In situ Conservation Assessment of Forage and Fodder CWR in Spain Using Phytosociological Associations
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Species and Phytosociological Associations
2.2. Ecogeographical Analyses
2.2.1. Creation of the Ecogeographical Land Characterization Map and Representativeness Analysis
2.2.2. Gap, Coverage, and Complementarity Analyses
3. Results
3.1. Selection of Species and Phytosociological Associations
3.2. Ecogeographical Analyses
3.2.1. Creation of Ecogeographical Land Characterization Map and Representativeness Analysis
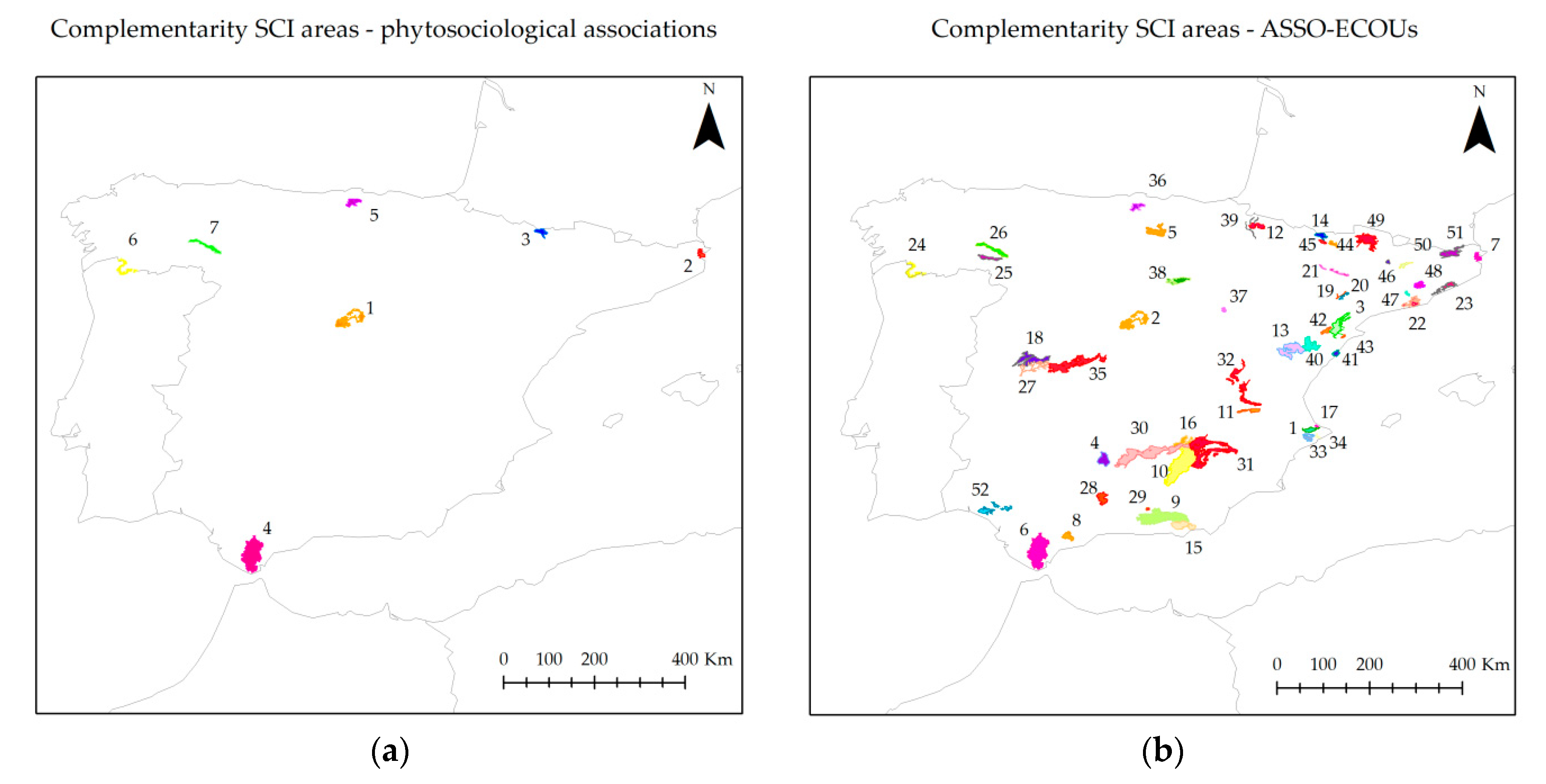
3.2.2. Gap Analysis, Coverage, and Complementarity Analyses
4. Discussion
4.1. Relevance of Genetic Diversity in the Conservation of Crop Wild Relatives
4.2. Phytosociological Associations as a Means for Multiple Species Conservation Management
4.3. Ecogeographical Analyses
4.3.1. Creation of ELC Map and Representativeness Analysis
4.3.2. Gap Analysis, Coverage, and Complementary Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heywood, V.; Casas, A.; Ford-Lloyd, B.; Kell, S.; Maxted, N. Conservation and sustainable use of crop wild relatives. Agric. Ecosyst. Environ. 2007, 121, 245–255. [Google Scholar] [CrossRef]
- Lidder, P.; Sonnino, A. Chapter 1: Biotechnologies for the Management of Genetic Resources for Food and Agriculture. In Advances in Genetics; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 78, pp. 1–167. ISBN 9780123943941. [Google Scholar]
- Esquinas-Alcázar, J. Science and society: Protecting crop genetic diversity for food security: Political, ethical and technical challenges. Nat. Rev. Genet. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Hawtin, G.; Iwanaga, M.; Hodgkin, T. Genetic resources in breeding for adaptation. Euphytica 1996, 92, 255–266. [Google Scholar] [CrossRef]
- Brozynska, M.; Furtado, A.; Henry, R.J. Genomics of crop wild relatives: Expanding the gene pool for crop improvement. Plant Biotechnol. J. 2016, 14, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Crouch, J.H.; Mackill, D.J.; Xu, Y.; Blair, M.W.; Ragot, M.; Upadhyaya, H.D.; Ortiz, R. The Molecularization of Public Sector Crop Breeding: Progress, Problems, and Prospects. Adv. Agron. 2007, 95, 163–318. [Google Scholar]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Maxted, N. Conserving the genetic resources of crop wild relatives in European protected areas. Biol. Conserv. 2003, 113, 411–417. [Google Scholar] [CrossRef]
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop Wild Relatives—Undervalued, Underutilized and under Threat? Bioscience 2011, 61, 559–565. [Google Scholar] [CrossRef]
