Changes in Relative Fish Density Around a Deployed Tidal Turbine during on-Water Activities
Abstract
1. Introduction
2. Materials and Methods
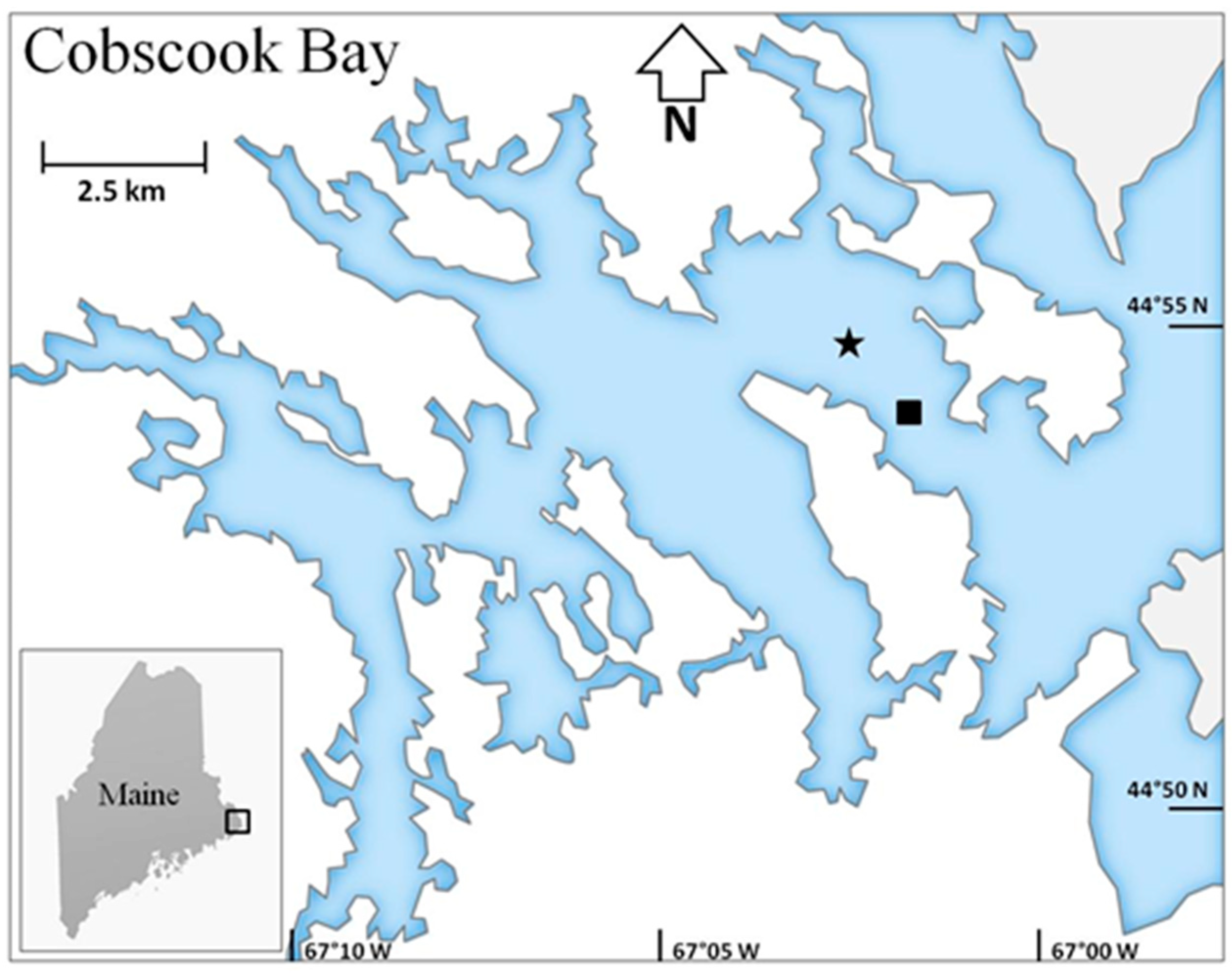
2.1. Data Collection and Study Area
2.2. Data Processing and Analysis
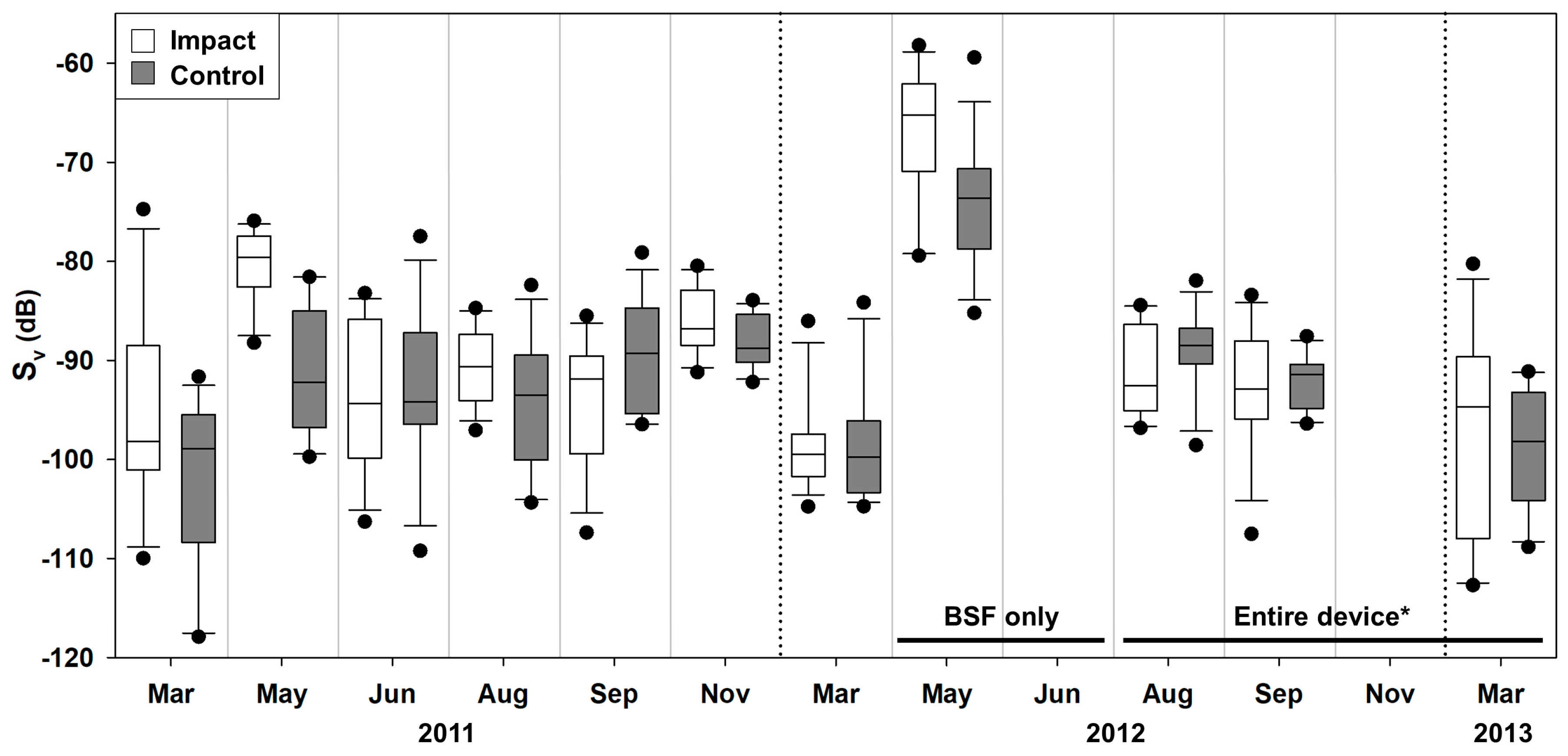
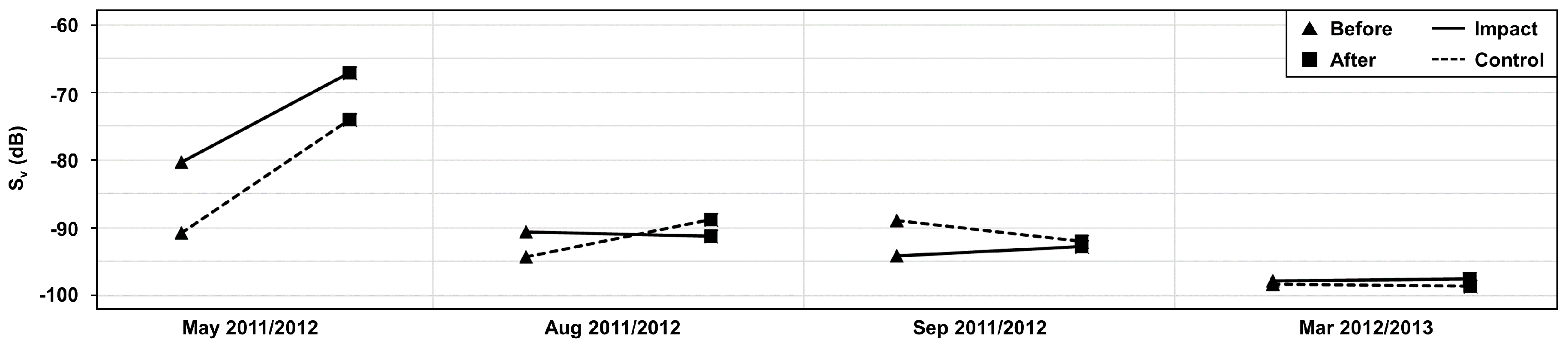
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Willman, L. Feasibility analysis of offshore renewables penetrating local energy systems in remote oceanic areas–a case study of emissions from an electricity system with tidal power in Southern Alaska. Appl. Energy 2014, 117, 42–53. [Google Scholar] [CrossRef]
- Roberts, A.; Thomas, B.; Sewell, P.; Khan, Z.; Balmain, S.; Gillman, J. Current tidal power technologies and their suitability for applications in coastal and Marine areas. J. Ocean Eng. Mar. Energy 2016, 2, 227–245. [Google Scholar] [CrossRef]
- Zhou, Z.; Benbouzid, M.; Charpentier, J.-F.; Scuiller, F.; Tang, T. Developments in large marine current turbine technologies–A review. Renew. Sustain. Energy Rev. 2017, 71, 852–858. [Google Scholar] [CrossRef]
- Copping, A.; Sather, N.; Hanna, L.; Whiting, J.; Zydlewski, G.; Staines, G.; Gill, A.; Hutchison, I.; O′Hagan, A.; Simas, T. Annex IV 2016 State of the Science Report: Environmental effects of Marine renewable energy development around the world. Available online: https://tethys.pnnl.gov/publications/state-of-the-science-2016 Ocean Energy Systems (accessed on 8 October 2019).
- Tethys. Available online: http://www.tethys.pnnl.gov (accessed on 8 October 2019).
- Jansujwicz, J.S.; Johnson, T.R. The Maine Tidal Power Initiative: Transdisciplinary Sustainability Science Research for the responsible development of tidal power. Sustain. Sci. 2015, 10, 75–86. [Google Scholar] [CrossRef]
- Gregory, R.; Ohlson, D.; Arvai, J. Deconstructing adaptive Management: Criteria for applications to environmental Management. Ecol. Appl. 2006, 16, 2411–2425. [Google Scholar] [CrossRef]
- Forward, R.B.; Tankersley, R.A. Selective tidal-stream transport of Marine animals. Ocean Mar. Biol. 2001, 39, 305–353. [Google Scholar]
- Shields, M.; Ford, A.; Woolf, D. Ecological considerations for tidal energy development in Scotland. In Proceedings of the 10th World Renewable Energy Conference, Glasgow, Scotland, 19–25 July 2008. [Google Scholar]
