Median to Strong Rainfall Intensity Favors Carbon Sink in a Temperate Grassland Ecosystem in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Measurement of CO2, and Abiotic and Biotic Variables
2.4. Eddy Flux Measurement (EC)
2.5. Statistical Analysis
3. Results
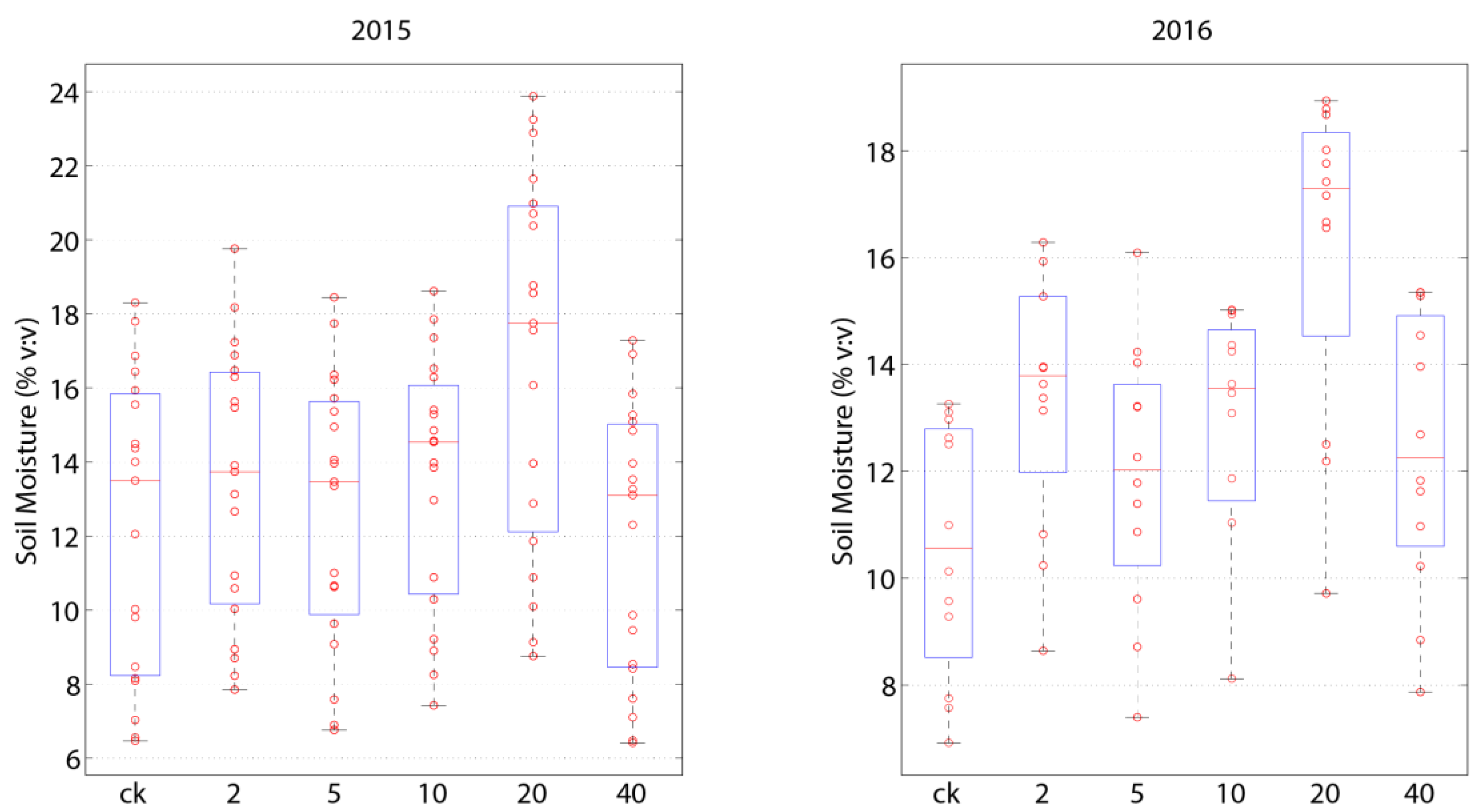
3.1. Influence of Rainfall Additions on Abiotic Factors
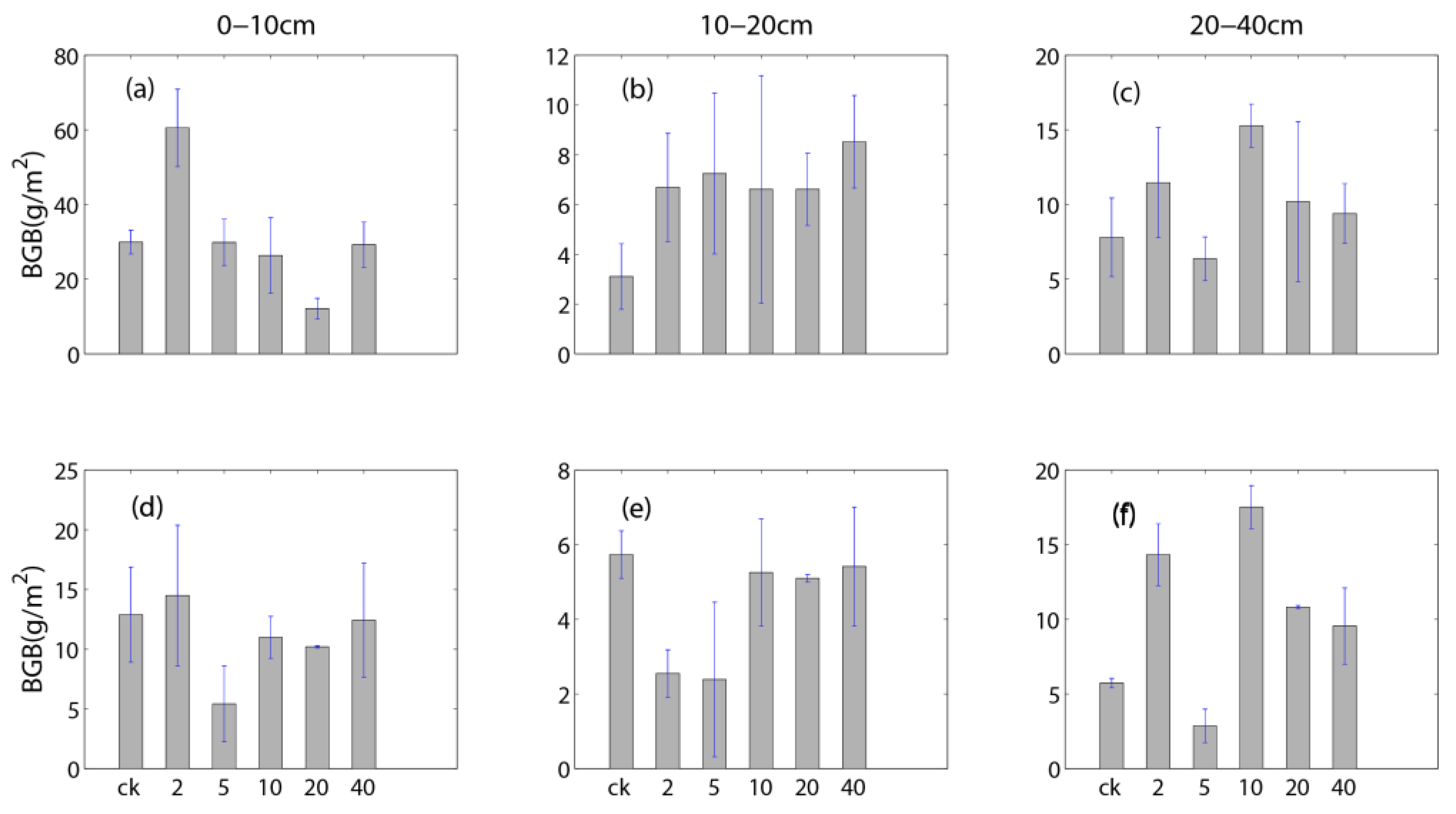
3.2. Influences of Rainfall Additions on Plant Biomass
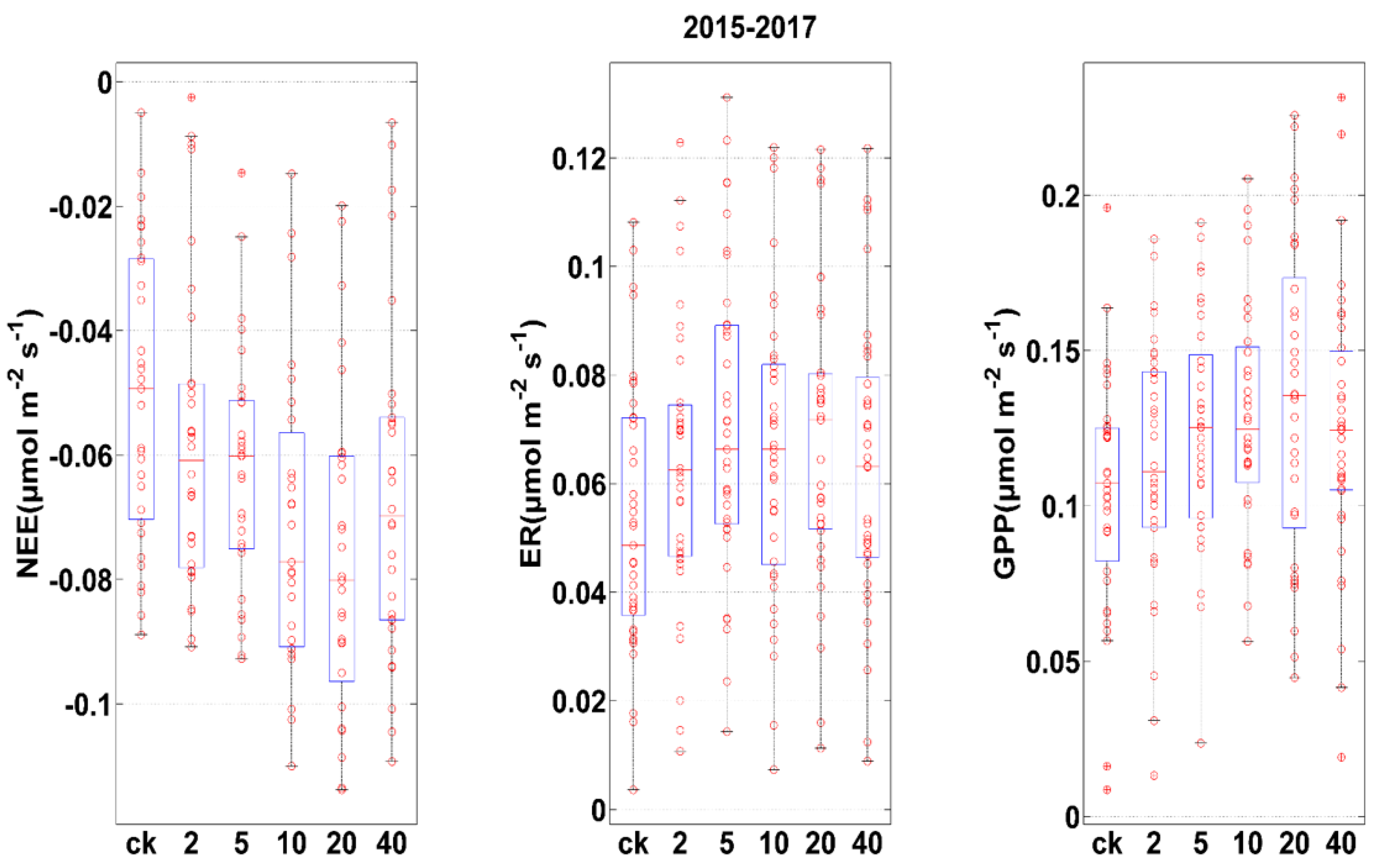
3.3. Influences of Rainfall Additions on C Balance
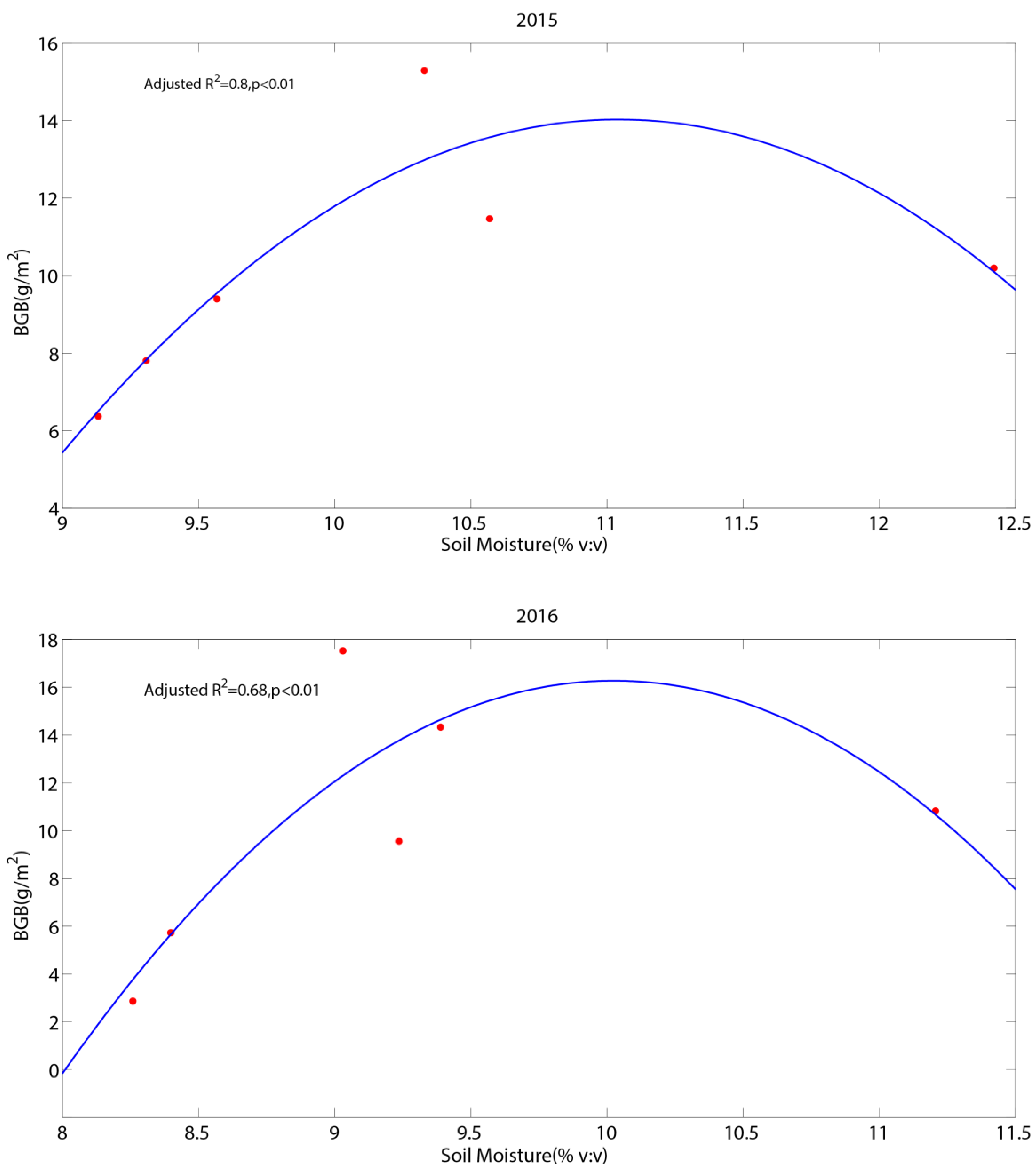
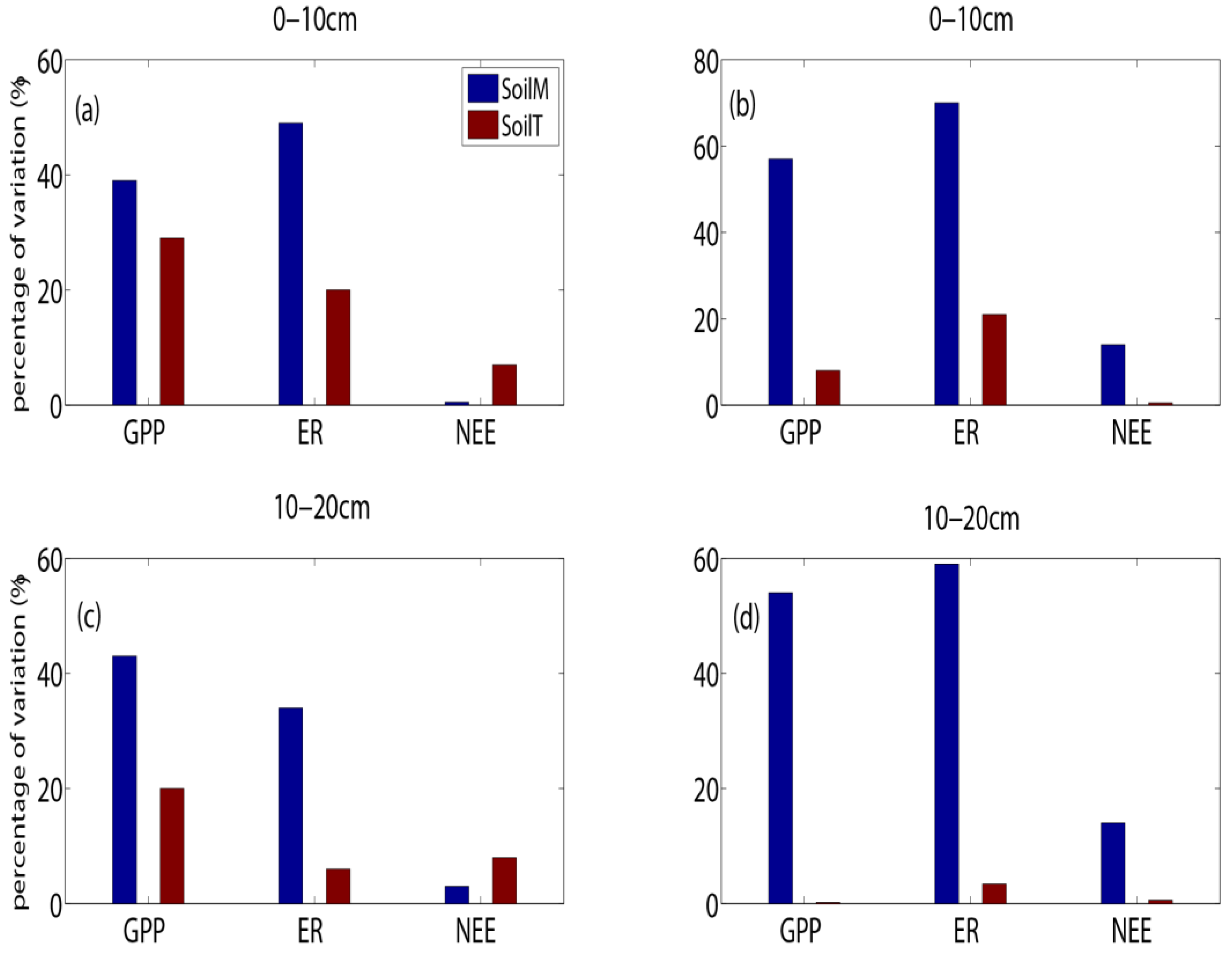
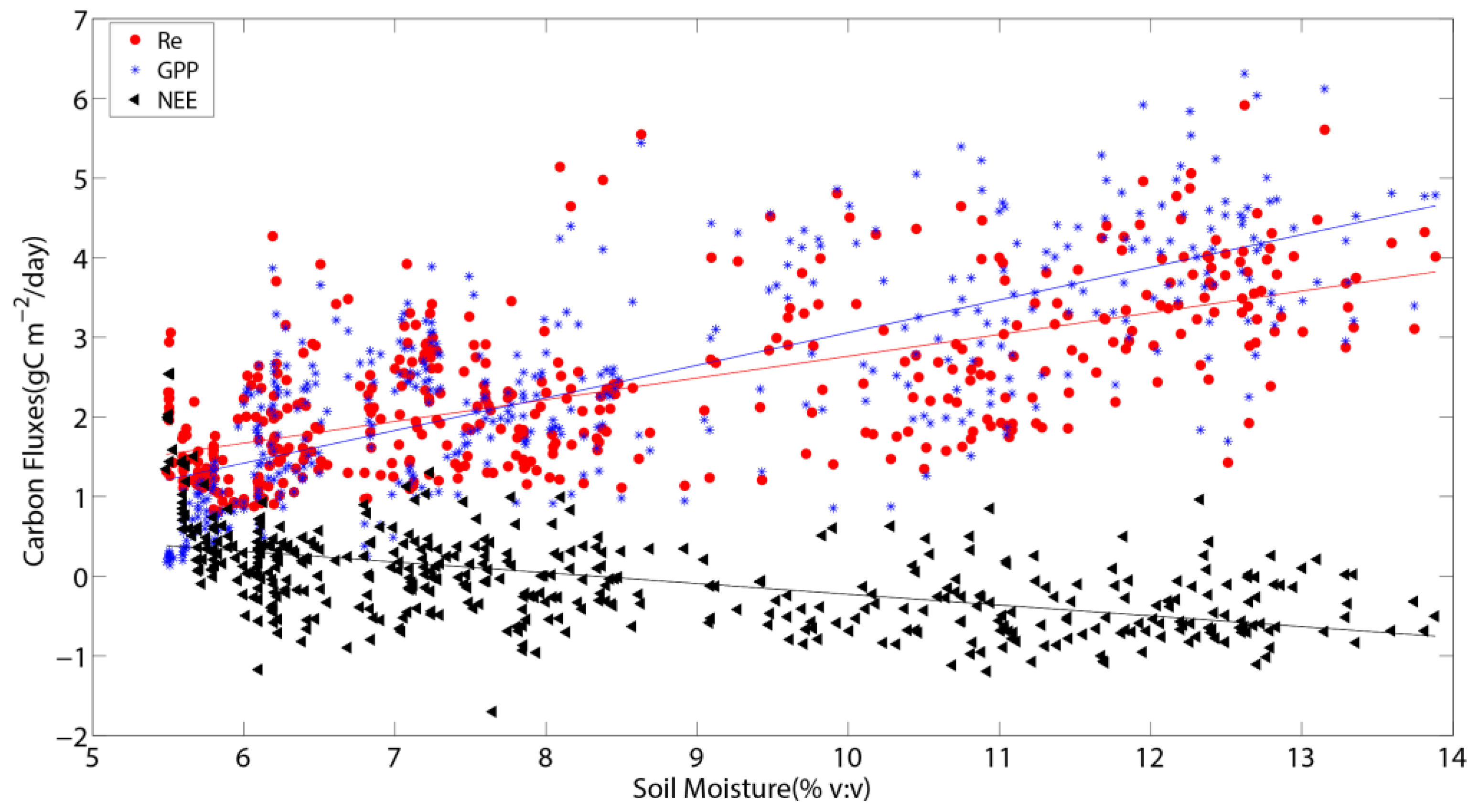
3.4. The Relationship Between Abiotic Factors and Carbon Fluxes
4. Discussions
4.1. Effect of Change in Rainfall Patterns on Carbon Fluxes
4.2. Effects of Change in Precipitation Patterns on Biomass
4.3. Different Responses of Carbon Fluxes and Root Biomass
4.4. The Effect of Soil Moisture on Carbon Fluxes
4.5. Implications for Grassland C Cycling to Precipitation Patterns Change
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huxman, T.E.; Cable, J.M.; Ignace, D.D.; Eilts, J.A.; English, N.B.; Weltzin, J.; Williams, D.G. Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: The role of native versus non-native grasses and soil texture. Oecologia 2004, 141, 295–305. [Google Scholar] [CrossRef]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef]