- MAGRAMA. Ministerio de Agricultura Alimentación y Medio Ambiente Estrategia Española de Conservación Vegetal 2014–2020. Principios y Orientaciones para la Conservación de la Diversidad Vegetal Silvestre en España; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014; p. 77. [Google Scholar]
- Labokas, J.; Maxted, N.; Kell, S.; Brehm, J.M.; Iriondo, J.M. Development of national crop wild relative conservation strategies in European countries. Genet. Resour. Crop Evol. 2018, 65, 1385–1403. [Google Scholar] [CrossRef]
- Fielder, H.; Hopkins, J.; Smith, C.; Kell, S.; Ford-Lloyd, B.; Al, E. UK wild species to underpin food security: Species selection, genetic reserves and targeted collection. Crop Wild Relat. 2012, 8, 24–27. [Google Scholar]
- Pinheiro de Carvalho, M.Â.; Nóbrega, H.; Freitas, G.; Fontinha, S.; Frese, L. Towards the establishment of a genetic reserve for Beta patula Aiton. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., Dulloo, M.E., Ford-Lloyd, B.V., Frese, L., Iriondo, J.M., Al, E., Eds.; CAB International: Wallingford, UK, 2012; pp. 36–44. [Google Scholar]
- Iriondo, J.M.; Fielder, H.; Fitzgerald, H.; Kell, S.P.; Labokas, J.; Negri, V.; Phillips, J.; Teso, M.L.R.; Sensen, S.; Taylor, N.; et al. National Strategies for the Conservation of Crop Wild Relatives. In Enhancing Crop Genepool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; Maxted, N., Dulloo, M.E., Ford-Lloyd, B.V., Eds.; CAB International: Oxfordshire, UK, 2016; pp. 161–171. [Google Scholar]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop Evol. 2012, 59, 205–217. [Google Scholar] [CrossRef]
- Maxted, N.; Kell, S.; Ford-Lloyd, B.; Dulloo, E.; Toledo, Á. Toward the systematic conservation of global crop wild relative diversity. Crop Sci. 2012, 52, 774–785. [Google Scholar] [CrossRef]
- Garcia, R.M.; Parra-Quijano, M.; Iriondo, J.M. Identification of ecogeographical gaps in the Spanish Aegilops collections with potential tolerance to drought and salinity. PeerJ 2017, 5, e3494. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Asdal, Å.; Magos Brehm, J.; Rasmussen, M.; Maxted, N. In situ and ex situ diversity analysis of priority crop wild relatives in Norway. Divers. Distrib. 2016, 22, 1112–1126. [Google Scholar] [CrossRef]
- Rubio Teso, M.L.; Iriondo, J.M.; Parra-Quijano, M.; Torres Lamas, E. National Strategy for the Conservation of Crop Wild Relatives of Spain. 2013. Available online: https://pgrsecure.bham.ac.uk/sites/default/files/documents/public/National_CWR_Conservation_Strategy_Spain.pdf (accessed on 10 September 2019).
