Abstract
Unavailability of balanced nutrients in nutrient-deficient soils is the key reason in reduced yields of spring maize. After application to soil, most of the phosphorus (80–90%) is lost in the environment because of runoff losses and chemically bonding. So, this makes the phosphorus unavailable for plant use. However, soil microorganisms may provide a biological rescue system which is able to solubilize the soil-bound phosphorus (p). Keeping this in view, the present study is designed to meet the following objectives; (1) to improve physico-chemical properties of soil (e.g., soil water retention, soil enzyme activities), and (2) to improve growth and yield of spring maize (cv. Hybrid YSM-112) through the inoculation of phosphorus solubilization bacteria (PSB). A pot experiment was carried out with the following treatments; T1: control (uninoculated control, CT), T2: inoculation with PSB (Enterobacter sakazakii J129), T3: recommend level of NPK fertilizers (RNPK), T4: PSB + RNPK fertilizers, T5: rock phosphate (RP), T6: PSB + RP. Results showed that the addition of PSB together with RNPK improved the yield and yield-related characteristics of spring maize grown in sandy soil. Moreover, it also enhanced dry mater characteristics and maize grain quality. Soil fertility in the context of P-solubilization, soil organic acids, soil organic matter, enzyme activities, PSB colony, and rhizosphere moisture contents were significantly improved with PSB inoculation together with recommended dose of NPK fertilizers (RNPK) compared to PSB alone, rock phosphate (RP) alone, or PSB together with rock phosphate and control treatment. Maize digestibility attributes such as DM, CP, CF, EE (by 35%, 20%, 33%, and 28% respectively) and grain quality such as NPK, Mg, Ca, Fe, Mn, Cu, and Zn (by 88%, 92%, 71%, 68%, 78%, 90%, 83, 69%, 92%, 48%, and 90% respectively) were improved compared to control. In conclusion, improvement in maize crop yield and soil characteristics are more prominent and significant when RNPK is supplemented and inoculated. The present study suggests that PSB, together with RNPK, would improve the maize plant growth and soil fertility in sandy soil.
1. Introduction
Pakistan has a 1.3-million-ha area under maize with an annual production of about 6.13 million tonnes. It contributes 2.7% and 0.5% share in value addition and GDP, respectively [1]. Maize growth and development are critically influenced by the unavailability of phosphorus due to complex reactions with soil [2]. It is a significant constituent of almost all biochemical processes such as respiration, photosynthesis, signal transduction, macromolecule biosynthesis, and energy transfer [3]. Phosphorus has a contribution of about 0.2% in dry matter production, and the world’s 30–40% crop yield is associated with phosphorus availability [4]. However, phosphorus is incorporated in the form of soluble fertilizers, 1% of which becomes the constituents of the plant body and rest of the portion is converted into insoluble complexes by entering into the immobile pools through precipitation reactions with highly reactive Al3+ and Fe3+ in acidic and Ca2+ in calcareous soils [5]. Moreover, about 80% P in soil is precipitated with these metal ions, and about 20% of P is recovered from the applied source [6]. To overcome the above problems of P deficiency, the satisfactory amount is applied in order to combat P deficiency in plants [7].
Most of the soils in Pakistan are calcareous as well as alkaline in nature with pH > 7.0 [8]. Therefore, the fixation of phosphorus in soils is a severe problem [9]. On the basis of a single crop, about 15–20% of phosphorus is absorbed by the plants from the applied phosphorus fertilizer [10]. The mineralization process is mediated by the enzymes especially phosphatases [11] and phytases, which are released by the soil microbes [12]. Moreover, phosphatases (e.g., acid and alkaline phosphatases) released from the cell (exo-enzymes) use organic phosphorus as a substrate and transform into inorganic forms of phosphorus [13]. However, negative or positive activities of microorganisms have direct or indirect effects on the soil health [14]. Rhizospheric microorganisms improve the soil quality by mediating the soil processes viz. release and storage of water and nutrients, nutrients mobilization, root mineralization, organic matter decomposition, nitrogen fixation, sulfur reduction, nitrification, and exudation of soluble compounds [15].
The micro-organisms which have the ability to solubilize P in the soil have to be eco-friendly and economical in order to meet the nutrients requirement of a crop [12]. Consequently, these microorganisms, used solely [16] or in combination with other rhizosphere microbes [17], have shown substantial measurable effects on plants in conventional agronomic soils and increased the growth and productivity of many crops [18].
The prevalence of low P availability all over the world, together with low levels of its application and considerable rise in the price of phosphatic fertilizer over the past few years, have encouraged the agricultural scientist to develop techniques targeting at using P-compounds of low solubility and decreasing the dependence of farmers on synthetic phosphatic fertilizer [19]. Insoluble phosphate compounds such as rock phosphate (RP) may be effective as a P source, but its low solubility limits its direct use as a source of P [20]; therefore, RP needs to be processed to make it soluble and phytoavailable [5].
Phosphate dissolution rates can be greatly accelerated in soil in the presence of organic acids such as malate, citrate, and oxalat, leading to 10- to 1000-fold higher soil solution P concentrations depending upon soil type and concentration of organic acids released either as the result of decomposition of organic wastes or released by microorganisms (PSBs) [21] or plant roots in the rhizosphere [22]. Delvasto, et al. [23] reported that phosphorus solubilizing microorganisms may solubilize P from RP through acidification, chelation, exchange reaction, and polymeric substances formation. Phosphate solubilizing bacteria are used as inoculants, and they increased P uptake and crop yield [24]. Rock phosphate inoculated with phosphate solubilizing bacteria can mobilize the insoluble form of P through the release of organic acids such as citric, oxalic, gluconic, lactic, succinic, and propionic acids and, among these, citric acid showed the maximum reduction in pH [16]. Release of these acids creates a localized high acidity in the immediate vicinity of RP and some organic acids even lead to complexion of Ca2+ [25]. Savini, et al. [26] also reported that RP, when used in combination with organic wastes P from RP, can be increased due to the release of organic acids which may reduce the pH of soil in addition to chelation of Ca+2 and Mg+2 ions and eventually improves the availability of P to plants.
Keeping the above discussion in view, the main objective the present study was to evaluate the effect of phospho-bacteria to solubilize plant-available phosphate from rock phosphate in maize (Zea mays L.) production under calcareous to assess the effectiveness of P-solubilizing bacteria in soil amended with rock phosphate for the uptake of N, P and K.
2. Materials and Methods
Two experiments were performed during the spring season 2018 at the research area of COMSATS University Islamabad, Vehari Campus (CUIV) (32.08° N latitude, 72.67° E longitude) under the sub-tropical climate of the Punjab, Pakistan. The top soil layer (0–15 cm) was collected from the Cholistan Desert, Bahawalpur, Punjab-Pakistan. The soil was taken back to CUIV and then cleaned, air-dried, and passed through the sieve (2 mm). A subsample of this large sample is used for the experiment and used for soil physico-chemical properties (Table 1).

Table 1.
Physico-chemical properties of soil used in the study.
2.1. Experiment 1: Maize Growth
2.1.1. Experimental Site and Design
The experiment was laid out in CRD design, having four replications with six treatments viz., T1: control (CT; un-inoculated), T2: phosphorus solubilization bacteria (PSB), T3: recommend nitrogen phosphorus potash level (RNPK), T4: PSB + RNPK, T5: rock phosphate (RP; @ 2.5g kg−1 of soil), T6: RP + PSB. Soil was sandy clay loam having following physico-chemical characteristics (Table 1). According to our best of knowledge, this is the first ever study about the application of PSB to sandy soil.
2.1.2. Crop Husbandry
Hybrid maize seeds (YSM-112) were inoculated with PSB and sown in the specified treatments. Nitrogen fertilizer was applied in three splits in the form of urea (250 kg nitrogen ha−1, 46% nitrogen), i.e., 1/3rd at the time of sowing, 1/3rd when the crop was at knee height, and 1/3rd was at tasseling stage. The whole of the phosphorus and potash fertilizers were applied in the form of di-ammonium phosphate (300 kg P2O5; 46% phosphorus and 18% nitrogen) and murate of potash (K2O; 50%). The growth period of the crop started from 7 March 2018 and ended on 10 June 2018. The phosphorus solubilization bacteria strain (Enterobacter sakazakii J129) was taken from the Soil Microbiology Laboratory, Institute of Soil and Environmental Science, University of Agriculture Faisalabad, Pakistan.