- IPCC Climate Change. The Physical Science Basis; Cambridge Univ. Press: Cambridge, UK, 2007. [Google Scholar]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Rasmussen, R.M.; Parsons, D.B. The changing character of precipitation. Bull. Am. Meteorol. Soc. 2003, 84, 1205–1217. [Google Scholar] [CrossRef]
- Tan, K.; Ciais, P.; Piao, S.; Wu, X.; Tang, Y.; Vuichard, N.; Liang, S.; Fang, J. Application of the ORCHIDEE global vegetation model to evaluate biomass and soil carbon stocks ofQinghai-Tibetan grasslands. Glob. Biogeochem. Cycles 2010, 24, GB1013. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Wever, L.A.; Carlson, P.J. Seasonal and inter-annual variation in carbon dioxide exchange and carbon balance in a northern temperate grassland. Glob. Chang. Biol 2012, 8, 599–615. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den Berg, E.; Reich, P. DIVISION S-5 NOTES. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Ahlstrom, A. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science (80-) 2015, 120, 4503–4518. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Hu, Z.; Zhao, W.; Yu, G.; Sun, X.; Li, L.; Liang, N.; Bai, W. Responses of gross primary productivity to different sizes of precipitation events in a temperate grassland ecosystem in Inner Mongolia, China. J. Arid Land 2016, 8, 36–46. [Google Scholar] [CrossRef]