- Taylor, N.G.; Kell, S.P.; Holubec, V.; Parra-Quijano, M.; Chobot, K.; Maxted, N. A systematic conservation strategy for crop wild relatives in the Czech Republic. Divers. Distrib. 2017, 23, 448–462. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Fitosociología. Bases Para el Estudio de Comunidades Vegetales; Lalucat Jo, J., de Bolos Capdevilla, J.O., Eds.; H. Blume Ediciones: Madrid, Spain, 1979; ISBN 9781604138795. [Google Scholar]
- Caro, T. Reserve Site Selection. In Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship and Other Surrogate Species; Island Press: Whasington, DC, USA, 2010; pp. 61–98. [Google Scholar]
- Rubio Teso, M.L.; Torres, E.; Parra-Quijano, M.; de la Rosa, L.; Fajardo, J.; Iriondo, J.M. National inventory and prioritization of crop wild relatives in Spain. Genet. Resour. Crop Evol. 2018, 65, 1237–1253. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 506–517. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Torres Lamas, E.; Iriondo Alegría, J.M.; López, F. Herramientas CAPFITOGEN. V. 2.0. Manual. In Herramientas Capfitogen; FAO: Rome, Italy, 2016; p. 292. [Google Scholar]
- Ketchen, D.J.; Shook, C.L. The Application of Cluster Analysis in Management Strategic Research: An Analysis and Critique. Strateg. Manag. J. 1996, 17, 441–458. [Google Scholar] [CrossRef]
- Scott, J.M.; Davis, F.; Csuti, B.; Noss, R.; Butterfield, B.; Anderson, H.; Caicco, S.; Erchia, F.D.; Edwards, T.C.; Ulliman, J. Gap analysis: A Geographic approach to protection of Biological Diversity. Wildl. Monogr. 1993, 123, 3–41. [Google Scholar]
- Rebelo, A.G. Iterative selection procedures: Centres of endemism and optimal placement of reserves. In Botanical Diversity in Southern Africa; Huntley, B.J., Ed.; National Botanical Institute: Pretoria, South Africa, 1994; pp. 231–257. [Google Scholar]
- Maxted, N.; Brehm, J.M.; Kell, S. Resource Book for the Preparation of National Plans for Conservation of Crop Wild Relatives and Landraces; FAO: Rome, Italy, 2013. [Google Scholar]
- Turner, N.J.; Łuczaj, Ł.J.; Migliorini, P.; Pieroni, A.; Dreon, A.L.; Sacchetti, L.E.; Paoletti, M.G. Edible and Tended Wild Plants, Traditional Ecological Knowledge and Agroecology. Crit. Rev. Plant Sci. 2011, 30, 198–225. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Soussana, J.-F.; Howden, S.M. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Improving representativeness of genebank collections through species distribution models, gap analysis and ecogeographical maps. Biodivers. Conserv. 2012, 21, 79–96. [Google Scholar] [CrossRef]
