Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1: Maize Growth
2.1.1. Experimental Site and Design
2.1.2. Crop Husbandry
2.1.3. Determination of Macro and Micro-Nutrients from Grain and Dry Matter
2.1.4. Determination of Soil Enzymes Activities after Crop Harvesting
2.1.5. Selected Soil Physio-Chemical Properties (Pre- and Post-harvest)
2.2. Experiment 2: Incubation of PSB Inoculated Sandy Soil
Experimental Set up
2.3. Experiment 3: Influence of fresh PSB Inoculation on Sandy Soil Water Retention
2.4. Data Analysis
3. Results
3.1. Experiment 1: Physiological and Yield Related Attributes in Response to PSB
3.2. Phosphorus Solublization in Response to PSB
3.3. Influence of PSB Inoculation on Root Exudate and Moisture Contents of Rhizoseath
3.4. Soil Physio-Chemical Properties and Bacterial Colonization in Response to PSB
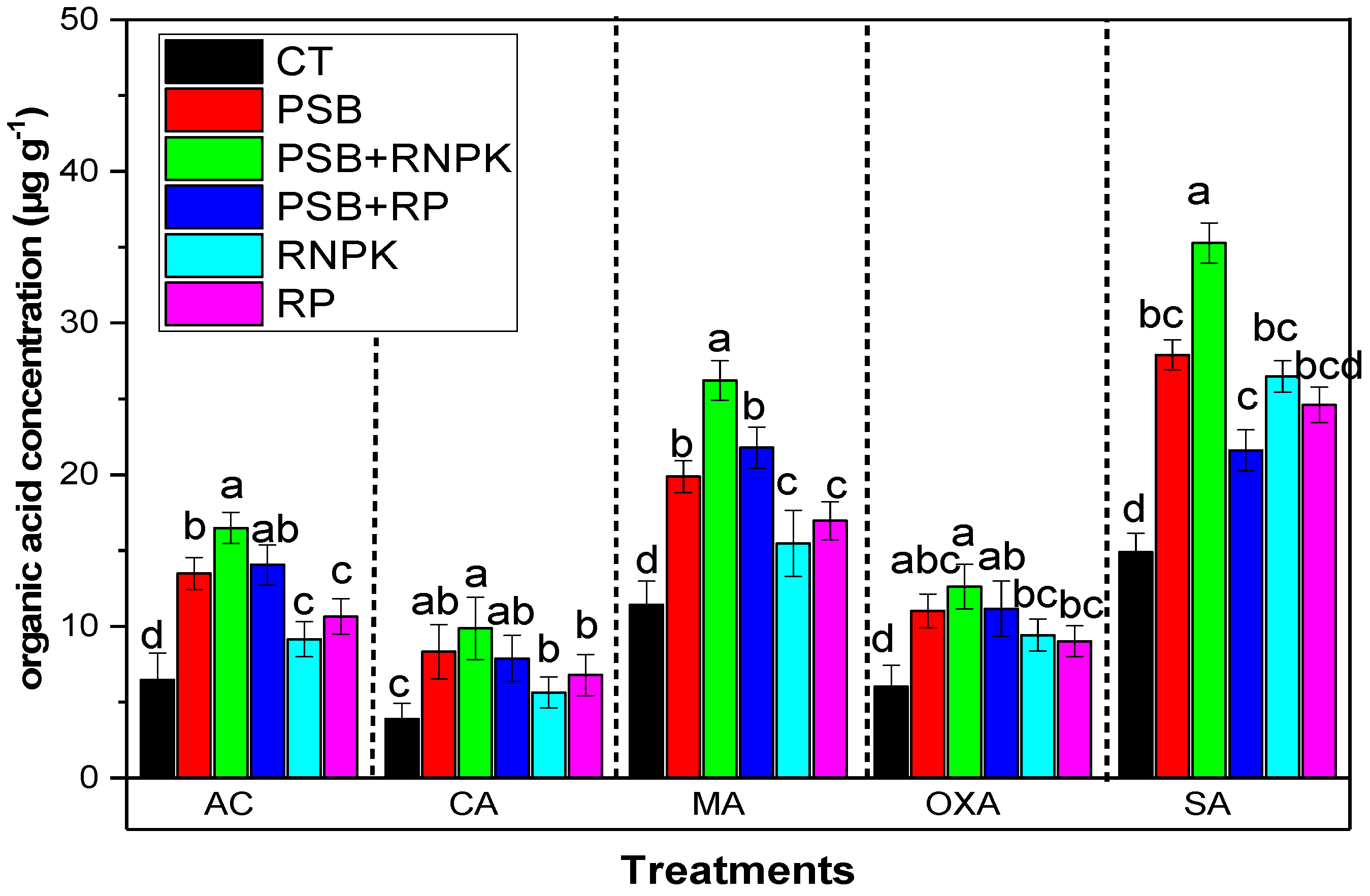
3.5. Effects of PSB Inoculation on Organic Acid in Rhizosphere
3.6. Soil Enzymes Activities in Response PSB Inoculation
3.7. Effect of PSB Inoculated and Non-Inoculated on Dry Matter Characteristics
3.8. Impacts of PSB Inoculation on Maize Grain Quality
3.9. Incubation Study of PSB Inoculated Sandy Soil
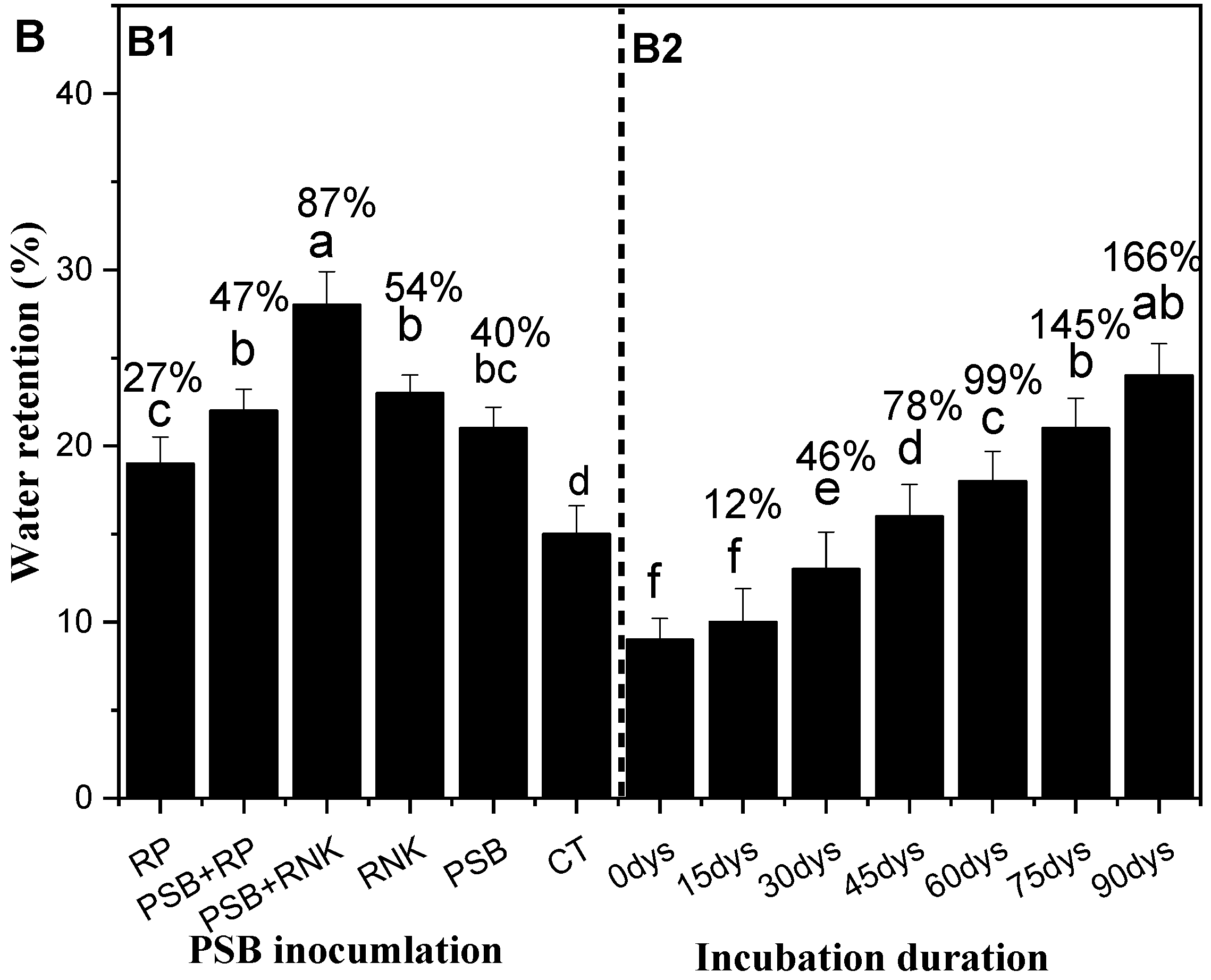
Impacts of Incubation on the Sandy Soil Water Retention and Selected Soil Properties
3.10. Fresh Inoculation Study and Water Retention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GOP. Economic Survey of Pakistan 2016–2017; Ministry of food, Agriculture and Livestock, Fedral Berean of Statistics Islamabad, Pakistan: Islamabad, Pakistan, 2017. [Google Scholar]
- Ezawa, T.; Smith, S.E.; Smith, F.A. P metabolism and transport. Am. Fungi. Plant. Soil 2002, 244, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant. Physiol. 2003, 127, 390–397. [Google Scholar] [CrossRef]