2.1.3. Determination of Macro and Micro-Nutrients from Grain and Dry Matter
After drying of the plants, maize straw and grains were ground in a Wiley mill, with sieves of 0.25 mm (60 mesh). Then the material was crushed, sieved, and digested by the di-acid mixture (HNO3:HCLO3 = 2:1) then analyzed for macro- and microelement analysis (atomic absorption spectrophotometer; PerkinElmer, Singapore) [27]. Nitrogen content was determined by the method of Nessler, phosphorus was determined using molecular absorption spectrophotometer (calorimetry) at a wavelength of 725 nm and potassium by flame photometry. Moisture, crude protein (CP, %), ether extract (EE, %), ash (%), crude fiber (CF, %), neutral detergent fiber (NDF, %), acid detergent fiber (ADF, %), acid detergent lignin (ADL, %), non-structural carbohydrates (NSC, %), were measured by following the methods of AOAC [28]. NSC were determined as a difference.
2.1.4. Determination of Soil Enzymes Activities after Crop Harvesting
The rhizosphere soil samples were collected after the crop harvesting, and the samples were stored at 4 °C. The soil enzymes activities were performed within a week of soil sample collection to avoid any change in soil characteristics, i.e., phosphomonoestrase [29], urease [30], acid phosphate [31], dehydrogenase [32], β-glucosidase [33], and catalase [33], while the other parameters like bulk density, total porosity, chitinase, PSB colony [34], soil organic carbon [35], and rhizosheath.
For urease activity measurement, the soil (1 g) was incubated into the 0.5 mL urease solution (into a flask), and then 4 mL borate buffer (pH 10.0) was added for approx. 2 h at 37 ± 2 °C. After the end of the incubation period, 6 mL KCl (1 M) was added into the flask and remained for 30 min for reaction (A mixture). The ammonium contents were assessed from the mixture (A + NaOH + sodium dichloroisocyanurate) at 690 nm. Alkaline phosphate (ALP) and β-glucosidase (βGS) were determined by adding 1 g soil into nitropheny phosphate (pH 4.0) and p-nitrophenyl-β-d-glucopyranoside, respectively. Both are incubated at 37 °C for one 1 h and then added tris pH (12.0) to stop the reaction of β-glucosidase activities. The βGS and ALP activities were measured at 464 and 505 nm, respectively. Similarly, the activities of catalase (CAT) were incubated by mixing 5 g soil into 25 mL H2O2 (3%) for 30 min at 4 °C. After the end of incubation, 25 mL H2SO4 (1 M) was added and filtered the solution. Then the 20 mL H2SO4 (0.5 M) was added into 5 mL filtrate, and the solution was titrated as KMnO4 (5 mM) again to determine the unreacted H2O2. The PSB colonies were determined using Pikovskaya’s agar plates.
2.1.5. Selected Soil Physio-Chemical Properties (Pre- and Post-harvest)
The texture of sandy soil (pre-harvest) was measured by the pipette method [36] while the pH and EC of soil (pre-and post-harvest) were measured by shaking the soil–water suspension for 1 h (1:2.5, 25 °C). Similarly, CEC (pre- and post-harvest) was determined by mixing the two buffer (sodium acetate, pH 8.2; and ammonium acetate, pH 7.0) [37]. The OC contents (pre- and post-harvest) were determined by wet oxidation methods [37]. The available phosphorus (P) was measured from the soil extract by using the sodium carbonate [38]. The potassium (k) was noted from the soil sample by using the sodium acetate and then used the atomic absorption spectrophotometer [39]. Similarly, the soil porosity was measured by the Kay method [40]. The soil aggregation percentage (SAS) was determined by dry and wet sieving [41]. The BD was carried out by the method followed by Chaturvedi and Sankar [42].
2.2. Experiment 2: Incubation of PSB Inoculated Sandy Soil
The PSB was inoculated with the same treatments as was applied in Experiment 1. The treatments were incubated for 8 months and simulated the cholistan desert environment by the applying water as average rainfall at cholistan (166 mm annum−1) to create its possible field application after the completion of the study. During the incubation period, the temperature was at 28 ± 2 °C (average cholistan temperature). The experiment was laid out in CRD with factorial arrangements replicated four times. The experiment set up was carried out at COMSATS University Islamabad, Vehari Campus. The soil physico-chemical properties and water retention were recorded using the standard procedures. The purpose of this experiment was to suggest the farmers inoculate the PSBs into sandy soil before the onset of rainfall showers.
Experimental Set up
A column experiment (PVC; plastic column) was carried out to determine the influence of PSB or without PSB on the sandy soil water retention and replicated four times. For this purpose, the PSB colonies or RP or RNPK or mixture of all these were mixed well (plastic tub; v. 30 L) into measured soil 500 g soil (sieved and sir dried soil) and left for 15 days. Then the soil and PSB mixture was filled into a plastic column (25 cm long; 12.5 cm diameter) and an un-inoculated plastic column acted as the control. The headspace (3 cm) of each column was left above the treated or non-PSB-treated soils for the addition of water. All the PVC columns were covered at the bottom with cheesecloth to stop the sandy soil leaching during the experiment. The columns were placed on the wooden rack at the height of 500 m and allowed to settle and established their colonies; plastic bottles were placed to collect the leachates. The distilled water was added to each PVC cylinder until 50% FC was achieved and then left for equilibrium approximately 24 h, after which leaching process was started. The leaching process was started by adding water, and this process lasted overnight. Leachates were collected in the plastic bottles at the bottom of cylinders and then, volume was recorded. To determine the aging of DBCs on the sandy soil water retention, the leaching process was performed five times at the start of this experiment, and after this, it was carried out after 15, 30, 45, 60, 75, and 90 days (crop duration). The retained water (RW) was calculated by using the following equation as described by Novak and D. Rehrah [43]
RW% = (volume of added water − volume of leachates) × 100.
2.3. Experiment 3: Influence of fresh PSB Inoculation on Sandy Soil Water Retention
Experimental Set up: In Pakistan, PSB is mostly applied directly to soil without any incubation. So, this study is conducted to evaluate the effects of fresh PSB application on the sandy soil water retention. A similar experimental set up was used as was discussed in Section 2.2.1.
2.4. Data Analysis
The data of experiment was statistically analyzed by using the Statistix software package (version 8.1; Analytical Software, Tallahassee, FL, USA). The two-way factorial analysis of variance was used for the data of Experiment 1 where the plant and soil attributes were analyzed. One-way ANOVA was used for the analysis of data of Experiment 2 where the PSB or RC or RNPK or their treatments were analyzed. The statistical data are represented either in the form of tables or figures. The probability level for the data was 5% [44], and all the graphs were constructed by using Origin Pro software (version 2018).
3. Results
3.1. Experiment 1: Physiological and Yield Related Attributes in Response to PSB
PSB application together with RNPK significantly (p < 0.05) improved the physiological- and yield-related attributes compared to sole application of RNPK or RP or a combination of these two (Table 2). Inoculation, together with PSB and RNPK fertilization, increased the physiological parameters such as shoot length (34%), root length (62%), cob diameter (29%), fresh biomass (32%), and dry biomass (54%) (Table 1).

Table 2.
Influence of phosphor bacteria on the physiological characteristics of spring maize.
The yield-related attributes such as kernel rows cob−1 (12%), kernel cob−1 (50%), and 100 kernel weight (55%) and biological yield (34%) increased, as compared to control (Table 2). Similarly, maize grain yield increased by 37% and by 10% over the control and sole NPK application, respectively. Moreover, the maize grain yield was decreased by 35% and by 18% compared to the sole application of RP and PSB. So, PSB application, along with RNPK, can improve the yield compared to the sole application of RNPK and other sources in the alkaline soil of tropical areas.
3.2. Phosphorus Solublization in Response to PSB
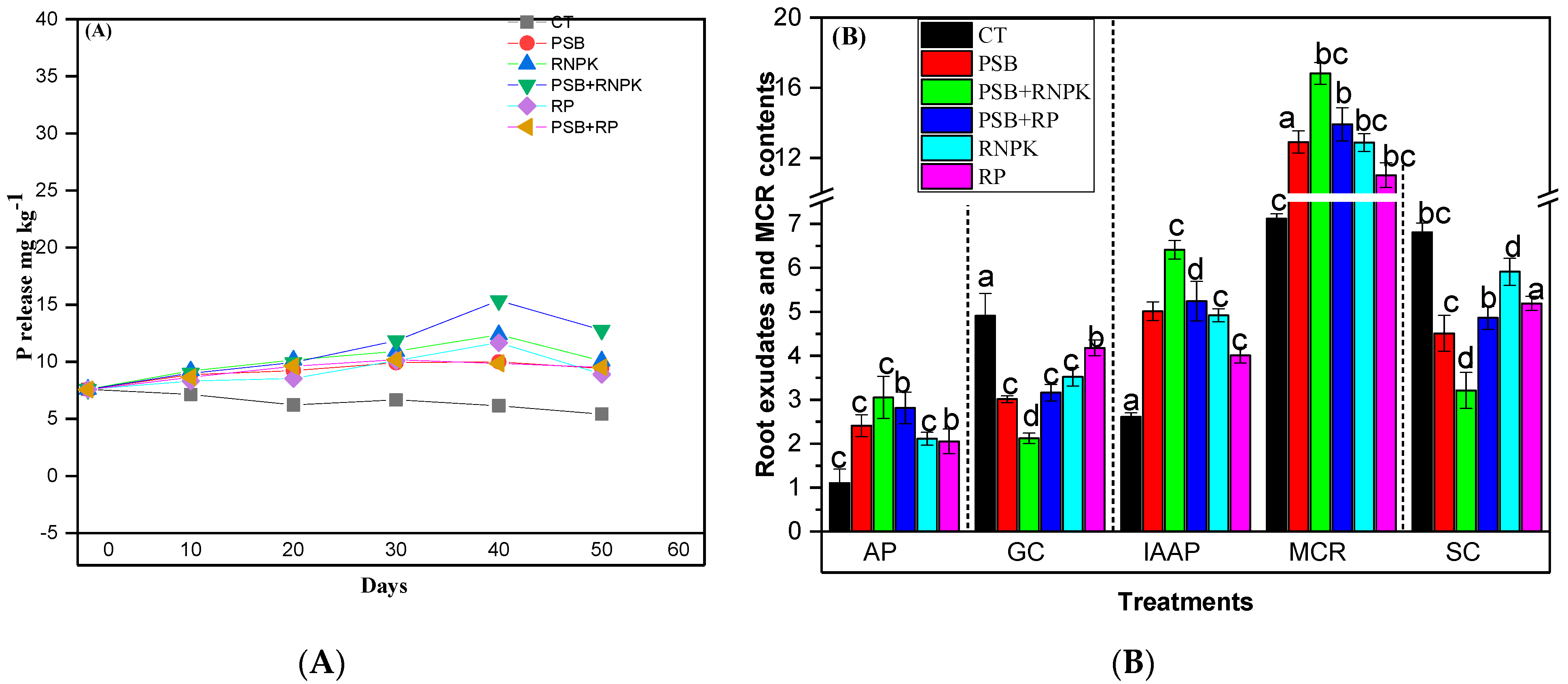
The phosphorous solubilization (PS) was increased in PSB together with the application of RNPK and also on the PSB-PR as compared to the sole application of RNPK or RP or control at the end of soil sampling duration (50 days; Figure 1A). Similarly, the PS was improved with the passage of time (days) in all treatments, but the maximum was observed in PSB + RNPK treatments, followed by PSB + RP, and PSB that was statistically similar to RNPK. Figure 1A indicates that after 10 days from seedling emergence, the maximum PS was noted in the RNPK (by 4.5%, 8.3%, and 11.4%, respectively), compared to PSB + RNPK, PSB + RP, or PSB, but after 20 days, the gap between RNPK and PSB + RNPK was reduced to 2.2%. Moreover, after 30 and 40 days from crop emergence, the PS was increase in PSB + RNPK (by 8% and 19.4% higher vs. RNPK) as compared to all other treatments i.e., RNPK > PSB + PR > PSB. As far as after 50 days, the PS was recorded as the following order PSB + RNPK > RNPK > PSB + PR > PSB > control. The data exhibited the positive impacts of PSB on the long term availability of P over non-inoculant soil (Figure 1A).
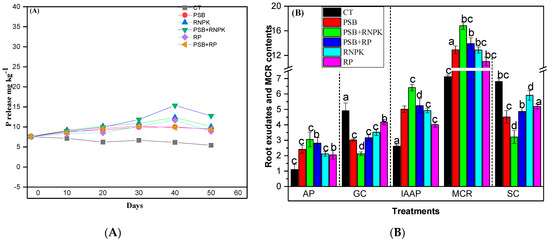
Figure 1.
Effect of phosphorus solubilization bacteria (PSB) on the phosphorus (P) release with the passage of time (days; 10 days to 50 days after plant emergence; (A) and some root exudates (AP, GC, IAAP, SC) and moisture contents of rhizoseath (MCR; %) (B). All the bars (B) having similar betters among the other bars are not signifincalty similar according to DMR test (p ≤ 0.05) while the error bars show the standard errors of the mean values. AP, auxin production (mg mL−1); GC, glucose contents (µg g−1); IAAP indole acetic acid production (µg mL−1); and SC score contents (µg g−1). CT, control; PSB, phosphorus solublization bacteria; PSB + RNPK (PSB + recommended nitrogen, phosphorus and potash), PSB + RP (PSB + rock phosphate).
3.3. Influence of PSB Inoculation on Root Exudate and Moisture Contents of Rhizoseath
PSB together with RNPK or PSB alone reduced the root exudates (GC and SC) and increased the AP, IAAP, and MCR of the sandy soil, as in the case of our study (Figure 1B). The figure exhibits that AP (mg mL−1) and IAAP (µg mL−1) were increased by 44.5% and 23.2% (PSB + RNPK vs. RNPK), 21% and 21.8% (PSB + RNPK vs. PSB), and 63.6% and 59.3% (PSB + RNPK vs. CT). Similarly, GC (µg g−1) and SC (µg g−1) were increased in the CT treatments compared to sole or combined application of PSB + RNPK (by 57% and 53%) or PSB + RP (by 36% and 29%) or PSB (by 39% and 34%) or RNP (by 28% and 13%), or RP (24% and 15%), respectively (Figure 1B). Moreover, the GC and SC concentrations were also increased in the sole application of RNPK (by 60% and 84%), or PSB (by 42% and 40%), or RP (by 97% and 63%) over the PSB together applied with RNPK. The higher concentration of GC and SC in control soil indicates that the plants are under stress, as seen in our study. Rhizoseath moisture contents (MCR) are very important for bacterial growth and colony formation. The higher MCR (%) was recorded in PSB applied together with RNPK over all other treatments such as RNPK (by 23%) or PSB + RP (by 17%) or PSB (by 23%) or control (by 58%). However, PSB akine application also increased the MSC over other treatments, i.e., RNPK (by 6%), or RP (by 15), or control (by 45%). The increased in MCR in our study improved the growth of PSB colonies (Table 3).

Table 3.
Influence of phosphor bacteria on the yield and yield-related attributes of spring maize.
3.4. Soil Physio-Chemical Properties and Bacterial Colonization in Response to PSB
The data in Table 3 indicate that the soil physico-chemical properties and bacterial colonization improved with the addition of PSB along with RNPK. The values of SBD, SP, ACC act. SOC, Rhiz. from 1.40 to 1.53, 0.44 to 0.51, 145.89 to 332.48, 7.61 to 8.94, 1.06 to 3.45, respectively. The most promising increase of these properties was recorded in PSB, together with RNPK, to RNPK alone (by 3.5%, 19%, 7%, and 32%, respectively) compared to sole RNPK application. Moreover, the PSB alone application increased the SPD (by 2%), SP (by 4%), ACC act. (by 13%), SOC (by 2%), and Rhiz. (by 15%) over the RNPK, respectively. Interestingly, the values of Ch. Act and PSB colonies were higher in PSB together with RNPK over RNPK (negative, by 71%) or PSB (positive, by 58%) or PSB + RP (positive, by 68%) treatments.