- Rubio Teso, M.L.; Kinoshita Kinoshita, K.; Iriondo, J.M. Optimized Site Selection for the In Situ Conservation of Forage CWRs: A Combination of Community- and Genetic-level Perspectives. In Enhancing Crop Genepool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; Maxted, N., Dulloo, M.E., Ford-Lloyd, B.V., Eds.; CAB International: Oxfordshire, UK, 2016; pp. 199–205. [Google Scholar]
- Biondi, E. Phytosociology today: Methodological and conceptual evolution. Plant Biosyst. 2011, 145, 19–29. [Google Scholar] [CrossRef]
- European Commission Council Directive 92/43/ECC. Off. J. Eur. Union 1992, 94, 40–52.
- Trochet, A.; Schmeller, D.S. Effectiveness of the Natura 2000 network to cover threatened species. Nat. Conserv. 2013, 4, 35–53. [Google Scholar] [CrossRef]
- Dimitrakopoulos, P.G.; Memtsas, D.; Troumbis, A.Y. Questioning the effectiveness of the Natura 2000 species areas for conservation strategy: The case of Crete. Glob. Ecol. Biogeogr. 2004, 13, 199–207. [Google Scholar] [CrossRef]
- Maiorano, L.; Falcucci, A.; Garton, E.O.; Boitani, L. Contribution of the Natura 2000 network to biodiversity conservation in Italy. Conserv. Biol. 2007, 21, 1433–1444. [Google Scholar] [CrossRef]
- Rubio-Salcedo, M.; Martínez, I.; Carreño, F.; Escudero, A. Poor effectiveness of the Natura 2000 network protecting Mediterranean lichen species. J. Nat. Conserv. 2013, 21, 1–9. [Google Scholar] [CrossRef]
- D’Amen, M.; Bombi, P.; Campanaro, A.; Zapponi, L.; Bologna, M.A.; Mason, F. Protected areas and insect conservation: Questioning the effectiveness of natura 2000 network for saproxylic beetles in italy. Anim. Conserv. 2013, 16, 370–378. [Google Scholar] [CrossRef]
- Faith, D.P.; Carter, G.; Cassis, G.; Ferrier, S.; Wilkie, L. Complementarity, biodiversity viability analysis, and policy-based algorithms for conservation. Environ. Sci. Policy 2003, 6, 311–328. [Google Scholar] [CrossRef]
- Heywood, V. Conservation strategies for species/populations occurring outside protected areas. In Crop Wild Relatives: A Manual of in Situ Conservation; Hunter, D., Heywood, V., Eds.; Earthscan: London, UK; Washington, DC, USA, 2011; pp. 253–294. [Google Scholar]
- Traba, J.; García De La Morena, E.L.; Morales, M.B.; Suárez, F. Determining high value areas for steppe birds in Spain: Hot spots, complementarity and the efficiency of protected areas. Biodivers. Conserv. 2007, 16, 3255–3275. [Google Scholar] [CrossRef]
- Marignani, M.; Blasi, C. Looking for important plant areas: Selection based on criteria, complementarity, or both? Biodivers. Conserv. 2012, 21, 1853–1864. [Google Scholar] [CrossRef]
- Vane-Wright, R.I.; Humphries, C.J.; Williams, P.H. What to protect? Systematics and the agony of choice. Biol. Conserv. 1991, 55, 235–254. [Google Scholar] [CrossRef]
- Maxted, N.; Dulloo, E.; