synergistic effects of anaerobic co-digestion of whey, manure and fish ensilage. Bioresour. Technol. 2018, 249, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Sanz, J.L.; Lundin, M.; Taherzadeh, M.J.; Horváth, I.S. A comparison of process performance during the anaerobic mono- and co-digestion of slaughterhouse waste through different operational modes. J. Environ. Sci. 2018, 64, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).