- Abbasi, M.; Musa, N.; Manzoor, M. Phosphorus release capacity of soluble P fertilizers and insoluble rock phosphate in response to phosphate solubilizing bacteria and poultry manure and their effect on plant growth promotion and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosci. Discssion 2015, 12, 1839–1873. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.A.; Naveed, M.; Zahir, Z.A.; Hussain, M.B.; Sessitsch, A.; Mitter, B. L-Tryptophan-dependent biosynthesis of indole-3-acetic acid (IAA) improves plant growth and colonization of maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2012, 65, 1381–1389. [Google Scholar]
- Halajnia, A.; Khorasani, R. Phosphorus fractions in calcareous soils amended with P fertilizer and cattle manure. Gerderma 2009, 150, 209–213. [Google Scholar] [CrossRef]
- Memon, M.; Mashori, N.M.; Memon, K.S.; Kakar, H. Maize dry matter yield and P uptake as influenced by rock phosphate and single super phosphate treated with farm manure. Soil Environ. 2011, 32, 130–134. [Google Scholar]
- Li, J.; Marschner, P. Phosphorus pools and plant uptake in manure-amended soil. J. Soil Sci. Plant. Nutr. 2019, 19, 175–186. [Google Scholar] [CrossRef]
- Wu, S.C.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Gerderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Yadav, H.; Fatima, R.; Sharma, A.; Mathur, S. Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl. Soil Ecol. 2017, 113, 80–85. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Zhu, H.T.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Beech, I.B.; Paiva, M.; Caus, M.; Coutinho, C. Enzymatic activity and within biofilms of sulphate-reducing bacteria. In Biofilm Community Interactions: Chance or Necessity? Gilbert, P.G., Allison, D., Brading, M., Verran, J., Walker, J., Eds.; BioLine: Cardiff, UK, 2001; pp. 231–239. [Google Scholar]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. Biotechnology 2018, 8, 461. [Google Scholar]
- Khan, M.; Sharif, M. Solubility enhancement of phosphorus from rock phosphate through composting with poultry litter. Sarhad. J. Agric. 2012, 28, 415–420. [Google Scholar]
- Chen, Y.; Rekha, P.; Arun, A.; Shen, F.; Lai, W.A.; Young, C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidia, A. Co-inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron. Acad. Sci. Hung. 2007, 55, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Taurian, T.; Anzuay, M.S.; Angelini, J.G.; Tonelli, M.L.; Luduena, L.; Pen, D.; Inanez, F.; Fabra, A. Phosphate-solubilizing peanut associated bacteria: Screening for plant growth-promoting activities. Plant. Soils 2010, 329, 421–431. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Silva, U.C.; Medeiros, J.D.; Leite, L.R.; Morais, D.K.; Cuadros-Orellana, S.; Oliveira, C.A.; de Paula Lana, U.G.; Gomes, E.A.; Dos Santos, V.L. Long-Term Rock Phosphate Fertilization Impacts the Microbial Communities of Maize Rhizosphere. Front. Microbiol. 2017, 8, 1266. [Google Scholar] [CrossRef]
- Laxminarayana, K. Effect of P solubilizing microorganisms on yield of rice and nutrient availability in an acid soil of Mizoram. J. Indian Soc. Soil Sci. 2005, 53, 240–243. [Google Scholar]
- Chaiharn, M.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181. [Google Scholar] [CrossRef]
- Delvasto, P.; Valverde, A.; Ballester, A.; Igual, J.; Muñoz, J.; González, F.; Blázquez, M.; García, C. Characterization of brushite as a re-crystallization product formed during bacterial solubilization of hydroxyapatite in batch cultures. Soil Biol. Biochem. 2006, 38, 2645–2654. [Google Scholar] [CrossRef]
- Gulati, A.; Rahi, P.; Vyas, P. Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr. Microbiol. 2008, 56, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Reddy, M.S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 2011, 47, 30–34. [Google Scholar] [CrossRef]
- Savini, I.; Smithson, P.; Karanja, N. Effects of added biomass, soil pH and calcium on the solubility of Minjingu phosphate rock in a Kenyan Oxisol: Einfluss von zugeführter Biomasse, Boden pH und Calcium-Löslichkeit von Minjingu Phosphatgestein in einem Keniatischen Oxisol. Arch. Agron. Soil Sci. 2006, 52, 19–36. [Google Scholar] [CrossRef]
- Da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica; Rio de Janeiro: Embrapa Solos Brasília: Brasília, Brazil, 2009; Volume 627. [Google Scholar]
- AOAC, Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002.
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Casida, L.E., Jr. Microbial metabolic activity in soil as measured by dehydrogenase determinations. Appl Env. Microbiol 1977, 34, 630–636. [Google Scholar]
- Xu, G.; Zheng, H. Analysis method handbook of soil microorganisms. In References; Agriculture Press: Beijing, China, 1986; pp. 287–289. [Google Scholar]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods Soil Analytical Part 3—Chemical Methods, SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Olsen, S.R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USA Dept. Agric. 1954, 939, 1–19. [Google Scholar]
- Haluschak, P. Laboratory Methods of Soil Analysis, Canada-Manitoba Soil Survey; Haluschak, P., Ed.; Government of Manitoba: Winnipeg, MB, Canada, 2006. [Google Scholar]
- Kay, B. Rates of change of soil structure under different cropping systems. In Advances in Soil Science 12; Springer: Berlin/Heidelberg, Germany, 1990; pp. 1–52. [Google Scholar]
- De Leenheer, L.; De Boodt, M. Determination of aggregate stability by the change in mean weight diameter. Meded. Landbouwhogesch Opzoekingstations Staat Gent. 1959, 24, 290–300. [Google Scholar]
- Chaturvedi, R.; Sankar, K. Laboratory Manual for the Physico-Chemical Analysis of Soil, Water and Plant; Wildlife Institute of India: Dehradun, India, 2006; p. 97. [Google Scholar]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; Schomberg, H.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2013, 3, 195–206. [Google Scholar]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biological Approach; McGraw-Hill College: New York, PA, USA, 1997. [Google Scholar]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Gurdeep, K.; Reddy, M.S. Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate-solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop. Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Saxena, J.; Chandra, S.; Nain, L. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J. Soil Sci. Plant Nutr. 2013, 13, 511–525. [Google Scholar]
- Jha, A.; Saxena, J.; Sharma, V. Investigation on phosphate solubilization potential of agricultural soil bacteria as affected by different phosphorus sources, temperature, salt, and pH. Commun. Soil Sci. Plant. Anal. 2013, 44, 2443–2458. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Amjad, M.; Murtaza, B.; Khan, W.U.D.; Ahmad, N. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant. Nutr. 2017, 42, 112–122. [Google Scholar] [CrossRef]
- Asghar, M.J.; Mehdi, S.S. Selection indices for yield and quality traits in sweet corn. Pak. J. Bot. 2010, 42, 775–789. [Google Scholar]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- El-Tarabily, K.A.; Nassar, A.H.; Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 2008, 39, 161–171. [Google Scholar] [CrossRef]
- Young, C.C.; Lai, W.A.; Shen, F.T.; Hung, M.H.; Hung, W.S.; Arun, A.B. Exploring the microbial potentially to augment soil fertility in Taiwan. In Proceedings of the 6th ESAFS International Conference: Soil Management Technology on Low Productivity and Degraded Soils, Taipei, Taiwan, 24–29 November 2003; pp. 25–27. [Google Scholar]
- Singh, H.; Reddy, S.M. Improvement of wheat and maize crops by inoculating Aspergillus spp. in alkaline soil fertilized with rock phosphate. Arch. Agron. Soil Sci. 2012, 58, 535–546. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Prasanna, R.; Kumar, A.; Pattnaik, S.; Chakravarty, K.; Shivay, Y.S.; Singh, R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur. J. Soil Biol. 2013, 55, 124–130. [Google Scholar] [CrossRef]
- Noumavo, P.A.; Kochoni, E.; Didagbé, Y.O.; Adjanohoun, A.; Allagbé, M.; Sikirou, R.; Gachomo, E.W.; Kotchoni, S.O.; Baba-Moussa, L. Effect of different plant growth promoting rhizobacteria on maize seed germination and seedling development. Am. J. Plant. Sci. 2013, 4, 1013. [Google Scholar] [CrossRef] [Green Version]
- Baldotto, M.A.; Baldotto, L.E.B.; Santana, R.B.; Marciano, C.R. Initial performance of maize in response to NPK fertilization combined with Herbaspirillum seropedicae. Rev. Ceres 2012, 59, 841–849. [Google Scholar] [CrossRef]
- Biswas, J.; Ladha, J.; Dazzo, F. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Kumar, P.; Yadava, R.; Gollen, B.; Kumar, S.; Verma, R.K.; Yadav, S. Nutritional contents and medicinal properties of wheat: A review. Life Sci. Med. Res. 2011, 22, 1–10. [Google Scholar]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef]
- Yadav, V.; Jain, S.; Mishra, P.; Khare, P.; Shukla, A.K.; Karak, T.; Singh, A.K. Amelioration in nutrient mineralization and microbial activities of sandy loam soil by short term field aged biochar. Appl. Soil Ecol. 2019, 138, 144–155. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant. Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Drijber, R.; Zhang, J.; Li, X. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Głab, T.P.J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Gerderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Arif, M.S.; Riaz, M.; Shahzad, S.M.; Yasmeen, T.; Akhtar, M.J.; Riaz, M.A.; Jassey, V.E.; Bragazza, L.; Buttler, A. Associative interplay of plant growth promoting rhizobacteria (Pseudomonas aeruginosa QS40) with nitrogen fertilizers improves sunflower (Helianthus annuus L.) productivity and fertility of aridisol. Appl. Soil Ecol. 2016, 108, 238–247. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Addition of biochar to a sandy desert soil: Effect on crop growth, water retention and selected properties. Agronomy 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Alshankiti, A.; Gill, S. Integrated plant nutrient management for sandy soil using chemical fertilizers, compost, biochar and biofertilizers. J. Arid Studis. 2016, 26, 101–106. [Google Scholar]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De-Pasquale, C. Effect of biochar on the physical and structural properties of a desert sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Uzoma, K.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil. Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]


| Soil Properties | Values | |
|---|---|---|
| Pre-Sowing | Post-Harvest | |
| Physical properties | ||
| Sand (g kg−1) | 470 ± 3.1 | |
| Silt (g kg−1) | 240 ± 2.3 | |
| Clay (g kg−1) | 290 ± 1.6 | |
| Textural class | Sandy clay loam | |
| Chemical properties | ||
| Saturation percentage | 39.20 ± 1.20 | 41.73 ± 1.15 |
| pH | 7.73 ± 0.03 | 7.01 ± 0.05 |
| ECe ( µS cm−1) | 16.39 ± 24.1 | 18.79 ± 29.78 |
| Soil organic matter (g kg−1) | 8.43 ± 0.68 | 8.99 ± 0.39 |
| Total soil N (mg kg−1) | 4.12 ± 9.17 | 4.72 ± 8.34 |
| NaHCO3 extractable-P (mg kg−1 soil) | 7.59 ± 0.12 | 8.70 ± 0.29 Potash |
| SAR (m molc L−1)1/2 | 4.56 ± 0.23 | 4.52 ± 0.30 |
| ESP (m molc L−1/100 g) | 0.43 ± 0.09 | 0.41 ± 0.08 |
| Calcium carbonate (%) | 6.23 ± 0.15 | 6.76 ± 0.21 |
| Rock Phosphate characteristics | ||
| P concentration before PSB inoculation (P mg L−1) | 40.23 ± 4.1 | |
| P concentration after PSB inoculation (P mg L−1) | 482.5 ± 13.6 | |
| Physiological Characteristics | Shoot Length (cm) | Root Length (cm) | Cob Diameter (cm) | Fresh Biomass (g Plant−1) | Dry Biomass (g Plant−1) |
|---|---|---|---|---|---|
| CT | 124.78e | 20.85d | 2.84c | 379.91e | 302.44e |
| PSB | 136.14c | 21.55c | 3.32ab | 401.66d | 341.66c |
| RNPK | 161.32a | 28.43b | 3.54a | 451.23b | 431.28b |
| PSB + RNPK | 167.48a | 33.70a | 3.66a | 501.08a | 466.22a |
| RP | 131.81cd | 20.48cd | 3.05bc | 388.64e | 333.89d |
| PSB + RP | 147.05b | 25.15b | 3.29ab | 428.78c | 414.78b |
| LSD p < 0.05 | 7.62 | 4.31 | 0.31 | 10.47 | 15.11 |
| PSB * RNPK | * | * | * | ** | ** |
| PSB * RP | * | * | * | * | * |
| RNPK * RP | ** | * | * | ** | ** |
| Yield and Yield Attributes | Kernel Rows Cob−1 | Kernels Cob−1 | 100-Kernel Weight (g) | Kernel Yield Plant−1 (g) | Biological Yield Plant−1 (g) |
|---|---|---|---|---|---|
| CT | 15.7d | 311.35e | 11.00e | 118.93e | 241.64d |
| PSB | 16.1c | 348.81d | 14.99c | 138.79d | 282.06c |
| RNPK | 17.4ab | 442.18bc | 16.92ab | 149.56b | 301.23.69b |
| PSB + RNPK | 17.6a | 466.71a | 17.12a | 164.12a | 322.84a |
| RP | 17.1b | 411.24c | 13.01d | 121.04e | 236.88d |
| PSB + RP | 16.9ab | 428.74bc | 15.11c | 143.11cd | 296.19bc |
| LSD p < 0.05 | 0.32 | 11.05 | 1.91 | 5.41 | 15.01 |
| PSB * RNPK | NS | NS | * | ** | *** |
| PSB * RP | NS | NS | * | * | ** |
| RNPK * RP | * | * | * | ** | ** |
| Treatments | SBD | SP | ACC act. | Ch. Act. | PSB-Cloy | SOC | Rhiz |
|---|---|---|---|---|---|---|---|
| CT | 1.53e | 0.51e | 145.89e | Negative | 3.45 × 104d | 7.61d | 1.06d |
| PSB | 1.43b | 0.44a | 305.68b | Positive | 6.82 × 105b | 8.42b | 2.01b |
| RNPK | 1.46c | 0.46b | 268.89c | Negative | 5.56 × 104b | 8.30b | 2.36b |
| PSB + RNPK | 1.41a | 0.44a | 332.48a | Positive | 7.55 × 105a | 8.94a | 3.45a |
| RP | 1.49d | 0.49d | 221.26d | Negative | 4.32 × 104c | 8.08c | 1.92c |
| PSB + RP | 1.43b | 0.47c | 296.68b | Positive | 5.42 × 105b | 8.33b | 2.45b |
| LSD p < 0.05 | 0.018 | 0.001 | 15.29 | 1.40 × 105 | 0.21 | 0.41 | |
| PSB * RNPK | NS | NS | * | * | *** | *** | |
| PSB * RP | NS | NS | ** | * | ** | ** | |
| RNPK * RP | NS | NS | * | * | * | * |
| Treatments | PHE | UE | CL | APH | DHG | βGS |
|---|---|---|---|---|---|---|
| CT | 0.44e | 1.44d | 0.15d | 14.69e | 0.09e | 2.01d |
| PSB | 0.84c | 1.81 | 0.28bc | 21.47 | 0.15d | 3.21c |
| RNPK | 0.91b | 2.22b | 0.44b | 28.79b | 0.22b | 3.51b |
| PSB + RNPK | 1.11a | 2.51a | 0.51a | 30.51a | 0.27a | 3.66ab |
| RP | 0.76d | 1.54d | 0.25c | 19.48d | 0.13d | 3.15c |
| PSB + RP | 0.96b | 2.31c | 0.33bc | 25.01c | 0.18c | 3.26c |
| LSD p < 0.05 | 0.06 | 0.21 | 0.09 | 1.51 | 0.02 | 0.06 |
| PSB * RNPK | *** | *** | ** | *** | *** | * |
| PSB * RP | ** | ** | ** | *** | ** | NS |
| RNPK * RP | * | ** | * | ** | * | NS |
| Treatments | Macronutrients | Micronutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Mg | Ca | Fe | Mn | Cu | Zn | |
| CT | 7.61e | 2.47e | 2.91f | 1.61e | 1.91d | 10.82e | 2.56c | 1.09c | 12.91e |
| PSB | 12.01bc | 3.01c | 3.14d | 1.96c | 2.64c | 16.22d | 3.81b | 1.29a | 15.89d |
| RNPK | 13.01b | 3.51b | 4.41b | 2.15b | 2.07b | 17.51b | 4.41ab | 1.51a | 21.76b |