- Guo, Q.; Hu, Z.M.; Li, S.G.; Yu, G.R.; Sun, X.M.; Li, L.H.; Liang, N.S.; Bai, W.M. Exogenous N addition enhances the responses of gross primary productivity to individual precipitation events in a temperate grassland. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, Q.; Li, S.; Piao, S.; Knapp, A.K.; Ciais, P.; Li, X.; Yu, G. Shifts in the dynamics of productivity signal ecosystem state transitions at the biome-scale. Ecol. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. dependence of carbon sequestration on the differential response of ecosystem photosynthesis and respiration to rain pulses in a semiarid steppe. GCB 2009, 110, 115–126. [Google Scholar] [CrossRef]
- Thomey, M.L.; Collins, S.L.; Vargas, R.; Johnson, J.E.; Brown, R.F.; Natvig, D.O.; Friggens, M.T. Effect of precipitation variability on net primary production and soil respiration in a Chihuahuan Desert grassland. Glob. Chang. Biol. 2011, 17, 1505–1515. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Chen, Q.; Lin, S.; Giese, M.; Brueck, H. Resource manipulation effects on net primary production, biomass allocation and rain-use efficiency of two semiarid grassland sites in Inner Mongolia, China. Oecologia 2011, 165, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.B.; Zhou, C.T.; Liu, W.J.; Li, L.F.; Kang, X.M.; Jiang, L.L.; Cui, X.Y.; Wang, Y.F.; Zhou, X.Q.; Xu, C.Y. Aboveground net primary productivity and carbon balance remain stable under extreme precipitation events in a semiarid steppe ecosystem. Agric. For. Meteorol. 2017, 240–241, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Z.; Biederman, J.A.; Xu, C.; Xu, Z.; Che, R.; Wang, Y.; Cui, X.; Hao, Y. Ecological responses to heavy rainfall depend on seasonal timing and multi-year recurrence. New Phytol. 2019. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Mei, X.; Cui, X. The response of ecosystem CO2 exchange to small precipitation pulses over a temperate steppe. Plant Ecol. 2010, 209, 335–347. [Google Scholar] [CrossRef]
- Bai, W.; Wan, S.; Niu, S.; Liu, W.; Chen, Q.; Wang, Q.; Zhang, W.; Han, X.; Li, L. Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: Implications for ecosystem C cycling. Glob. Chang. Biol. 2010, 16, 1306–1316. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef]
- Potts, D.L.; Huxman, T.E.; Enquist, B.J.; Weltzin, J.F.; Williams, D.G. Resilience and resistance of ecosystem functional response to a precipitation pulse in a semi-arid grassland. J. Ecol. 2006, 94, 23–30. [Google Scholar] [CrossRef]
- Bae, K.; Koo, D.; Timothy, L.; Lee, Y. Seasonal variation of soil respiration rates in a secondary forest and agroforestry systems. Agrofor. Syst. 2013, 87, 131–139. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO 2 sink may be largely offset by stimulated N2O and CH 4 emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef]