- Boehlert, G.W.; Gill, A.B. Environmental and ecological effects of ocean renewable energy development: A current synthesis. Oceanography 2010, 23, 68–81. [Google Scholar] [CrossRef]
- Hammar, L.; Andersson, S.; Eggertsen, L.; Haglund, J.; Gullström, M.; Ehnberg, J.; Molander, S. Hydrokinetic turbine effects on fish swimming behaviour. PLoS ONE 2013, 8, e84141. [Google Scholar] [CrossRef]
- Broadhurst, M.; Barr, S.; Orme, C.D.L. In-situ ecological interactions with a deployed tidal energy device; an observational pilot study. Ocean Coast. Manag. 2014, 99, 31–38. [Google Scholar] [CrossRef]
- Viehman, H.A.; Zydlewski, G.B. Fish interactions with a commercial-scale tidal energy device in the natural environment. Estuaries Coasts 2015, 38, 241–252. [Google Scholar] [CrossRef]
- Stokesbury, M.J.; Logan-Chesney, L.M.; McLean, M.F.; Buhariwalla, C.F.; Redden, A.M.; Beardsall, J.W.; Broome, J.E.; Dadswell, M.J. Atlantic sturgeon spatial and temporal distribution in Minas Passage, Nova Scotia, Canada, a region of future tidal energy extraction. PLoS ONE 2016, 11, e0158387. [Google Scholar] [CrossRef] [PubMed]
- Bevelhimer, M.; Scherelis, C.; Colby, J.; Adonizio, M.A. Hydroacoustic assessment of behavioral responses by fish passing near an operating tidal turbine in the east river, New York. Trans. Am. Fish. Soc. 2017, 146, 1028–1042. [Google Scholar] [CrossRef]
- Shen, H.; Zydlewski, G.B.; Viehman, H.A.; Staines, G. Estimating the probability of fish encountering a marine hydrokinetic device. Renew. Energy 2016, 97, 746–756. [Google Scholar] [CrossRef]
- Fraser, S.; Williamson, B.J.; Nikora, V.; Scott, B.E. Fish distributions in a tidal channel indicate the behavioural impact of a Marine renewable energy installation. Energy Rep. 2018, 4, 65–69. [Google Scholar] [CrossRef]
- Wiesebron, L.E.; Horne, J.K.; Scott, B.E.; Williamson, B.J. Comparing nekton distributions at two tidal energy sites suggests potential for generic environmental monitoring. Int. J. Mar. Energy 2016, 16, 235–249. [Google Scholar] [CrossRef]
- Copping, A.; Battey, H.; Brown-Saracino, J.; Massaua, M.; Smith, C. An international assessment of the environmental effects of Mar. energy development. Ocean Coast. Manag. 2014, 99, 3–13. [Google Scholar] [CrossRef]
- Trump, C.L.; Leggett, W.C. Optimum swimming speeds in fish: The problem of currents. Can. J. Fish. Aquat. Sci. 1980, 37, 1086–1092. [Google Scholar] [CrossRef]
- Weihs, D. Tidal stream transport as an efficient method for migration. ICES J. Mar. Sci. 1978, 38, 92–99. [Google Scholar] [CrossRef]
- Vieser, J.D. Collaborative Research on Finfish, Their Distribution, and Diversity in Cobscook Bay, Maine. Master′s Thesis, University of Maine, Orono, ME, USA, 2014. [Google Scholar]
- Redden, A.M.; Broome, J.; Keyser, F.; Stokesbury, M.; Bradford, R.; Gibson, J.; Halfyard, E. Use of animal tracking technology to assess potential risks of tidal turbine interaction with fish. In Proceedings of the 2nd International Conference on Environmental Interactions of Mar. Renewable Energy Technologies (EIMR2014), Outer Hebrides, Scotland, 28 April–2 May 2014. [Google Scholar]
- Keyser, F.M.; Broome, J.E.; Bradford, R.G.; Sanderson, B.; Redden, A.M. Winter presence and temperature-related diel vertical migration of striped bass (Morone saxatilis) in an extreme high-flow passage in the inner Bay of Fundy. Can. J. Fish. Aquat. Sci. 2016, 73, 1777–1786. [Google Scholar] [CrossRef]
- Broome, J.E.; Redden, A.M.; Keyser, F.M.; Stokesbury, M.J.W.; Bradford, R.G. Passive acoustic telemetry detection of Striped Bass at the FORCE TISEC test site in Minas Passage, Nova Scotia, Canada. In Proceedings of the 3rd Mar. Energy Technology Symposium, Washington, DC, USA, 27–29 April 2015; pp. 1–5. [Google Scholar]
- Viehman, H.A.; Zydlewski, G.B.; McCleave, J.D.; Staines, G.J. Using hydroacoustics to understand fish presence and vertical distribution in a tidally dynamic region targeted for energy extraction. Estuaries Coasts 2015, 38, 215–226. [Google Scholar] [CrossRef]
- Simmonds, J.; MacLennan, D.N. Fisheries Acoustics: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0-470-99529-7. [Google Scholar]
- Urmy, S.S.; Horne, J.K.; Barbee, D.H. Measuring the vertical distributional variability of pelagic fauna in Monterey Bay. ICES J. Mar. Sci. 2012, 69, 184–196. [Google Scholar] [CrossRef]
- Staines, G.; Zydlewski, G.; Viehman, H.; Shen, H.; McCleave, J. Changes in vertical fish distributions near a hydrokinetic device in Cobscook Bay, Maine, USA. In Proceedings of the 11th European Wave and Tidal Energy Conference (EWTEC2015), Nantes, France, 6–11 September 2015; pp. 6–11. [Google Scholar]
- Matveev, V.F.; Steven, A.D.L. The effects of salinity, turbidity and flow on fish biomass estimated acoustically in two tidal rivers. Mar. Freshw. Res. 2014, 65, 267–274. [Google Scholar] [CrossRef]
- Park, J.M.; Huh, S.-H.; Baeck, G.W. Temporal variations of fish assemblage in the surf zone of the Nakdong River Estuary, southeastern Korea. Anim. Cells Syst. 2015, 19, 350–358. [Google Scholar] [CrossRef]
- Viehman, H.A.; Zydlewski, G.B. Multi-scale temporal patterns in fish presence in a high-velocity tidal channel. PLoS ONE 2017, 12, e0176405. [Google Scholar] [CrossRef]
- Stanley, D.R.; Wilson, C.A. Seasonal and spatial variation in the abundance and size distribution of fishes associated with a petroleum platform in the northern Gulf of Mexico. Can. J. Fish. Aquat. Sci. 1997, 54, 1166–1176. [Google Scholar]
- Handegard, N.O.; Michalsen, K.; Tjøstheim, D. Avoidance behaviour in cod (Gadus morhua) to a bottom-trawling vessel. Aquat. Living Resour. 2003, 16, 265–270. [Google Scholar] [CrossRef]
- Draštík, V.; Kubečka, J. Fish avoidance of acoustic survey boat in shallow waters. Fish. Res. 2005, 72, 219–228. [Google Scholar] [CrossRef]
- Smith, E.P.; Orvos, D.R.; Cairns Jr, J. Impact assessment using the before-after-control-impact (BACI) model: Concerns and comments. Can. J. Fish. Aquat. Sci. 1993, 50, 627–637. [Google Scholar] [CrossRef]
- Marks, J.C.; Haden, G.A.; O′Neill, M.; Pace, C. Effects of flow restoration and exotic species removal on recovery of native fish: Lessons from a dam decommissioning. Restor. Ecol. 2010, 18, 934–943. [Google Scholar] [CrossRef]
- Echoview Software, Version 8.0.; Echoview Software Pty Ltd.: Hobart, Australia, 2015.
- Anderson, C.I.H.; Brierley, A.S.; Armstrong, F. Spatio-temporal variability in the distribution of epi-and meso-pelagic acoustic backscatter in the Irminger Sea, North Atlantic, with implications for predation on Calanus finmarchicus. Mar. Biol. 2005, 146, 1177–1188. [Google Scholar] [CrossRef]
- Ryan, T.E.; Downie, R.A.; Kloser, R.J.; Keith, G. Reducing bias due to noise and attenuation in open-ocean echo integration data. ICES J. Mar. Sci. 2015, 72, 2482–2493. [Google Scholar] [CrossRef]
- Madureira, L.S.; Everson, I.; Murphy, E.J. Interpretation of acoustic data at two frequencies to discriminate between Antarctic krill (Euphausia superba Dana) and other scatterers. J. Plankton Res. 1993, 15, 787–802. [Google Scholar] [CrossRef]
- Kang, M.; Furusawa, M.; Miyashita, K. Effective and accurate use of difference in mean volume backscattering strength to identify fish and plankton. ICES J. Mar. Sci. 2002, 59, 794–804. [Google Scholar] [CrossRef]
- Korneliussen, R.J.; Ona, E. An operational system for processing and visualizing multi-frequency acoustic data. ICES J. Mar. Sci. 2002, 59, 293–313. [Google Scholar] [CrossRef]
- Korneliussen, R.J.; Ona, E. Verified Acoustic Identification of Atlantic Mackerel; ICES: Sale, UK, 2004. [Google Scholar]
- Korneliussen, R.J.; Heggelund, Y.; Eliassen, I.K.; Johansen, G.O. Acoustic species identification of schooling fish. ICES J. Mar. Sci. 2009, 66, 1111–1118. [Google Scholar] [CrossRef]
- Foote, K.G. Linearity of fisheries acoustics, with addition theorems. J. Acoust. Soc. Am. 1983, 73, 1932–1940. [Google Scholar] [CrossRef]
- Misund, O.A. Sonar Observations of Schooling Herring: School Dimensions, Swimming Behaviour, and Avoidance of Vessel and Purse Seine; ICES: Sale, UK, 1990. [Google Scholar]
- Stanley, D.R.; Wilson, C.A. Effect of scuba divers on fish density and target strength estimates from stationary dual-beam hydroacoustics. Trans. Am. Fish. Soc. 1995, 124, 946–949. [Google Scholar] [CrossRef]
- Crain, C.M.; Kroeker, K.; Halpern, B.S. Interactive and cumulative effects of multiple human stressors in Mar. systems. Ecol. Lett. 2008, 11, 1304–1315. [Google Scholar] [CrossRef]
- Feist, B.E.; Anderson, J.J.; Miyamoto, R. Potential impacts of pile driving on juvenile pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon behavior and distribution; University of Washington: Seattle, WA, USA, 1991. [Google Scholar]
- Perrow, M.R.; Gilroy, J.J.; Skeate, E.R.; Tomlinson, M.L. Effects of the construction of Scroby Sands offshore wind farm on the prey base of Little tern Sternula albifrons at its most important UK colony. Mar. Pollut. Bull. 2011, 62, 1661–1670. [Google Scholar] [CrossRef]
- Rudstam, L.G.; Lindem, T.; Hansson, S. Density and in situ target strength of herring and sprat: A comparison between two methods of analyzing single-beam sonar data. Fish. Res. 1988, 6, 305–315. [Google Scholar] [CrossRef]
- Gurshin, C.W.; Howell, W.H.; Jech, J.M. Synoptic acoustic and trawl surveys of spring-spawning Atlantic cod in the Gulf of Maine cod spawning protection area. Fish. Res. 2013, 141, 44–61. [Google Scholar] [CrossRef]
- Doty, A.C.; Martin, A.P. Assessment of bat and avian mortality at a pilot wind turbine at Coega, Port Elizabeth, Eastern Cape, South Africa. N. Z. J. Zool. 2013, 40, 75–80. [Google Scholar] [CrossRef]
- Kuvlesky Jr, W.P.; Brennan, L.A.; Morrison, M.L.; Boydston, K.K.; Ballard, B.M.; Bryant, F.C. Wind energy development and wildlife conservation: Challenges and opportunities. J. Wildl. Manag. 2007, 71, 2487–2498. [Google Scholar] [CrossRef]
- Neill, S.M.; Ylitalo, G.M.; West, J.E. Energy content of Pacific salmon as prey of northern and southern resident killer whales. Endanger. Species Res. 2014, 25, 265–281. [Google Scholar]
- Gill, A.B. Offshore renewable energy: Ecological implications of generating electricity in the coastal zone. J. Appl. Ecol. 2005, 42, 605–615. [Google Scholar] [CrossRef]

| Month | Before Device Installation | After Device Installation | Device Status in “After” Survey |
|---|---|---|---|
| May | 2011 | 2012 | BSF present Turbine absent Not generating |
| August | 2011 | 2012 | Turbine present Not rotating Not generating |
| September | 2011 | 2012 | Turbine present Rotating generating |
| March | 2012 | 2013 | Turbine present Rotating Not generating |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staines, G.; Zydlewski, G.; Viehman, H. Changes in Relative Fish Density Around a Deployed Tidal Turbine during on-Water Activities. Sustainability 2019, 11, 6262. https://doi.org/10.3390/su11226262
Staines G, Zydlewski G, Viehman H. Changes in Relative Fish Density Around a Deployed Tidal Turbine during on-Water Activities. Sustainability. 2019; 11(22):6262. https://doi.org/10.3390/su11226262
Chicago/Turabian StyleStaines, Garrett, Gayle Zydlewski, and Haley Viehman. 2019. "Changes in Relative Fish Density Around a Deployed Tidal Turbine during on-Water Activities" Sustainability 11, no. 22: 6262. https://doi.org/10.3390/su11226262
APA StyleStaines, G., Zydlewski, G., & Viehman, H. (2019). Changes in Relative Fish Density Around a Deployed Tidal Turbine during on-Water Activities. Sustainability, 11(22), 6262. https://doi.org/10.3390/su11226262