| PSB + RNPK | 14.32a | 4.42a | 5.12a | 3.06a | 3.51a | 18.26a | 4.92a | 1.61a | 24.56a |
| RP | 10.78d | 2.64d | 3.01e | 1.78d | 2.84c | 15.61d | 3.66b | 1.16b | 15.31d |
| PSB + RP | 11.41bc | 3.21c | 3.86c | 2.01c | 3.16b | 16.91c | 4.01b | 1.44a | 17.89c |
| LSD | 1.12 | 0.31 | 0.21 | 0.11 | 0.22 | 0.61 | 0.45 | 0.21 | 1.63 |
| PSB * RNPK | *** | *** | *** | ** | * | * | * | * | * |
| PSB * RP | * | ** | ** | * | NS | * | NS | NS | * |
| RNPK * RP | ** | ** | *** | ** | * | * | NS | NS | * |
| Treatment | DM | CP | EE | Ash | CF | NDF | ADF | ADL | NSC |
|---|---|---|---|---|---|---|---|---|---|
| CT | 81.56e | 6.51e | 3.01e | 1.34d | 3.44c | 17.61a | 4.81a | 1.68a | 70.36a |
| PSB | 94.56c | 7.11c | 3.41c | 1.51c | 3.61b | 17.51ab | 4.61bc | 1.44b | 66.01c |
| RNPK | 96.01ab | 7.51ab | 3.88b | 1.65ab | 3.76a | 17.26b | 4.36d | 1.28b | 63.42d |
| PSB + RNPK | 97.45a | 7.84a | 4.01a | 1.71a | 3.84a | 17.15c | 4.16e | 1.19c | 61.89e |
| RP | 90.12d | 6.91d | 3.14d | 1.41cd | 3.68b | 17.44ab | 4.68b | 1.61a | 69.01b |
| PSB + RP | 95.01ab | 7.71b | 3.94ab | 1.58b | 3.81a | 17.32b | 4.52c | 1.36b | 65.15c |
| LSD | 1.41 | 0.35 | 0.15 | 0.13 | 0.11 | 0.18 | 0.14 | 0.1 | 0.23 |
| PSB * RNPK | * | * | * | ** | ** | * | * | NS | * |
| PSB * RP | NS | NS | * | * | NS | NS | NS | NS | NS |
| RNPK * RP | NS | NS | * | NS | * | NS | NS | NS | NS |
| Treatments | pH | EC | OM | CEC | SAS | SBD | SP |
|---|---|---|---|---|---|---|---|
| dsm−1 | % | meq/100 | % | (Mg m−3) | (m3 m−3) | ||
| CT | 7.53a | 0.51d | 0.25 | 4.96d | 6.61d | 1.46a | 0.51a |
| PSB | 6.82c | 0.84c | 0.31 | 8.51b | 14.36bc | 1.34bc | 0.47c |
| RNPK | 6.66d | 0.91b | 0.39 | 8.73b | 13.66c | 1.38bc | 0.47c |
| PSB + RNPK | 6.51e | 1.16a | 0.41 | 9.17a | 16.44a | 1.26d | 0.39e |
| RP | 6.96b | 0.76c | 0.28 | 7.11c | 14.62bc | 1.41b | 0.49b |
| PSB + RP | 6.80c | 1.04b | 0.38 | 8.982b | 14.88bc | 1.31c | 0.44d |
| LSD (0.05) | 0.11 | 0.16 | NS | 0.51 | 1.25 | 0.08 | 0.01 |
| SL | RL | RDW | FBM | DBM | SY | GY | COD | GWT | SBD | SP | SWR | NCG | PCG | KCG | PSBC | SOC | SWRI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | 1 | *** 0.82 | *** 0.84 | *** 0.91 | *** 0.91 | *** 0.87 | *** 0.93 | *** 0.77 | *** 0.87 | *** 0.73 | *** 0.90 | *** 0.94 | *** 0.95 | *** 0.91 | *** 0.93 | *** 0.84 | *** 0.93 | *** 0.85 |
| RL | *** 0.82 | 1 | *** 0.86 | *** 0.87 | *** 0.86 | *** 0.85 | *** 0.86 | *** 0.81 | *** 0.80 | ** 0.60 | *** 0.82 | *** 0.83 | *** 0.84 | *** 0.88 | *** 0.84 | *** 0.79 | *** 0.86 | *** 0.87 |
| RDW | *** 0.84 | *** 0.86 | 1 | *** 0.92 | *** 0.91 | *** 0.89 | *** 0.89 | *** 0.80 | *** 0.82 | *** 0.68 | *** 0.89 | *** 0.87 | *** 0.85 | *** 0.88 | *** 0.87 | *** 0.81 | *** 0.85 | *** 0.86 |
| FBM | *** 0.91 | *** 0.87 | *** 0.92 | 1 | *** 0.99 | *** 0.97 | *** 0.98 | *** 0.81 | *** 0.90 | ** 0.69 | *** 0.95 | *** 0.98 | *** 0.96 | *** 0.96 | *** 0.91 | *** 0.93 | *** 0.97 | *** 0.92 |
| DBM | *** 0.91 | *** 0.86 | *** 0.91 | *** 0.99 | 1 | *** 0.99 | *** 0.98 | *** 0.83 | *** 0.88 | ** 0.68 | *** 0.94 | *** 0.97 | *** 0.96 | *** 0.96 | *** 0.90 | *** 0.92 | *** 0.97 | *** 0.91 |
| SY | *** 0.87 | *** 0.85 | *** 0.89 | *** 0.97 | *** 0.99 | 1 | *** 0.94 | *** 0.81 | *** 0.86 | ** 0.68 | *** 0.93 | *** 0.95 | *** 0.94 | *** 0.95 | *** 0.89 | *** 0.91 | *** 0.94 | *** 0.87 |
| GY | *** 0.93 | *** 0.86 | *** 0.89 | *** 0.98 | *** 0.98 | *** 0.94 | 1 | *** 0.81 | *** 0.89 | ** 0.67 | *** 0.92 | *** 0.96 | *** 0.97 | *** 0.96 | *** 0.89 | *** 0.91 | *** 0.97 | *** 0.94 |
| COD | *** 0.77 | *** 0.81 | *** 0.80 | *** 0.81 | *** 0.83 | *** 0.81 | *** 0.81 | 1 | ** 0.65 | ** 0.64 | *** 0.76 | *** 0.82 | *** 0.83 | *** 0.83 | *** 0.76 | *** 0.79 | *** 0.86 | *** 0.85 |
| GWT | *** 0.87 | *** 0.80 | *** 0.82 | *** 0.90 | *** 0.88 | *** 0.86 | *** 0.89 | ** 0.65 | 1 | ** 0.68 | *** 0.84 | *** 0.88 | *** 0.86 | *** 0.87 | *** 0.90 | *** 0.85 | *** 0.86 | *** 0.82 |
| SBD | *** 0.73 | ** 0.60 | ** 0.68 | ** 0.69 | ** 0.68 | ** 0.68 | ** 0.67 | ** 0.64 | ** 0.68 | 1 | ** 0.66 | *** 0.71 | *** 0.70 | *** 0.71 | ** 0.69 | *** 0.70 | ** 0.68 | ** 0.61 |
| SP | *** 0.90 | *** 0.82 | *** 0.89 | *** 0.95 | *** 0.94 | *** 0.93 | *** 0.92 | *** 0.76 | *** 0.84 | ** 0.66 | 1 | *** 0.93 | *** 0.92 | *** 0.93 | *** 0.90 | *** 0.86 | *** 0.90 | *** 0.85 |
| SWR | *** 0.94 | *** 0.83 | *** 0.87 | *** 0.98 | *** 0.97 | *** 0.95 | *** 0.96 | *** 0.82 | *** 0.88 | *** 0.71 | *** 0.93 | 1 | *** 0.99 | *** 0.96 | *** 0.91 | *** 0.96 | *** 0.98 | *** 0.90 |
| NCG | *** 0.95 | *** 0.84 | *** 0.85 | *** 0.96 | *** 0.96 | *** 0.94 | *** 0.97 | *** 0.83 | *** 0.86 | *** 0.70 | *** 0.92 | *** 0.99 | 1 | *** 0.96 | *** 0.90 | *** 0.94 | *** 0.98 | *** 0.91 |
| PCG | *** 0.91 | *** 0.88 | *** 0.88 | *** 0.96 | *** 0.96 | *** 0.95 | *** 0.96 | *** 0.83 | *** 0.87 | *** 0.71 | *** 0.93 | *** 0.96 | *** 0.96 | 1 | *** 0.87 | *** 0.92 | *** 0.96 | *** 0.93 |
| KCG | *** 0.93 | *** 0.84 | *** 0.87 | *** 0.91 | *** 0.90 | *** 0.89 | *** 0.89 | *** 0.76 | *** 0.90 | ** 0.69 | *** 0.90 | *** 0.91 | *** 0.90 | *** 0.87 | 1 | *** 0.83 | *** 0.88 | *** 0.81 |
| PSBC | *** 0.84 | *** 0.79 | *** 0.81 | *** 0.93 | *** 0.92 | *** 0.91 | *** 0.91 | *** 0.79 | *** 0.85 | *** 0.70 | *** 0.86 | *** 0.96 | *** 0.94 | *** 0.92 | *** 0.83 | 1 | *** 0.94 | *** 0.89 |
| SOC | *** 0.93 | *** 0.86 | *** 0.85 | *** 0.97 | *** 0.97 | *** 0.94 | *** 0.97 | *** 0.86 | *** 0.86 | ** 0.68 | *** 0.90 | *** 0.98 | *** 0.98 | *** 0.96 | *** 0.88 | *** 0.94 | 1 | *** 0.93 |
| SWRI | *** 0.85 | *** 0.87 | *** 0.86 | *** 0.92 | *** 0.91 | *** 0.87 | *** 0.94 | *** 0.85 | *** 0.82 | ** 0.61 | *** 0.85 | *** 0.90 | *** 0.91 | *** 0.93 | *** 0.81 | *** 0.89 | *** 0.93 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javeed, H.M.R.; Qamar, R.; Rehman, A.u.; Ali, M.; Rehman, A.; Farooq, M.; Zamir, S.I.; Nadeem, M.; Cheema, M.A.; Shehzad, M.; et al. Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability 2019, 11, 7049. https://doi.org/10.3390/su11247049
Javeed HMR, Qamar R, Rehman Au, Ali M, Rehman A, Farooq M, Zamir SI, Nadeem M, Cheema MA, Shehzad M, et al. Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability. 2019; 11(24):7049. https://doi.org/10.3390/su11247049
Chicago/Turabian StyleJaveed, Hafiz Muhammad Rashad, Rafi Qamar, Atique ur Rehman, Mazhar Ali, Abdul Rehman, Muhammad Farooq, Shahid Ibni Zamir, Muhammad Nadeem, Mumtaz Akhtar Cheema, Muhammad Shehzad, and et al. 2019. "Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria" Sustainability 11, no. 24: 7049. https://doi.org/10.3390/su11247049
APA StyleJaveed, H. M. R., Qamar, R., Rehman, A. u., Ali, M., Rehman, A., Farooq, M., Zamir, S. I., Nadeem, M., Cheema, M. A., Shehzad, M., Zakir, A., Sarwar, M. A., Iqbal, A., & Hussain, M. (2019). Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability, 11(24), 7049. https://doi.org/10.3390/su11247049










