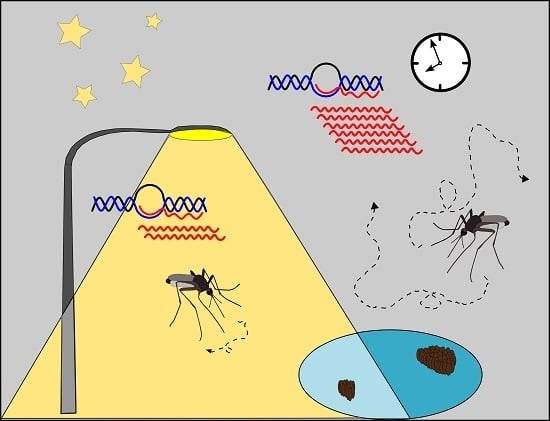
Artificial Light at Night Influences Clock-Gene Expression, Activity, and Fecundity in the Mosquito Culex pipiens f. molestus
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Colony
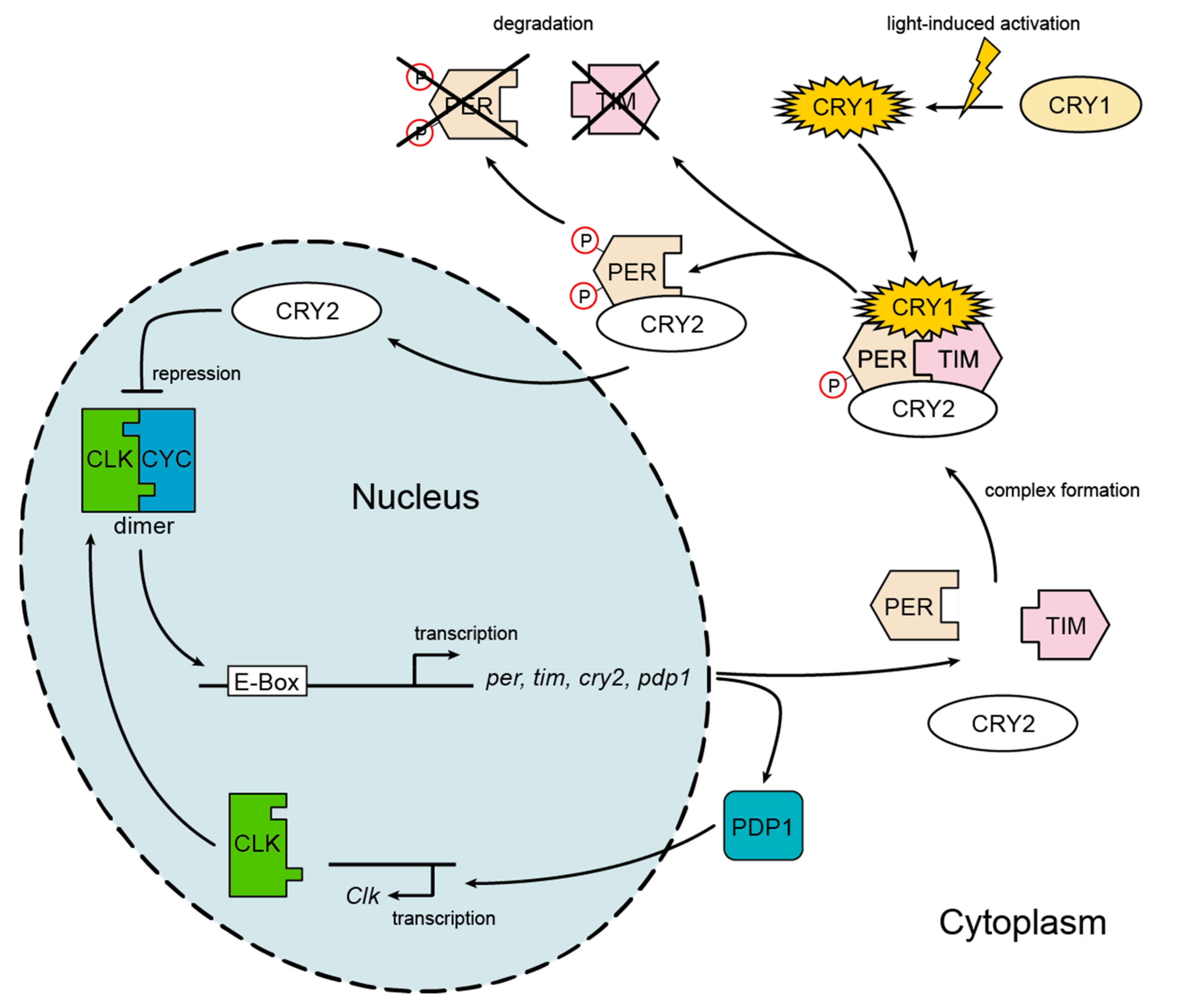
2.2. Expression of Circadian Clock Genes
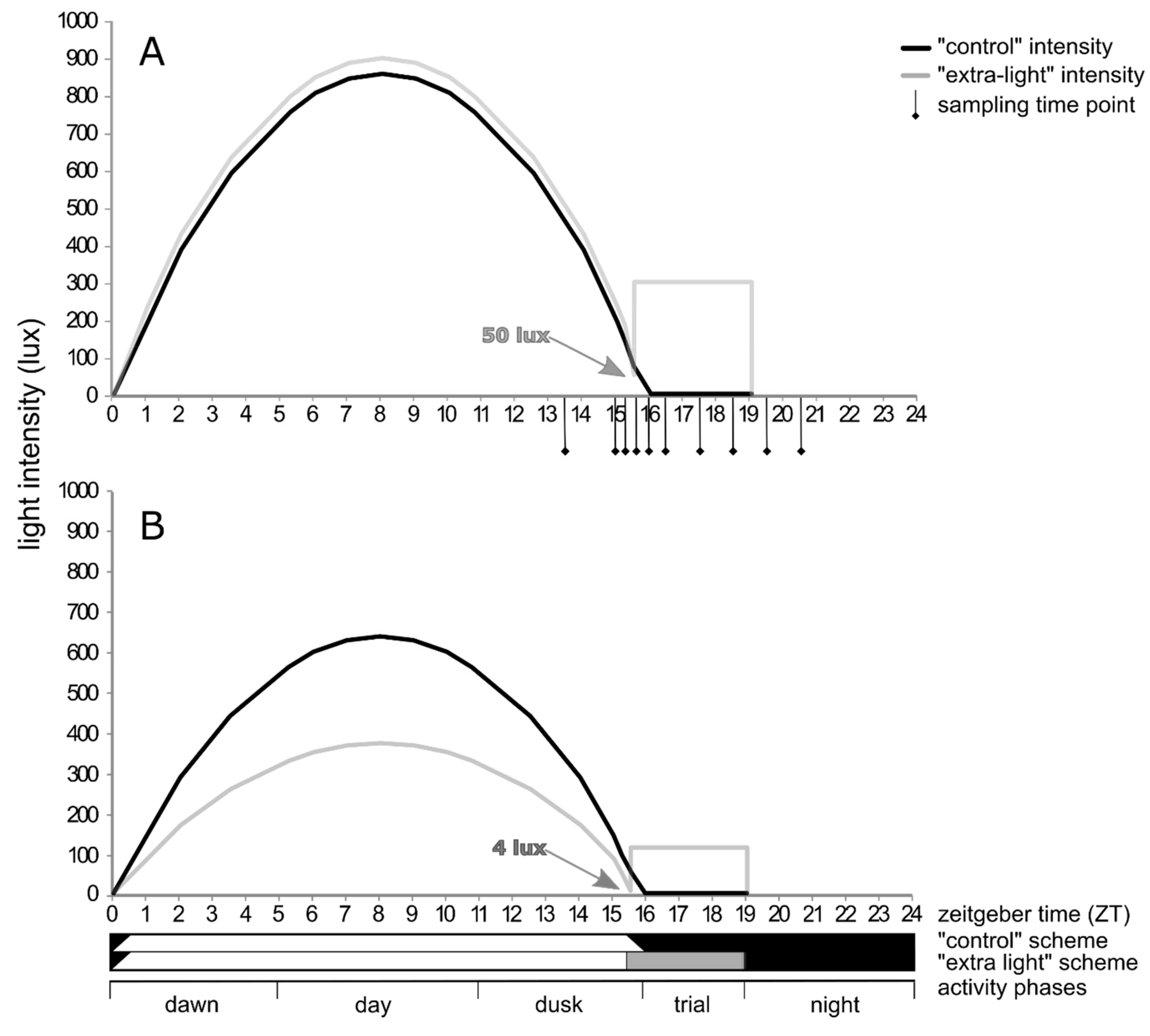
2.3. Diel Activity
2.4. Fecundity
3. Results
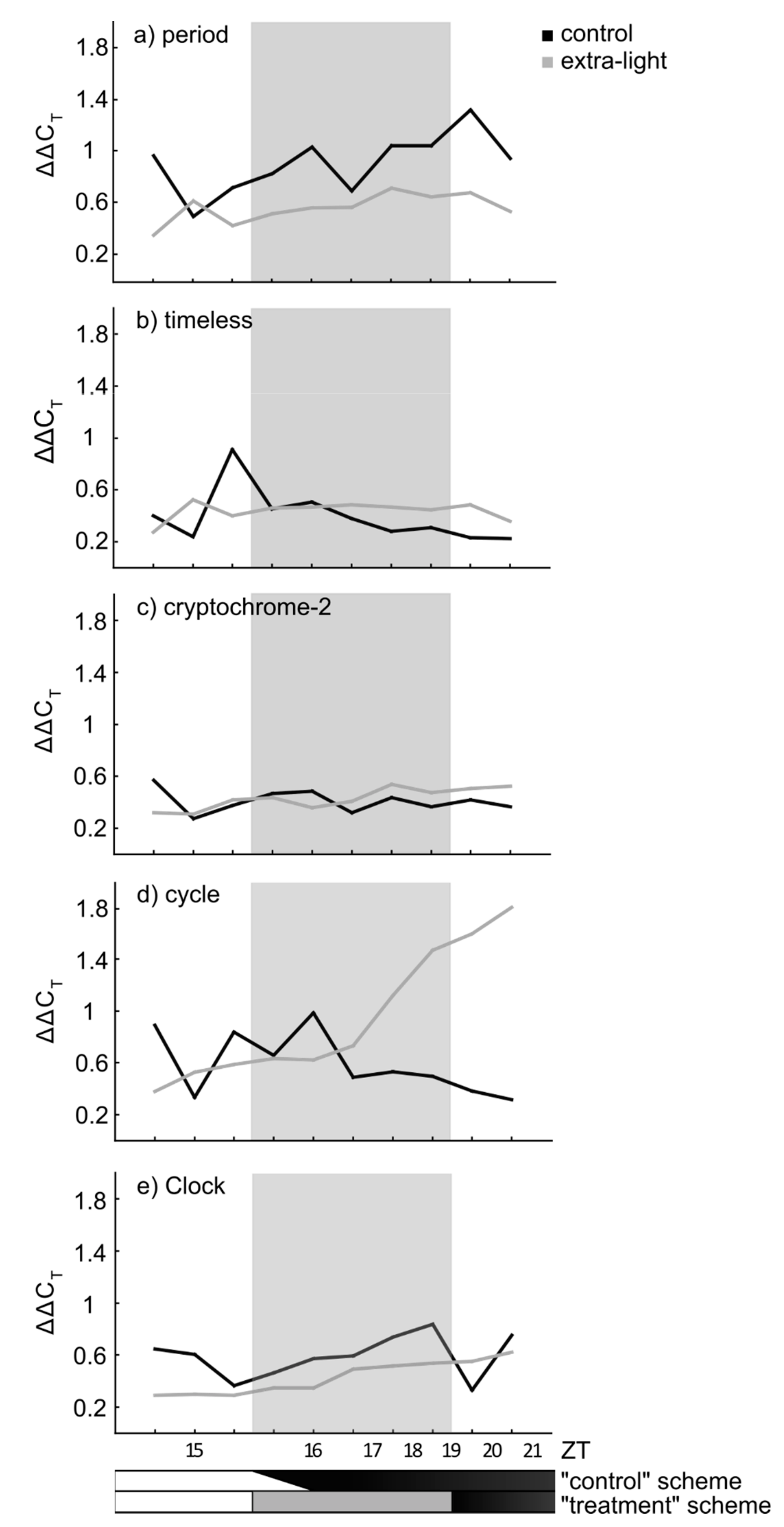
3.1. Expression of Circadian Clock Genes
3.2. Diel Activity Patterns
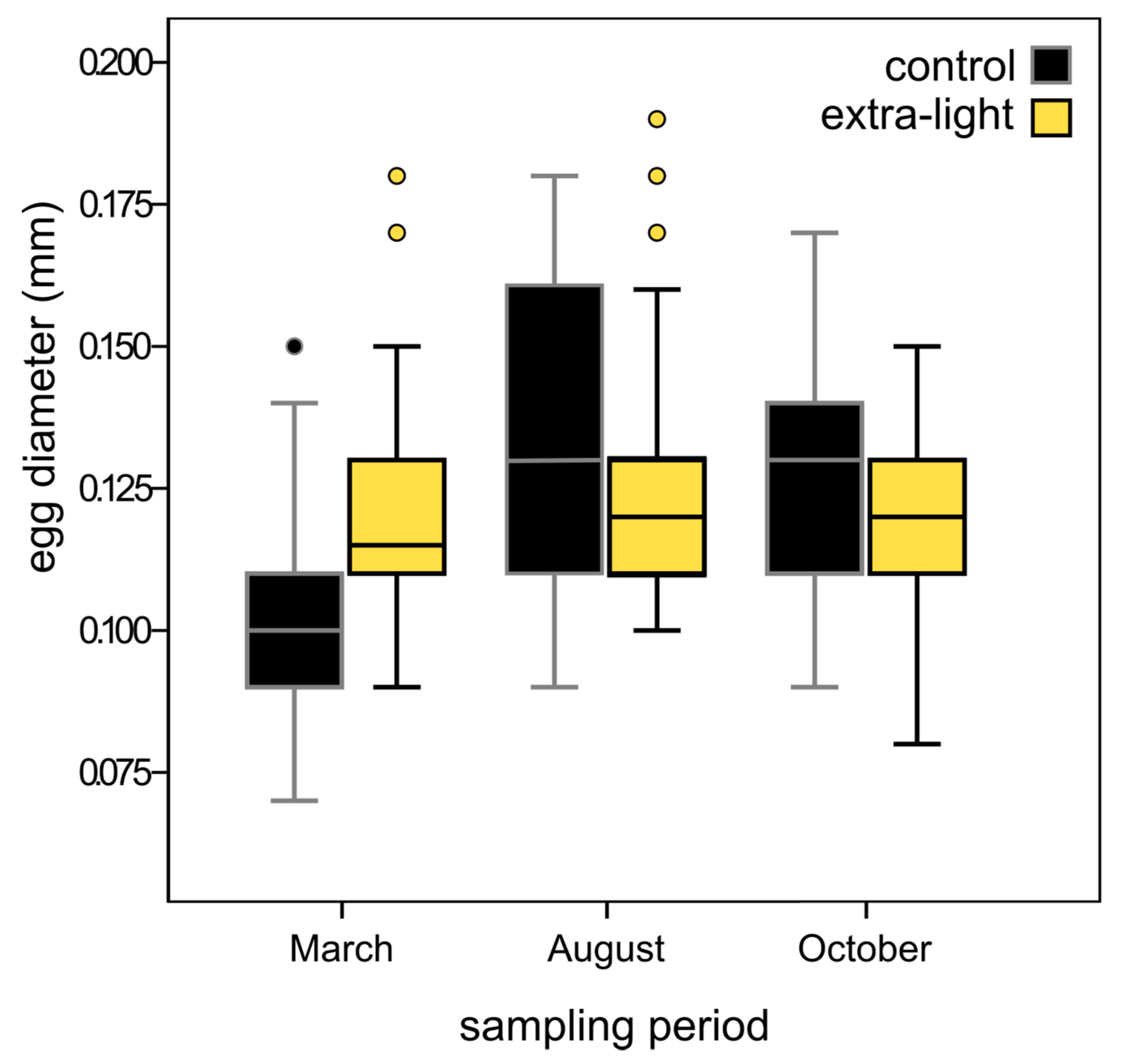
3.3. Fecundity
4. Discussion
4.1. Gene Expression
4.2. Activity
4.3. Fecundity
4.4. Linkages among the Components of the Study
4.5. Considerations of the Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs, Population Division. In World Urbanization Prospects: The 2014 Revision (ST/ESA/SER.A/366); United Nations: New York, NY, USA, 2015. [Google Scholar]
- Kyba, C.C.M.; Kuester, T.; de Miguel, A.S.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef] [PubMed]
- Hölker, F.; Moss, T.; Griefahn, B.; Kloas, W.; Voigt, C.C.; Henckel, D.; Hänel, A.; Kappeler, P.M.; Völker, S.; Schwope, A.; et al. The Dark Side of Light: A transdisciplinary research agenda for light pollution policy. Ecol. Soc. 2010, 15, 13. [Google Scholar] [CrossRef]
- Grubisic, M.; van Grunsven, R.H.A.; Kyba, C.C.M.; Manfrin, A.; Hölker, F. Insect declines and agroecosystems, does light pollution matter? Ann. Appl. Biol. 2018, 173, 180–189. [Google Scholar] [CrossRef]
- Brüning, A.; Kloas, W.; Preuer, T.; Hölker, F. Influence of artificially induced light pollution on the hormone system of perch and roach in a rural habitat. Conserv. Physiol. 2018, 6, coy016. [Google Scholar] [CrossRef] [PubMed]
- Emerson, K.J.; Bradshaw, W.E.; Holzapfel, C.M. Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution 2008, 62, 979–983. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night, the research challenge. Philos. Trans. R. Soc. B 2015, 370, 20140133. [Google Scholar] [CrossRef]
- Perkin, E.K.; Hölker, F.; Tockner, K. The effects of artificial lighting on adult aquatic and terrestrial insects. Freshw. Biol. 2014, 59, 368–377. [Google Scholar] [CrossRef]
- Longcore, T.; Aldern, H.L.; Eggers, J.F.; Flores, S.; Franco, L.; Hirshfield-Yamanishi, E.; Petrinec, L.N.; Yan, W.A.; Barroso, A.M. Tuning the white light spectrum of light emitting diode lamps to reduce attraction of nocturnal arthropods. Philos. Trans. R. Soc. B 2015, 370, 20140125. [Google Scholar] [CrossRef]
- Degen, T.; Mitesser, O.; Perkin, E.K.; Weiß, N.S.; Oehlert, M.; Mattig, E.; Hölker, F. Street lighting, sex-independent impacts on moth movement. J. Anim. Ecol. 2016, 85, 1352–1360. [Google Scholar] [CrossRef]
- Eisenbeis, G. Artificial night lighting and insects, attraction of insects to streetlamps in a rural setting in Germany. In Ecological Consequences of Artificial Night Lighting; Rich, G.C., Longcore, T.M., Eds.; Island Press: Washington, DC, USA, 2006; pp. 191–198. [Google Scholar]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Manfrin, A.; Lehmann, D.; van Grunsven, R.H.A.; Larsen, S.; Syväranta, J.; Whartin, G.; Voigt, C.C.; Monaghan, M.T.; Hölker, F. Dietary changes in predators and scavengers in a nocturnally illuminated riparian ecosystem. Oikos 2018, 127, 960–969. [Google Scholar] [CrossRef]
- Navara, K.J.; Nelson, R.J. The dark side of light at night, physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.V.; Pierce, S.M.; Walsh, H.M.; Kvalvik, S.K.; Lim, J.D. Urban light pollution alters the diel vertical migration of Daphnia. Verh. Der Int. Ver. Für Theor. Und Angew. Limnol. 2000, 27, 1–4. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Lewis, S.M. The impact of artificial light at night on nocturnal insects, A review and synthesis. Ecol. Evol. 2018, 1–22. [Google Scholar] [CrossRef]
- Firebaugh, A.; Haynes, K.J. Light pollution may create demographic traps for nocturnal insects. Basic Appl. Ecol. 2019, 34, 118–125. [Google Scholar] [CrossRef]
- Vezzani, D. Review, Artificial container-breeding mosquitoes and cemeteries, a perfect match. Trop. Med. Int. Health 2007, 12, 299–313. [Google Scholar] [CrossRef]
- Kraus, J.M.; Vonesh, J.R. Fluxes of terrestrial and aquatic carbon by emergent mosquitoes, a test of controls and implications for cross-ecosystem linkages. Oecologia 2012, 170, 1111–1122. [Google Scholar] [CrossRef]
- Klecka, J.; Boukal, D.S. Who eats whom in a pool? A comparative study of prey selectivity by predatory aquatic insects. PLoS ONE 2012, 7, e37741. [Google Scholar] [CrossRef]
- Githeko, A.K.; Lindsey, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases, a regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- Rudolf, M.; Czajka, C.; Börstler, J.; Melaun, C.; Jöst, H.; von Thien, H.; Badusche, M.; Becker, N.; Schmidt-Chanasit, J.; Krüger, A.; et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE 2013, 8, e71832. [Google Scholar] [CrossRef]
- Di Luca, M.; Toma, L.; Boccolini, D.; Severini, F.; La Rosa, G.; Minelli, G.; Bongiorno, G.; Montarsi, F.; Arnoldi, D.; Capelli, G.; et al. Ecological distribution and CQ11 genetic structure of Culex pipiens complex (Diptera, Culicidae) in Italy. PLoS ONE 2016, 11, e0146476. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Jöst, A.; Weitzel, T. The Culex pipiens complex in Europe. J. Am. Mosq. Control Assoc. 2012, 28, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.M.; Keyghobadi, N.; Malcolm, C.A.; Mehmet, C.; Schaffner, F.; Mogi, M.; Fleischer, R.C.; Wilkerson, R.C. Emerging vectors in the Culex pipiens complex. Science 2004, 303, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Sousa, A.C.; Vicente, J.L.; Pinho, L.; Calderón, I.; Arez, E.; Almeida, A.P.; Donnelly, M.J.; Pinto, J. Feeding patterns of molestus and pipiens forms of Culex pipiens (Diptera, Culicidae) in a region of high hybridization. Parasites Vectors 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Kothera, L.; Godsey, M.; Mutebi, J.-P.; Savage, H.M. A comparison of aboveground and belowground populations of Culex pipiens (Diptera, Culicidae) mosquitoes in Chicago, Illinois, and New York City, New York, using microsatellites. J. Med. Entomol. 2010, 47, 805–813. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Möhlmann, T.W.R.; Melsen, D.; Favia, G.; Wennergren, U.; Koenraadt, C.J.M. Latitudinal diversity of Culex pipiens biotypes and hybrids in farm, peri-urban, and wetland habitats in Europe. PLoS ONE 2016, 11, e0166959. [Google Scholar] [CrossRef]
- Lalubin, F.; Bize, P.; van Rooyen, J.; Christe, P.; Glaizot, O. Potential evidence of parasite avoidance in an avian malarial vector. Anim. Behav. 2012, 84, 539–545. [Google Scholar] [CrossRef]
- Danabalan, R.; Ponsonby, D.J.; Linton, Y.M. A critical assessment of available molecular identification tools for determining the status of Culex pipiens s.l. in the United Kingdom. J. Am. Mosq. Control Assoc. 2012, 28, 68–74. [Google Scholar] [CrossRef]
- Veronesi, R.; Gentile, G.; Carrieri, M.; Maccagnani, B.; Stermieri, L. Seasonal pattern of daily activity of Aedes caspius, Aedes detritus, Culex modestus, and Culex pipiens in the Po Delta of northern Italy and significance for vector-borne disease risk assessment. J. Vector Ecol. 2012, 37, 49–61. [Google Scholar] [CrossRef]
- Montarsi, F.; Mazzon, L.; Cazzin, S.; Ciocchetta, S.; Capelli, G. Seasonal and daily activity patterns of mosquito (Diptera, Culicidae) vectors of pathogens in Northeastern Italy. J. Med. Entomol. 2015, 52, 56–62. [Google Scholar] [CrossRef]
- Meier, J.M. Temporal Profiles of Urban Lighting, Proposal for a research design and first results from three sites in Berlin. Int. J. Sustain. Lighting 2018, 20, 11–28. [Google Scholar] [CrossRef]
- Mathias, D.; Jacky, L.; Bradshaw, W.E.; Holzapfel, C.M. Geographic and developmental variation in expression of the circadian rhythm gene, timeless, in the pitcher-plant mosquito, Wyeomyia smithii. J. Insect Physiol. 2005, 51, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Meireles-Filho, A.C.A.; Britto, C.; Lima, J.B.P.; Valle, D.; Peixoto, A.A. Cloning and daily expression of the timeless gene in Aedes aegypti (Diptera, Culicidae). Insect Biochem. Mol. Biol. 2006, 36, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Rivas, G.B.S.; Meireles-Filho, A.C.A.; Lima, J.B.P.; Peixoto, A.A. Circadian expression of clock genes in two mosquito disease vectors, cry2 is different. J. Biol. Rhythm. 2009, 24, 444–451. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Hou, T.Y.; Ward, S.M.; Collins, F.H.; Duffield, G.E. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2011, 108, E421–E430. [Google Scholar] [CrossRef]
- Meireles-Filho, A.C.A.; Kyriacou, C.P. Circadian rhythms in insect disease vectors. Memórias Do Inst. Oswaldo Cruz 2013, 108, 48–58. [Google Scholar] [CrossRef]
- Sandrelli, F.; Costa, R.; Kyriacou, C.P.; Rosato, E. Comparative analysis of circadian clock genes in insects. Insect Mol. Biol. 2008, 17, 447–463. [Google Scholar] [CrossRef]
- Zhu, H.; Sauman, I.; Yuan, Q.; Casselman, A.; Emery-Le, M.; Emery, P.; Reppert, S.M. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008, 6, e4. [Google Scholar] [CrossRef]
- Yuan, Q.; Metterville, D.; Briscoe, A.D.; Reppert, S.M. Insect cryptochromes, gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 2007, 24, 948–955. [Google Scholar] [CrossRef]
- Kyriacou, C.P. Clocks, cryptochromes and Monarch migrations. J. Biol. 2009, 8, 55. [Google Scholar] [CrossRef]
- Meuti, M.E.; Stone, M.; Ikeno, T.; Denlinger, D.L. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J. Exp. Biol. 2015, 218, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Meuti, M.E.; Denlinger, D.L. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 2013, 53, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmayer, W.L. The effect of moonlight on the flight activity of mosquitoes. Ecology 1964, 45, 87–94. [Google Scholar] [CrossRef]
- Howell, P.I.; Knols, B.G.J. Male mating biology. Malar. J. 2009, 8, S8. [Google Scholar] [CrossRef] [PubMed]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.; Quetting, M.; Partecke, J. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 2013, 280, 20123017. [Google Scholar] [CrossRef] [PubMed]
- Van Geffen, K.G.; Groot, A.T.; van Grunsven, R.H.A.; Donners, M.; Berendse, F.; Veenendaal, E.M. Artificial night lighting disrupts sex pheromone in a noctuid moth. Ecol. Entomol. 2015, 40, 401–408. [Google Scholar] [CrossRef]
- Van Geffen, K.G.; van Eck, E.; de Boer, R.A.; van Grunsven, R.H.A.; Salis, L.; Berendse, F.; Veenendaal, E.M. Artificial light at night inhibits mating in a Geometrid moth. Insect Conserv. Divers. 2015, 8, 282–287. [Google Scholar] [CrossRef]
- McLay, L.K.; Green, M.P.; Jones, T.M. Chronic exposure to dim artificial light at night decreases fecundity and adult survival in Drosophila melanogaster. J. Insect Physiol. 2017, 100, 15–20. [Google Scholar] [CrossRef]
- Hänel, A.; Posch, T.; Ribas, S.J.; Aubé, M.; Duriscoe, D.; Jechow, A.; Kollath, Z.; Lolkerna, D.E.; Moore, C.; Schmidt, N.; et al. Measuring night sky brightness, methods and challenges. J. Quant. Spectrosc. Radiat. Transf. 2018, 205, 278–290. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Mohar, A.; Posch, T. How bright is moonlight? Astron Geophys 2017, 58, 31–32. [Google Scholar] [CrossRef]
- Jechow, A.; Hölker, F.; Kyba, C.C.M. Using all-sky differential photometry to investigate how nocturnal clouds darken the night sky in rural areas. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Kobozev, S.A.J.L. Engineering. The reconstruction of illumination at the Moscow metropolitan stations. Light Eng. 2010, 18, 67. [Google Scholar]
- Baker, D.A.; Nolan, T.; Fischer, B.; Pinder, A.; Crisanti, A.; Russell, S. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genom. 2011, 12, 296. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Gentile, J.E.; Duffield, G.E. Extensive circadian and light regulation of the transcriptome in the malaria mosquito Anopheles gambiae. BMC Genom. 2013, 14, 218. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- R. Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2015.
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- De Rosario-Martinez, H. Phia: Post hoc Interaction AnalysisR Package Version 0.2-0. Available online: http://CRAN.R-project.org/package=phia (accessed on 30 June 2016).
- Degen, T.; Hovestadt, T.; Mitesser, O.; Hölker, F. Altered sex-specific mortality and female mating success: Ecological effects and evolutionary responses. Ecosphere 2017, 8, e01820. [Google Scholar] [CrossRef]
- Jhumur, U.S.; Dötterl, S.; Jürgens, A. Naïve and conditioned responses of Culex pipiens pipiens biotype f. molestus (Diptera, Culicidae) to flower odors. J. Med. Entomol. 2006, 43, 1164–1170. [Google Scholar]
- Foster, W.A. Phytochemicals as population sampling lures. J. Am. Mosq. Control Assoc. 2008, 24, 138–146. [Google Scholar] [CrossRef]
- Honnen, A.-C.; Johnston, P.R.; Monaghan, M.T. Sex-specific gene expression in the mosquito Culex pipiens f. molestus in response to artificial light at night. BMC Genom. 2016, 17, 22. [Google Scholar]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Marinotti o Calvo, E.; Nguyen, Q.K.; Dissanayake, S.; Ribeiro, J.M.C.; James, A.A. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol. Biol. 2006, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.J.; McBride, C.S.; DeGennaro, M.; Despo, O.; Vosshall, L.B. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genom. 2016, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Kaiser, C. Life-span of mosquitoes (Culicidae, Diptera) under laboratory conditions. Gerontologia 1973, 19, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.C.; Lee, S.J.; Bush, B.R.; Duffield, G.E. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 2012, 58, 1609–1619. [Google Scholar] [CrossRef]
- Wheeler, D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996, 41, 407–431. [Google Scholar] [CrossRef]
- Grech, K.; Maung, L.A.; Read, A.F. The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malar. J. 2007, 6, 130. [Google Scholar] [CrossRef]
- Telang, A.; Wells, M.A. The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. J. Insect Physiol. 2004, 50, 677–685. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J. Insect seasonal cycles, Genetics and evolution. Annu. Rev. Ecol. Syst. 1981, 12, 281–308. [Google Scholar] [CrossRef]
- Goto, S.G.; Denlinger, D.L. Short-day and long-day expression patterns of genes involved in the flesh fly clock mechanism, period, timeless, cycle and cryptochrome. J. Insect Physiol. 2002, 48, 803–816. [Google Scholar] [CrossRef]
- Chadee, D.; Martinez, R. Landing periodicita of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies. J. Vector Ecol. 2000, 25, 158–163. [Google Scholar] [PubMed]
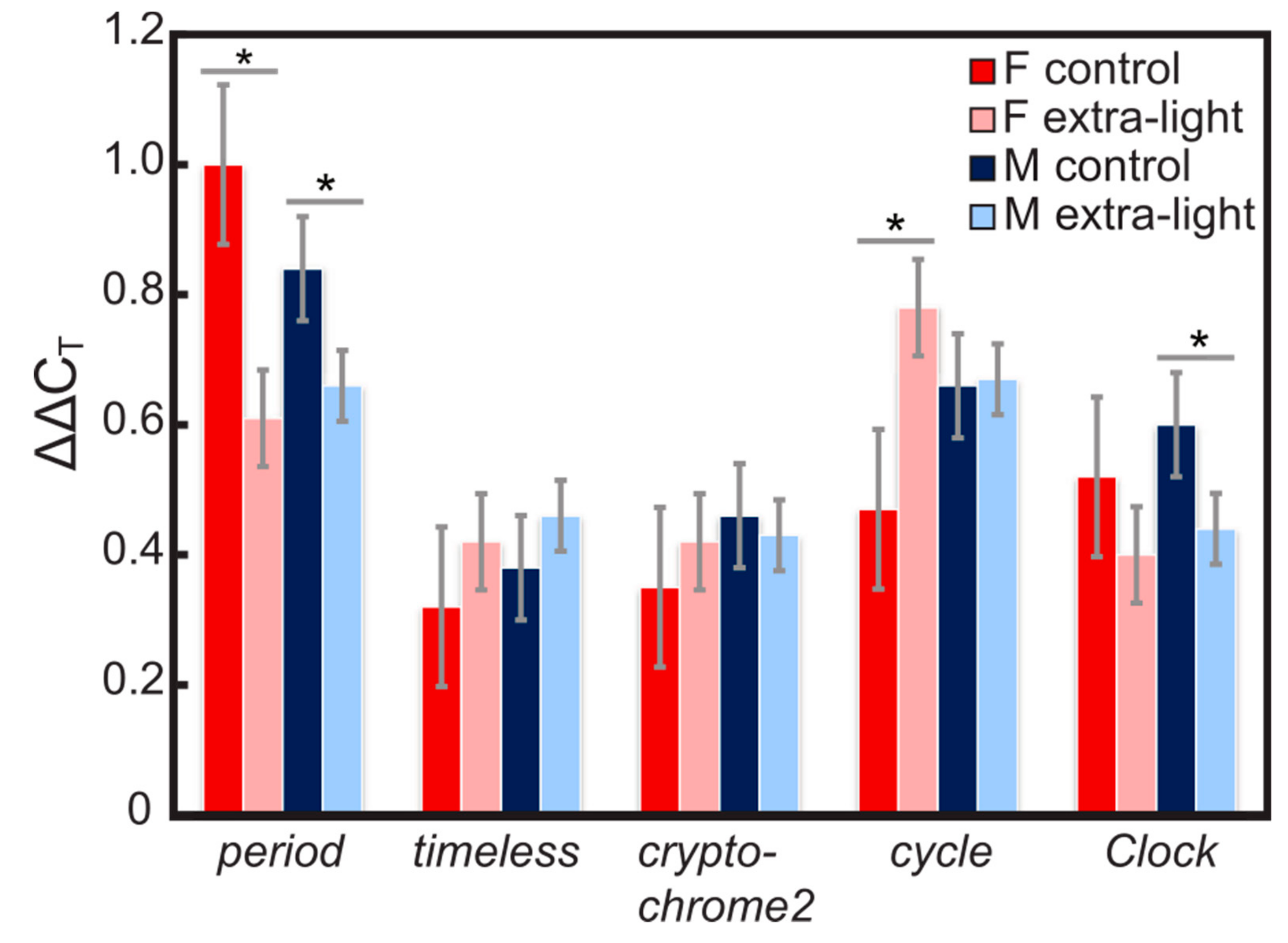

| Gene | AIC (Complete) | AIC (Best) | Residual Deviance | df | Significant Terms |
|---|---|---|---|---|---|
| period | 667.23 | 660.23 | 204.77 | 346 | treatment |
| timeless | 323.98 | 321.33 | 219.12 | 315 | sex |
| treatment x time | |||||
| cryptochrome2 | 194.63 | 193.07 | 139.58 | 315 | sex |
| treatment x time a | |||||
| cycle | 672.59 | 669.28 | 205.37 | 310 | sex |
| exposure | |||||
| treatment x time | |||||
| Clock | 222.79 | 219.80 | 137.08 | 315 | sex |
| treatment x time a |
| Stage | Phase | Mean Activity Control | Mean Activity Extra-Light | Z-Score | P2tailed |
|---|---|---|---|---|---|
| LD | dawn | 0.098 | 0.053 | −3469 | 0.001 |
| day | 0.010 | 0.008 | −1267 | 0.205 | |
| dusk | 0.105 | 0.038 | −15,030 | <0.0001 | |
| trial | 0.419 | 0.051 | −27,369 | <0.0001 | |
| night | 0.251 | 0.213 | −10,052 | <0.0001 | |
| DD | dawn | 0.209 | 0.074 | −19,549 | <0.0001 |
| day | 0.076 | 0.050 | −10,105 | <0.0001 | |
| dusk | 0.314 | 0.234 | −9537 | <0.0001 | |
| trial | 0.172 | 0.200 | −8695 | <0.0001 | |
| night | 0.295 | 0.114 | −30,723 | <0.0001 |
| Control | Extra-Light | ||||||
|---|---|---|---|---|---|---|---|
| Sampling Period | n | Mean Egg Diameter in mm (SD) | n | Mean Egg Diameter in mm (SD) | P-Value | χ2 | df |
| March | 93 | 0.105 (0.015) | 88 | 0.118 (0.017) | <0.0001 | 14.29 | 1 |
| August | 110 | 0.134 (0.025) | 125 | 0.128 (0.024) | 0.47 | 3.93 | 1 |
| October | 271 | 0.125 (0.015) | 224 | 0.119 (0.014) | <0.0001 | 23.98 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honnen, A.-C.; Kypke, J.L.; Hölker, F.; Monaghan, M.T. Artificial Light at Night Influences Clock-Gene Expression, Activity, and Fecundity in the Mosquito Culex pipiens f. molestus. Sustainability 2019, 11, 6220. https://doi.org/10.3390/su11226220
Honnen A-C, Kypke JL, Hölker F, Monaghan MT. Artificial Light at Night Influences Clock-Gene Expression, Activity, and Fecundity in the Mosquito Culex pipiens f. molestus. Sustainability. 2019; 11(22):6220. https://doi.org/10.3390/su11226220
Chicago/Turabian StyleHonnen, Ann-Christin, Janina L. Kypke, Franz Hölker, and Michael T. Monaghan. 2019. "Artificial Light at Night Influences Clock-Gene Expression, Activity, and Fecundity in the Mosquito Culex pipiens f. molestus" Sustainability 11, no. 22: 6220. https://doi.org/10.3390/su11226220
APA StyleHonnen, A.-C., Kypke, J. L., Hölker, F., & Monaghan, M. T. (2019). Artificial Light at Night Influences Clock-Gene Expression, Activity, and Fecundity in the Mosquito Culex pipiens f. molestus. Sustainability, 11(22), 6220. https://doi.org/10.3390/su11226220