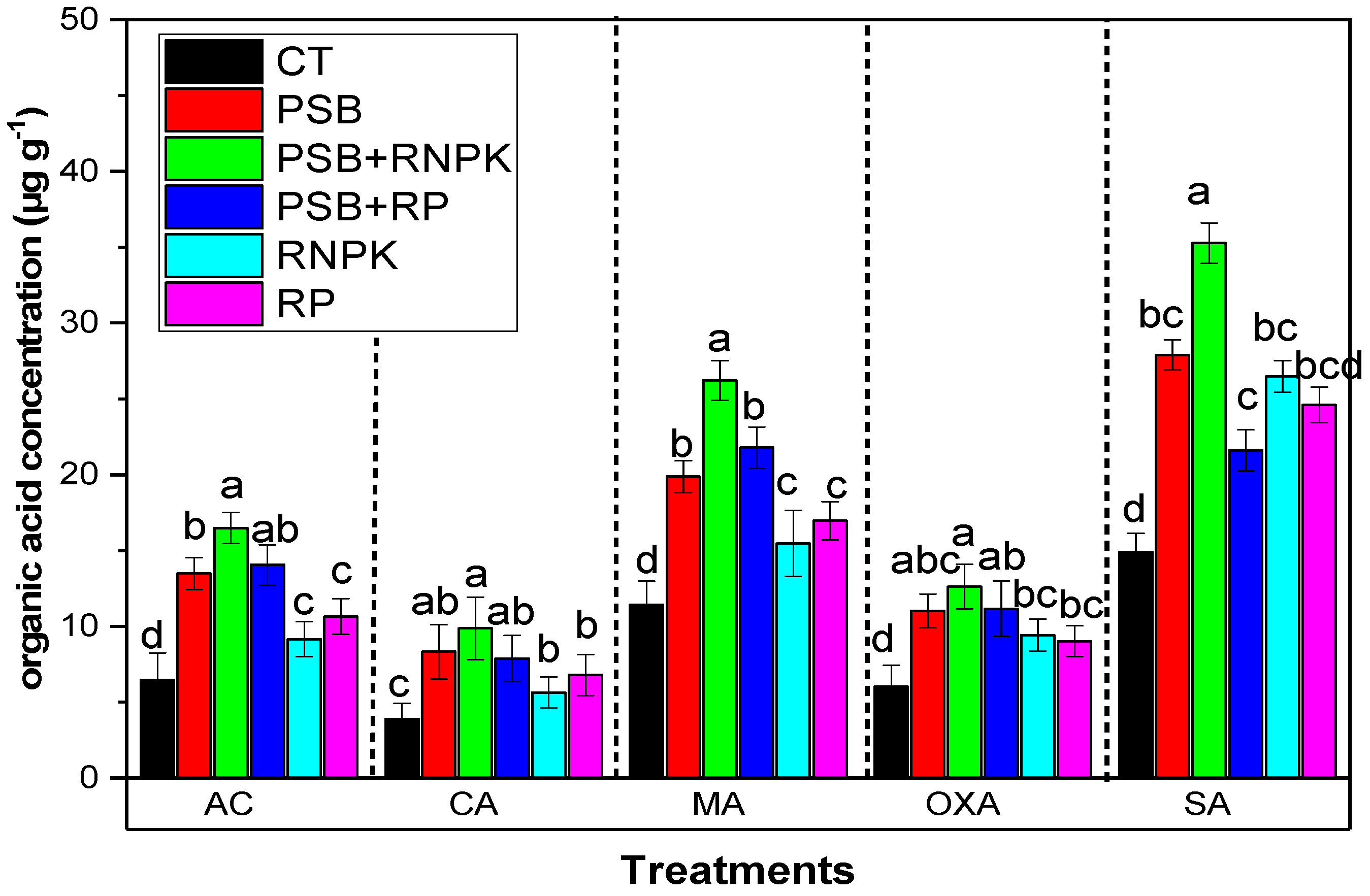
3.5. Effects of PSB Inoculation on Organic Acid in Rhizosphere
The current study indicates that PSB inoculation increases the organic acids in the rhizosphere compared to non-inoculant control (Figure 2). Moreover, the maximum organic acid was produced in the PSB addition together with RNPK in the sandy soil as compared to control and all other treatments RP alone, or PSB alone, or PSB together with RP. The data in Figure 2 show that AC by 155%, CA by 153%, MA by 129%, OXA by 39%, and SA by 137% was increased over the non-inoculant control. Moreover, when we compared the PSB treatments, PSB alone and PSB together with RP decreased the organic acid concentrations by 22% and 17% (AC), 19% and 25% (CA), 74% and 91% (MA), 25% and 33% (OXA), and 26% and 63% (SA) as compared to PSB together with RNPK, respectively (Figure 2).
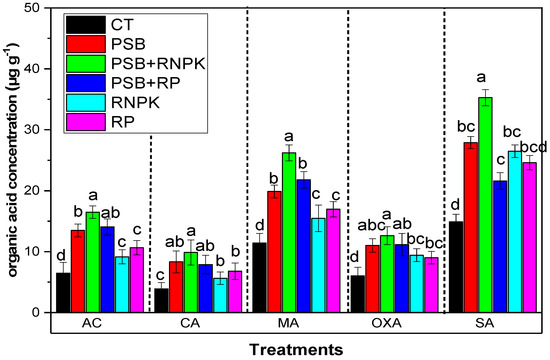
Figure 2.
Effect of phosphorus solubilization bacteria (PSB) on the organic acids in the rizosphere.All the bars having similar betters among the other bars are not significantly similar according to the DMR test (p ≤ 0.05), while the error bars show the standard errors of the mean values. AP, auxin production (mg mL−1); GC, glucose contents (µg g−1); IAAP indole acetic acid production (µg mL−1); and SC score contents (µg g−1). CT, control; PSB, phosphorus solubilization bacteria; PSB + RNPK (PSB + recommended nitrogen, phosphorus and potash), PSB + RP (PSB + rock phosphate).
3.6. Soil Enzymes Activities in Response PSB Inoculation
All the treatments significantly increased the soil enzymes activities over control (Table 4). The measured activities of phosphomonoestrase (PHE), urease (UE), catalase (CL), acid phosphate (APH, dehydrogenase (DHG), β-glucosidase (βGS) were in the ranges from 42% to 63%, 20% to 43%, 40% to 71%, 24% to 52%, 31% to 67%, and 36% to 46%, respectively, compared to control (Table 5). The most promising increase in soil enzymes activities was seen in the PSB together with RNPK (p < 0.05). The PSB together with RNPK proved the most effective treatment towards the increase of PHE (by 63%), UE (by 43%), CL (by 71%), APH (by 52%), DHG (by 67%) and βGS (by 46%) over the control, as in our study (Table 5). Moreover, the PSB + RNPK treatments were also significantly differed from RNPK alone or PSB alone or PSB together with RP treatments. The interaction pf PSB * RNPK, PSB * RP, and RNPK * RP were significant for all soil enzymes except the βGS treatments (p < 0.05).

Table 4.
Effects of PSB inoculation on soil physic-chemical properties and bacterial colonization.

Table 5.
Effects of PSB inoculation on soil enzyme activities after crop harvesting.
3.7. Effect of PSB Inoculated and Non-Inoculated on Dry Matter Characteristics
Often in Pakistan, the maize stem is used as green fodder for animals after removing the corn from the stem. So, the dry matter (DM) analysis is necessary for this scenario. The PSB alone or together with RNPK or RP are significant after the maize feeding characteristics over the control treatments (p < 0.05). DM, CP, EE, ash, and CF were higher in PSB together with RNPK by 36%, 21%, 33%, 28%, and 12% compared to control, while the NDF, ADF, ADL, and NSC had higher values than the all other PSB alone or together with SNPK or RP (Table 4). The interactive effect of PSB * RNPK was significant in almost all DM values, while the PSB * RP and RNPK * RP were found non-significant in most of the cases.
3.8. Impacts of PSB Inoculation on Maize Grain Quality
Grain quality is one of the vital parameters of any food product. The concentrations of all macro elements (NPK, Mg, Ca, g kg−1) were found in the ranges from 7.61 to 14.32, 2.47 to 4.42, 2.91 to 5.12, 1.61 to 3.06, and 1.91 to 3.51, respectively, while micronutrients (Fe, Mn, Cu, and Zn; mg kg−1) were in the ranges from 10.82 to 18.26, 2.56 to 4.92, 1.09 to 1.61, and 12.91 to 24.56, respectively (Table 6). Surprisingly, the concentration of all micro- and macronutrients increased over the application of PSB together with RNPK compared to PSB or RNPK alone. However, the increment in values were variable among the different treatments of PSB (with RP or alone). Moreover, the PSB together with RNPK was found to be the most promising treatment in enhancing the N by 88%, P by 79%, K by 76%, Mg by 90%, Ca by 84%, Fe by 69%, Mn by 92%, Cu by 48%, and Zn by 90%, respectively, in maize grain compared to control. In addition, the PSB alone or together with RP significantly improved the macro- (NPK, Mn, Ca) and micronutrients (Fe, Mn, Cu, and Zn) over the control treatments (Table 7).

Table 6.
Effects of PSB inoculation on the grain quality of maize.

Table 7.
Effects of PSB inoculation on maize dry matter characteristics.
3.9. Incubation Study of PSB Inoculated Sandy Soil
Impacts of Incubation on the Sandy Soil Water Retention and Selected Soil Properties
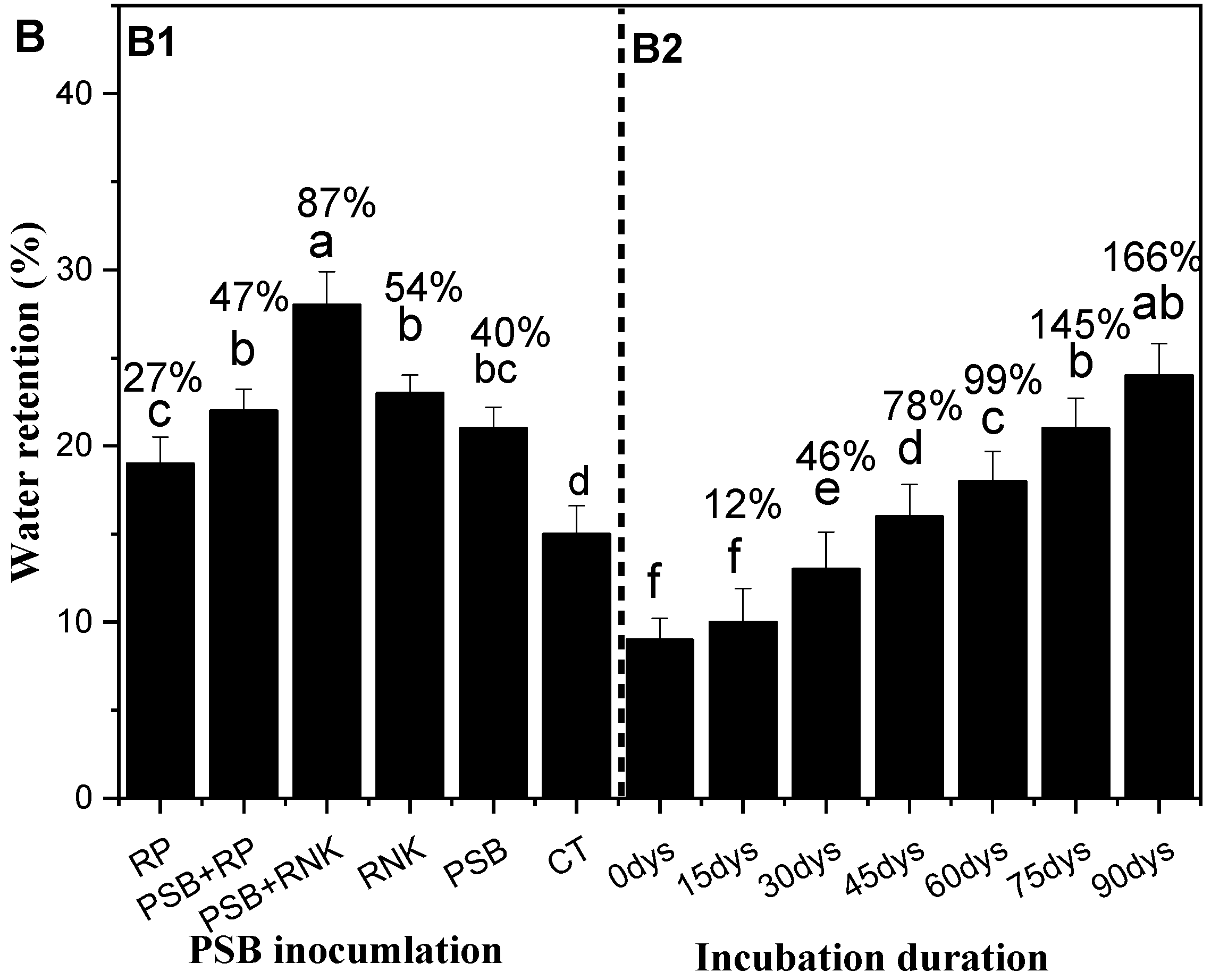
Using the PVC column experiment, the current study indicates the effect of PSB on soil water retention in the sandy soils. The data was collected up to 90 days after crop emergence, which was the actual crop water requirement duration to reach its maturity. Hence, the column experiment study had some inspiration for farmers for its field application. The incubation of PSB alone or together with RNPK or RP significantly increased the water retention (Figure 3A). In addition, the incubation also improved the soil properties (Table 8) compared to control. Our data results indicate that inoculation of PSB in sandy soil over some period (8 months) increased the water retention in PSB + RNPK by 205%, PSB + RP by 163%, RNPK by 138%, PSB by 120%, and RP by 78% compared to control. Moreover, the sole application of RNPK and RP into sandy soil decreased water retention by 29% and 73% compare to PSB + RNPK. Similarly, the selected soil properties, i.e., pH (by 14%), CE (by 127%), CEC (by 85%), SAS (by 148%), SBD (by 14%), and SP (by 24%) were improved in PSB together with RNPK over the control treatments. In addition, other soil properties were also improved by the sole application of PSB or PNPK compared to control (Table 8). Our study concludes that the aging of PBS increases the water retention of sandy soil and hence helps the farmers in increasing their crop productivity. The mixing of PSB before the onset of rainfall helps to increase soil water holding capacity.
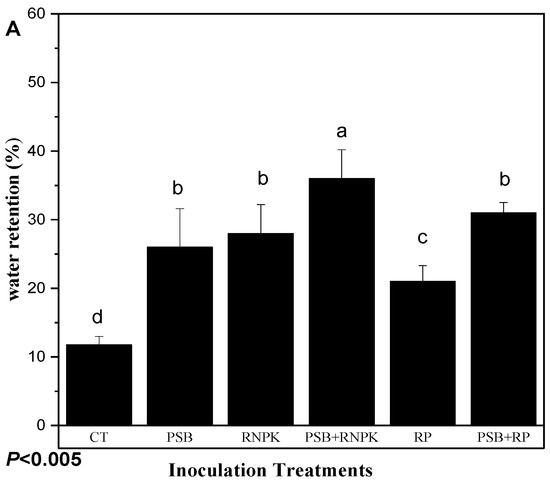
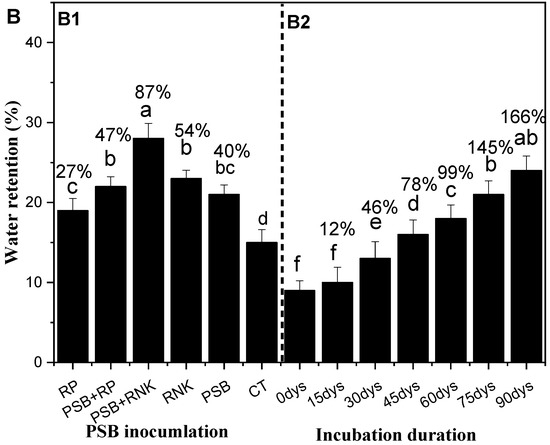
Figure 3.
Effect of phosphorus solubilization bacteria (PSB) incubation on the selected soil properties (Table 7) and water retention (A) and effect of PSB fresh inoculation on the soil water retention (B; (Figure B1,B2)). All the bars (A,B) having similar letters among the other bars are not significantly similar according to the DMR test (p ≤ 0.05), while the error bars show the standard errors of the mean values. CT, control; PSB, phosphorus solubilization bacteria; PSB + RNPK (PSB + recommended nitrogen, phosphorus, and potash), PSB + RP (PSB + rock phosphate).

Table 8.
Effects of PSB inoculation on the selected soil properties after incubation (8 months).
3.10. Fresh Inoculation Study and Water Retention
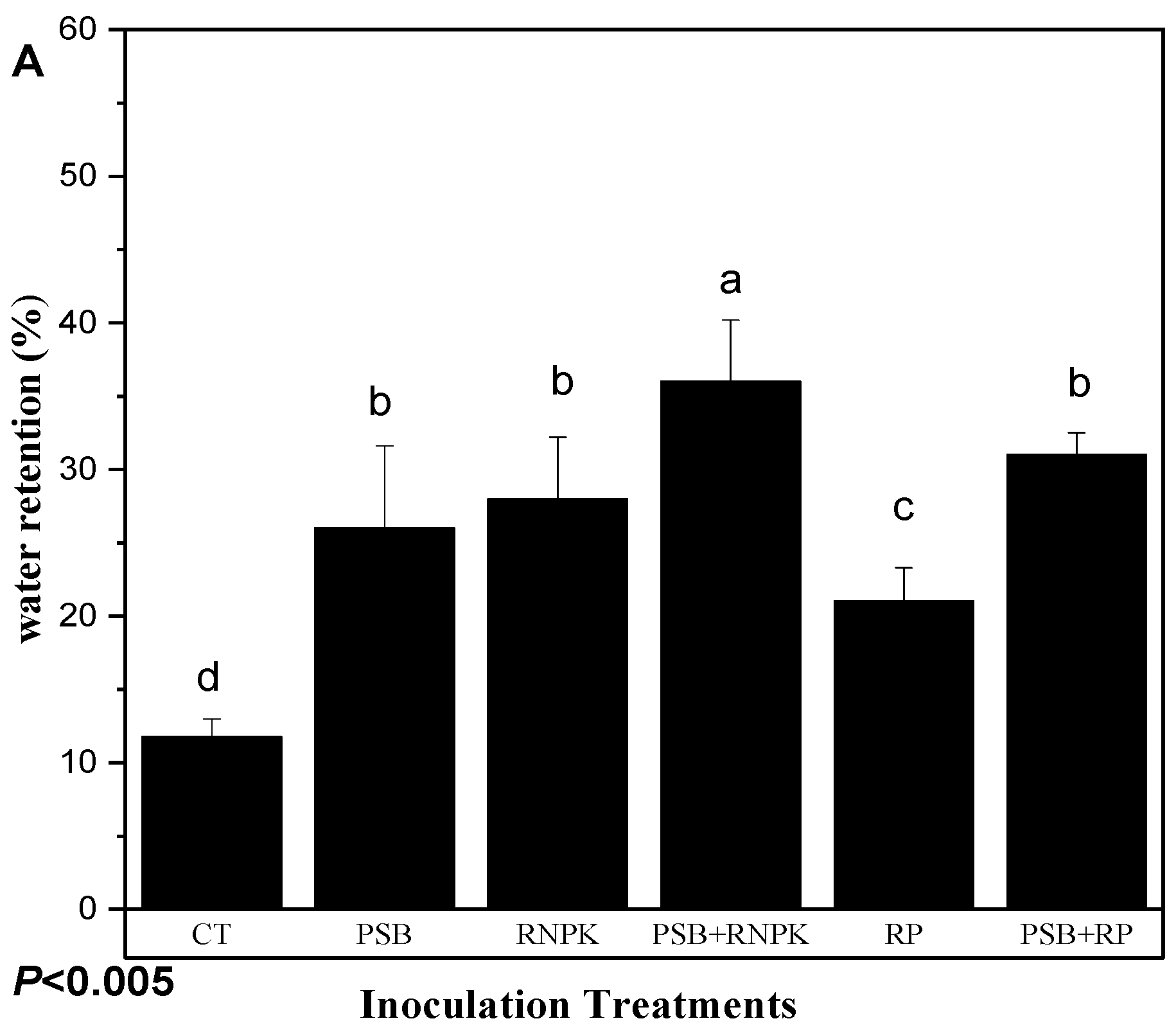
The PSB inoculation treatments, duration, and their interactive effects significantly affected the sandy soil water retention (Figure 3A,B). The study results exhibit that PSB together RNPK is the most effective treatment over all other treatments towards the increase of water retention. The incubation duration of fresh PSB inoculants also increases soil water retention (Figure 3B). This phenomenon of our study indicated that a fresh application of PSB to sandy soil also increased the sandy soil water retention over time (days) and supported early maize growth and development for a certain period of time. So, among all treatments, the interactive effects (Figure 3B, B1 and B2)) show that PSB + RNPK and the 75 or 90 days of fresh incubation is helpful to farmers. So, the water retention ability of the treatments are in the following order: PSB + RNPK > PSB + RP > RNPK ≥ RNPK > control. However, the incubation duration (days) also increased the water retention of fresh PSB inoculation in the following order: 90 days ≥ 75 days > 60 days > 45 days > 30 days > 15 days ≥ 0 day.
4. Discussion
Inoculation of PSB together with fertilizer application markedly improved the physiological and yield related parameters compared to an inoculated control. Among all treatments, PSB + RNPK proved the most effective treatment towards the increase of spring maize grain yield [45] compared to inoculation alone, RNPK alone [46] or PR alone and CT. In the present study, RNPK together with PSB resulted in maximum shoot length and fresh and dry biomass of shoot. This increase might be due to releases of higher chlorophyll activities (Supplementary Figures S1–S3) and more photosynthate availability that increased the growth of the plants [47]. These findings are also in agreement with Saxena, et al. [48], who reported that longer root length could be improved by PSB with the chemical fertilizer. P-solubilizing bacteria excrete hormones that induce longer root growth, which leads to an enhance uptake of nutrients [49]. Similarly, maximum maize fresh and dry biomass in PSB together with RNPK might be due to the availability of nutrients, especially P, by the inducing role of PSB. The use of PSB ensures the availability of nutrients in plants which result in maximum growth [15]. Such a synergistic effect of PSB application together with RNPK enhances the fresh and dry biomass compared to RP alone or PSB alone or CT [50]. The physiological attributes were improved in PSB together with RNPK treatments, which might due to the increase in the retention of assimilates that are essential for the cob production in the stem of the plant [51,52,53]. In addition, the application of PSB together with RNPK enhanced the availability and uptake of nutrients from the soil and, ultimately, better 100-grain weight was achieved. The study results related to grain yield was in accordance with those of Afzal and Bano [52] and Young, et al. [54] that PSB together with RNPK increased the yield components up to 30–50% as compared to RP alone or PSB alone or CT.
P-mobilization/solubilization was improved in the PSB treatments as compared to RNPK alone or PSB alone or CT, while the maximum P-solubilization was recorded in PSB together with RNPK. The study results are in accordance with those of Kaur and Reddy [46] who stated that application of fertilizer enriched with PSBs in the rhizosphere enhanced P-availability and increased the rice crop yield. Our study results indicate that highest yield was recorded in the inoculation treatments together with RNPK. Moreover, phosphorus (P) solubilization was increased with the passage of time when the inoculation of PSB was done along with RNPK compared to the control and other treatments, i.e., RNPK, PSB + RP, RP and PSB. The P solubilization in the soil increased with days and maximum P was recorded after 40 days of seedling emergence, and, afterward, this phenomenon was slowed down. This might be due to more accumulation of P in the soil which reduces the P solublization in the soil. After 45 days, the silking and tasseling process starts and the plant utilizes the photosynthates instead of soil P reservoirs. Similarly, the study results show that maximum P solubilization was when PSB was applied together with RNPK, and that was due to the availability of initial substrate resources to PSB. These study results conclude that the crop reaped the benefits imparted by the PSBs to the sandy soil in terms of available P contents and further improved the photosynthesis activity (see Supplementary Figure S1) and respiration (see Supplementary Figures S2 and S3) of the maize plant [55]. The soil P-availability showed in the form P uptake by the maize plant in various treatments. Swarnalakshmi, et al. [56] noted that PSB application together with chemical fertilizers enhanced P-availability and P-uptake compared to PSB alone or RNPK alone in wheat crops. Our study results confirmed the hypothesis that PSB amendment to sandy soil had strongly influenced the plant growth, physiology, yield, and yield-related attributes compared to other treatments. These positive effects were related to improved sandy soil structural stability (bulk density, porosity, soil organic carbon), higher nutrients retention capacity (CEC, PSB-colony, and rhizosheath) and superior production of organic acids (Figure 2; Table 9). As in the case of our study, PSB increased plant biomass but the activities of PSB towards P-solubilization in P-deficient soils could be different to different plant species and the prevailing environmental conditions [57,58]. One more phenomenon, i.e., production of organic acids by PSBs, enhances the P-mobilization/solubilization [59] in the sandy soil and finally enables the plant to uptake mineral nutrients [60]. In addition, the carboxylic groups of the acids increase the chelation of the cations, which further bind to phosphate and convert them into soluble forms [61,62]. From the results of the current study, it may be reaffirmed that P-solubilization by the different PSBs is involved in the production of organic acids [63].

Table 9.
Correlations coefficients (r) of different growth, yield, physiological, and nutrients contents related traits in maize as effected by PSB alone and in mixture with different doses of chemical fertilizers and rock phosphate (* p < 0.1, ** p < 0.01 n = 4).
Our results exhibit that the PSB inoculation on the maize root differentially affects dry matter characteristics (Table 6). More pronounced effects on DM, CP, CF, and EE were noted in the PSB addition together with RNPK to sandy soil and increased digestion in the animals [62]. These results are in agreement with the results of, who stated that PSB increases the nitrogen contents in dry matter characteristics. Moreover, the CP contents are co-related with P-solubilization [64] but even more improved when PSB is involved. Moreover, the PSB triggers the growth of already-available microbes, and then the P-uptake and P-transporter gene expression in maize. In addition, it has been noted that P-transporter gene expression was increased in the presence of PSB [65].
The maize grain is a vital portion for both humans and animals for food and feeding purposes. The more solubilization of nutrients by PSB together with RNPK emphatically enhanced the macro- and micronutrients in the grain over PSB alone or RP alone or CT [48]. Our study also shows that inoculation with microbes strains increases the accumulation of macro- and micronutrients in the kernels of spring maize grown in the sandy soils [62]. Improved maize grain quality might be due to an increased photosynthetic rate (Supplementary Figure S2) and plant growth and, ultimately, the availability of nutrients [66].
This study results conclude that the incubation process (8 months) improved the soil structural (SAS, SBD, SP) and chemical properties (pH, EC, CEC, and OM) by creating favorable niches for microbial activities and, subsequently, enhancing soil water retention and nutrient availability. In addition, this microbial growth promotes the physiology and, ultimately, the yield of maize plants. Our study results are in line with those of Yadav, Jain, Mishra, Khare, Shukla, Karak and Singh [63] who stated that PSB thrived efficiency on readily available subtracts (BC) and up-scaled the mobilization of P due to secretion of organic acids (anions). This readily available subtracts for PSB strengthened the plant–microbe interaction [67] in the sandy soils. Similarly, the use of PSB alone or in combination with NPK fertilizers [46] improved plant growth and yield [45]. As far as water infiltration in the incubation soil is concerned; all the incubation treatments significantly increased the soil water retention over the control (Table 9) but higher water retention was observed in the PSB addition to sandy soil together with RNPK where infiltration of water was much slower and less leachates were collected in the bottle at the bottom. At the start of the experiment, the PSB inoculated treatments took higher water to mist the whole column over the non-inoculant treatments.
The results of the current study indicate that fresh PSB together with RNPK significantly increases soil water retention (Table 9), relative to the RP alone or PSB alone or CT (non-inoculant control). These results are supported by a number of other studies [68,69]. The difference observed in higher water retention in PSB together with RNPK might be due to slow water movement by PSB colonialization in the column (Figure 3A) against different time durations compared to other treatments. Interestingly, it was observed that the water-staying time at head height was higher over other PSB alone and RP alone treatments, resulting in slow water drainage into the column.
In the end, when we compared the impact of fresh PSB and incubated PSB on sandy soil, it was clear that fresh PSB may be hydrophobic and then hydrophilic when PSB is incubated with prolonged contact with moist sandy soil, as was observed in other past studies [63,68,70,71]. The correlation amonf differet studied parameter are given in the Table 9.
5. Conclusions
In conclusion, the inoculation of PSB together with RNPK significantly increase physiology, growth, yield, and yield related characteristics, and the total P-uptake of maize grown in sandy soil. Bacterial strains used in the present study exhibited plant growth-promoting traits i.e., the production of IAA and solubilization of P and Zn. It also improves the sandy soil selected of physico-chemical properties, dry matter, and qualitative characteristics of maize. The digestibility of maize in term of dry matter attributes is also improved by the addition of PSB.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/11/24/7049/s1, Figure S1: Effect of different treatments of PSB alone and in mixture with different doses of chemical fertilizers and rock phosphate on total chlorophyll content of maize, Figure S2: Effect of different treatments of PSB alone and in mixture with different doses of chemical fertilizers and rock phosphate on photosynthetic rate of maize, Figure S3: Effect of different treatments of PSB alone and in mixture with different doses of chemical fertilizers and rock phosphate on transpiration rate of maize.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “conceptualization, H.M.R.J., M.A., R.Q. and A.R.; methodology, H.M.R.J. and M.A.; software, A.R.; validation, H.M.R.J. and M.A.; formal analysis, M.A., A.Z. and A.R.; investigation, H.M.R.J.; resources, H.M.R.J.; data curation, H.M.R.J. and M.H.; writing—original draft preparation, H.H.R.J., M.F., M.N., M.A.C. and M.A.; writing—review and editing, S.I.Z.; visualization, S.I.Z.; supervision, H.M.R.J., M.A., M.H. and R.Q.; project administration, H.M.R.J. and M.A.; funding acquisition, H.M.R.J.”
Funding
The Authors want to extend his thank to ORIC COMSATS University Islamabad, Pakistan for their lavish funding for research activities and analysis.
Acknowledgments
We want to acknowledge the Gohar Zaman, lab attendant for their support during the analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GOP. Economic Survey of Pakistan 2016–2017; Ministry of food, Agriculture and Livestock, Fedral Berean of Statistics Islamabad, Pakistan: Islamabad, Pakistan, 2017. [Google Scholar]
- Ezawa, T.; Smith, S.E.; Smith, F.A. P metabolism and transport. Am. Fungi. Plant. Soil 2002, 244, 221–230. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant. Physiol. 2003, 127, 390–397. [Google Scholar] [CrossRef]
- Abbasi, M.; Musa, N.; Manzoor, M. Phosphorus release capacity of soluble P fertilizers and insoluble rock phosphate in response to phosphate solubilizing bacteria and poultry manure and their effect on plant growth promotion and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosci. Discssion 2015, 12, 1839–1873. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Naveed, M.; Zahir, Z.A.; Hussain, M.B.; Sessitsch, A.; Mitter, B. L-Tryptophan-dependent biosynthesis of indole-3-acetic acid (IAA) improves plant growth and colonization of maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2012, 65, 1381–1389. [Google Scholar]
- Halajnia, A.; Khorasani, R. Phosphorus fractions in calcareous soils amended with P fertilizer and cattle manure. Gerderma 2009, 150, 209–213. [Google Scholar] [CrossRef]
- Memon, M.; Mashori, N.M.; Memon, K.S.; Kakar, H. Maize dry matter yield and P uptake as influenced by rock phosphate and single super phosphate treated with farm manure. Soil Environ. 2011, 32, 130–134. [Google Scholar]
- Li, J.; Marschner, P. Phosphorus pools and plant uptake in manure-amended soil. J. Soil Sci. Plant. Nutr. 2019, 19, 175–186. [Google Scholar] [CrossRef]
- Wu, S.C.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Gerderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Yadav, H.; Fatima, R.; Sharma, A.; Mathur, S. Enhancement of applicability of rock phosphate in alkaline soils by organic compost. Appl. Soil Ecol. 2017, 113, 80–85. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Zhu, H.T.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Beech, I.B.; Paiva, M.; Caus, M.; Coutinho, C. Enzymatic activity and within biofilms of sulphate-reducing bacteria. In Biofilm Community Interactions: Chance or Necessity? Gilbert, P.G., Allison, D., Brading, M., Verran, J., Walker, J., Eds.; BioLine: Cardiff, UK, 2001; pp. 231–239. [Google Scholar]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. Biotechnology 2018, 8, 461. [Google Scholar]