- Niu, S.; Luo, Y.; Fei, S.; Montagnani, L.; Bohrer, G.; Janssens, I.A.; Gielen, B.; Rambal, S.; Moors, E.; Matteucci, G. Seasonal hysteresis of net ecosystem exchange in response to temperature change: Patterns and causes. Glob. Chang. Biol. 2011, 17, 3102–3114. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, G.; Fu, Y.; Sun, X.; Li, Y.; Shi, P.; Wang, Y.; Zheng, Z. Effects of vegetation control on ecosystem water use efficiency within and among four grassland ecosystems in China. Glob. Chang. Biol. 2008, 14, 1609–1619. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.; Gong, J.; Zhang, Y.; Wu, B.; Qin, S.; Peltola, H. Multi-scale dynamics and environmental controls on net ecosystem CO2exchange over a temperate semiarid shrubland. Agric. For. Meteorol. 2018, 259, 250–259. [Google Scholar] [CrossRef]
- Yuan, W.; Piao, S.; Qin, D.; Dong, W.; Xia, J.; Lin, H.; Chen, M. Influence of Vegetation Growth on the Enhanced Seasonality of Atmospheric CO2. Glob. Biogeochem. Cycles 2018, 32, 32–41. [Google Scholar] [CrossRef]
- Davidson, E.A.; Samanta, S.; Caramori, S.S.; Savage, K. The Dual Arrhenius and Michaelis-Menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob. Chang. Biol. 2012, 18, 371–384. [Google Scholar] [CrossRef]
- Parton, W.; Morgan, J.; Smith, D.; Del Grosso, S.; Prihodko, L.; Lecain, D.; Kelly, R.; Lutz, S. Impact of precipitation dynamics on net ecosystem productivity. Glob. Chang. Biol. 2012, 18, 915–927. [Google Scholar] [CrossRef]
- Yuhui, W.; Jiquan, C.; Guangsheng, Z.; Changliang, S.; Jun, C.; Yu, W.; Jianmin, S. Predominance of precipitation event controls ecosystem CO2exchange in an Inner Mongolian desert grassland, China. J. Clean. Prod. 2018, 197, 781–793. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. A Synthesis is Emerging between Biodiversity-Ecosystem Function and Ecological Resilience Research: Reply to Mori. Trends Ecol. Evol. 2016, 31, 89–92. [Google Scholar] [CrossRef][Green Version]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Rastetter, E.B.; King, A.W.; Cosby, B.J.; Hornberger, G.M.; Neill, R.V.O.; Hobbie, J.E. Aggregating Fine-Scale Ecological Knowledge to Model Coarser-Scale Attributes of Ecosystems Published by: Ecological Society of America Stable URL: http://www.jstor.org/stable/1941889 Your use of the JSTOR archive indicates your acceptance of JSTOR’ s. America (N. Y.) 1992, 2, 55–70. [Google Scholar]
- Harpole, W.S.; Potts, D.L.; Suding, K.N. Ecosystem responses to water and nitrogen amendment in a California grassland. Glob. Chang. Biol. 2007, 13, 2341–2348. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat. Geosci. 2014, 7, 920–924. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.; Xia, J.; Zhang, Z.; Yang, H.; Wan, S. Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Glob. Chang. Biol. 2010, 16, 144–155. [Google Scholar] [CrossRef]
- Xu, W.; Yuan, W. Responses of microbial biomass carbon and nitrogen to experimental warming: A meta-analysis. Soil Biol. Biochem. 2017, 115, 265–274. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; De Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Bai, Y.; Xingguo, H.; Jianguo, W.; Zuozhong, C.; Linghao, L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 43, 181–184. [Google Scholar] [CrossRef]
- Green, J.K.; Seneviratne, S.I.; Berg, A.M.; Findell, K.L.; Lawrence, D.M.; Gentine, P. Large influence of soil moisture variability on long-term terrestrial carbon uptake. Nature 2019. accepted. [Google Scholar] [CrossRef]
- Williams, C.A.; Hanan, N.; Scholes, R.J.; Kutsch, W. Complexity in water and carbon dioxide fluxes following rain pulses in an African savanna. Oecologia 2009, 161, 469–480. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, S.; Luo, Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob. Chang. Biol. 2007, 13, 761–775. [Google Scholar]
- Wan, S.; Luo, Y. Substrate regulation of soil respiration in a tallgrass prairie: Results of a clipping and shading experiment. Glob. Biogeochem. Cycles 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garrten, C.T.; Andrews, J.A. Separating Root and Soil Microbial Contributions to Soil Respiration: A Review of Methods and Observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Reich, P.B.; Hungate, B.A.; Luo, Y. Carbon-Nitrogen Interactions in Terrestrial Ecosystems in Response to Rising Atmospheric Carbon Dioxide. Ann. Rev. Ecol. Evol. Syst. 2006, 37, 611–636. [Google Scholar] [CrossRef]
| Rainfall Treatment | 2 mm | 5 mm | 10 mm | 20 mm | 40 mm |
|---|---|---|---|---|---|
| Number | 40 | 12 | 6 | 3 | 2 |
| Frequency | 2 days | 3 days | 7 days | 15 days | 1 month |
| Start date | June 1 | June 1 | June 1 | June 1 | Mid-July |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, G.; Hu, Z.; Guo, Q.; Di, K.; Li, S. Median to Strong Rainfall Intensity Favors Carbon Sink in a Temperate Grassland Ecosystem in China. Sustainability 2019, 11, 6376. https://doi.org/10.3390/su11226376
Hao G, Hu Z, Guo Q, Di K, Li S. Median to Strong Rainfall Intensity Favors Carbon Sink in a Temperate Grassland Ecosystem in China. Sustainability. 2019; 11(22):6376. https://doi.org/10.3390/su11226376
Chicago/Turabian StyleHao, Guangcun, Zhongmin Hu, Qun Guo, Kai Di, and Shenggong Li. 2019. "Median to Strong Rainfall Intensity Favors Carbon Sink in a Temperate Grassland Ecosystem in China" Sustainability 11, no. 22: 6376. https://doi.org/10.3390/su11226376
APA StyleHao, G., Hu, Z., Guo, Q., Di, K., & Li, S. (2019). Median to Strong Rainfall Intensity Favors Carbon Sink in a Temperate Grassland Ecosystem in China. Sustainability, 11(22), 6376. https://doi.org/10.3390/su11226376