- Khan, M.; Sharif, M. Solubility enhancement of phosphorus from rock phosphate through composting with poultry litter. Sarhad. J. Agric. 2012, 28, 415–420. [Google Scholar]
- Chen, Y.; Rekha, P.; Arun, A.; Shen, F.; Lai, W.A.; Young, C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidia, A. Co-inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron. Acad. Sci. Hung. 2007, 55, 315–323. [Google Scholar] [CrossRef]
- Taurian, T.; Anzuay, M.S.; Angelini, J.G.; Tonelli, M.L.; Luduena, L.; Pen, D.; Inanez, F.; Fabra, A. Phosphate-solubilizing peanut associated bacteria: Screening for plant growth-promoting activities. Plant. Soils 2010, 329, 421–431. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Silva, U.C.; Medeiros, J.D.; Leite, L.R.; Morais, D.K.; Cuadros-Orellana, S.; Oliveira, C.A.; de Paula Lana, U.G.; Gomes, E.A.; Dos Santos, V.L. Long-Term Rock Phosphate Fertilization Impacts the Microbial Communities of Maize Rhizosphere. Front. Microbiol. 2017, 8, 1266. [Google Scholar] [CrossRef]
- Laxminarayana, K. Effect of P solubilizing microorganisms on yield of rice and nutrient availability in an acid soil of Mizoram. J. Indian Soc. Soil Sci. 2005, 53, 240–243. [Google Scholar]
- Chaiharn, M.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181. [Google Scholar] [CrossRef]
- Delvasto, P.; Valverde, A.; Ballester, A.; Igual, J.; Muñoz, J.; González, F.; Blázquez, M.; García, C. Characterization of brushite as a re-crystallization product formed during bacterial solubilization of hydroxyapatite in batch cultures. Soil Biol. Biochem. 2006, 38, 2645–2654. [Google Scholar] [CrossRef]
- Gulati, A.; Rahi, P.; Vyas, P. Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr. Microbiol. 2008, 56, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Reddy, M.S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 2011, 47, 30–34. [Google Scholar] [CrossRef]
- Savini, I.; Smithson, P.; Karanja, N. Effects of added biomass, soil pH and calcium on the solubility of Minjingu phosphate rock in a Kenyan Oxisol: Einfluss von zugeführter Biomasse, Boden pH und Calcium-Löslichkeit von Minjingu Phosphatgestein in einem Keniatischen Oxisol. Arch. Agron. Soil Sci. 2006, 52, 19–36. [Google Scholar] [CrossRef]
- Da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica; Rio de Janeiro: Embrapa Solos Brasília: Brasília, Brazil, 2009; Volume 627. [Google Scholar]
- AOAC, Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002.
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Casida, L.E., Jr. Microbial metabolic activity in soil as measured by dehydrogenase determinations. Appl Env. Microbiol 1977, 34, 630–636. [Google Scholar]
- Xu, G.; Zheng, H. Analysis method handbook of soil microorganisms. In References; Agriculture Press: Beijing, China, 1986; pp. 287–289. [Google Scholar]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods Soil Analytical Part 3—Chemical Methods, SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Olsen, S.R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USA Dept. Agric. 1954, 939, 1–19. [Google Scholar]
- Haluschak, P. Laboratory Methods of Soil Analysis, Canada-Manitoba Soil Survey; Haluschak, P., Ed.; Government of Manitoba: Winnipeg, MB, Canada, 2006. [Google Scholar]
- Kay, B. Rates of change of soil structure under different cropping systems. In Advances in Soil Science 12; Springer: Berlin/Heidelberg, Germany, 1990; pp. 1–52. [Google Scholar]
- De Leenheer, L.; De Boodt, M. Determination of aggregate stability by the change in mean weight diameter. Meded. Landbouwhogesch Opzoekingstations Staat Gent. 1959, 24, 290–300. [Google Scholar]
- Chaturvedi, R.; Sankar, K. Laboratory Manual for the Physico-Chemical Analysis of Soil, Water and Plant; Wildlife Institute of India: Dehradun, India, 2006; p. 97. [Google Scholar]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; Schomberg, H.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2013, 3, 195–206. [Google Scholar]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biological Approach; McGraw-Hill College: New York, PA, USA, 1997. [Google Scholar]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Gurdeep, K.; Reddy, M.S. Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate-solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop. Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Saxena, J.; Chandra, S.; Nain, L. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J. Soil Sci. Plant Nutr. 2013, 13, 511–525. [Google Scholar]
- Jha, A.; Saxena, J.; Sharma, V. Investigation on phosphate solubilization potential of agricultural soil bacteria as affected by different phosphorus sources, temperature, salt, and pH. Commun. Soil Sci. Plant. Anal. 2013, 44, 2443–2458. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Amjad, M.; Murtaza, B.; Khan, W.U.D.; Ahmad, N. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant. Nutr. 2017, 42, 112–122. [Google Scholar] [CrossRef]
- Asghar, M.J.; Mehdi, S.S. Selection indices for yield and quality traits in sweet corn. Pak. J. Bot. 2010, 42, 775–789. [Google Scholar]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- El-Tarabily, K.A.; Nassar, A.H.; Sivasithamparam, K. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 2008, 39, 161–171. [Google Scholar] [CrossRef]
- Young, C.C.; Lai, W.A.; Shen, F.T.; Hung, M.H.; Hung, W.S.; Arun, A.B. Exploring the microbial potentially to augment soil fertility in Taiwan. In Proceedings of the 6th ESAFS International Conference: Soil Management Technology on Low Productivity and Degraded Soils, Taipei, Taiwan, 24–29 November 2003; pp. 25–27. [Google Scholar]
- Singh, H.; Reddy, S.M. Improvement of wheat and maize crops by inoculating Aspergillus spp. in alkaline soil fertilized with rock phosphate. Arch. Agron. Soil Sci. 2012, 58, 535–546. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Prasanna, R.; Kumar, A.; Pattnaik, S.; Chakravarty, K.; Shivay, Y.S.; Singh, R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur. J. Soil Biol. 2013, 55, 124–130. [Google Scholar] [CrossRef]
- Noumavo, P.A.; Kochoni, E.; Didagbé, Y.O.; Adjanohoun, A.; Allagbé, M.; Sikirou, R.; Gachomo, E.W.; Kotchoni, S.O.; Baba-Moussa, L. Effect of different plant growth promoting rhizobacteria on maize seed germination and seedling development. Am. J. Plant. Sci. 2013, 4, 1013. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Baldotto, L.E.B.; Santana, R.B.; Marciano, C.R. Initial performance of maize in response to NPK fertilization combined with Herbaspirillum seropedicae. Rev. Ceres 2012, 59, 841–849. [Google Scholar] [CrossRef]
- Biswas, J.; Ladha, J.; Dazzo, F. Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Kumar, P.; Yadava, R.; Gollen, B.; Kumar, S.; Verma, R.K.; Yadav, S. Nutritional contents and medicinal properties of wheat: A review. Life Sci. Med. Res. 2011, 22, 1–10. [Google Scholar]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef]
- Yadav, V.; Jain, S.; Mishra, P.; Khare, P.; Shukla, A.K.; Karak, T.; Singh, A.K. Amelioration in nutrient mineralization and microbial activities of sandy loam soil by short term field aged biochar. Appl. Soil Ecol. 2019, 138, 144–155. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant. Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Drijber, R.; Zhang, J.; Li, X. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Głab, T.P.J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Gerderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Arif, M.S.; Riaz, M.; Shahzad, S.M.; Yasmeen, T.; Akhtar, M.J.; Riaz, M.A.; Jassey, V.E.; Bragazza, L.; Buttler, A. Associative interplay of plant growth promoting rhizobacteria (Pseudomonas aeruginosa QS40) with nitrogen fertilizers improves sunflower (Helianthus annuus L.) productivity and fertility of aridisol. Appl. Soil Ecol. 2016, 108, 238–247. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Addition of biochar to a sandy desert soil: Effect on crop growth, water retention and selected properties. Agronomy 2019, 9, 327. [Google Scholar] [CrossRef]
- Alshankiti, A.; Gill, S. Integrated plant nutrient management for sandy soil using chemical fertilizers, compost, biochar and biofertilizers. J. Arid Studis. 2016, 26, 101–106. [Google Scholar]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De-Pasquale, C. Effect of biochar on the physical and structural properties of a desert sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Uzoma, K.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil. Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).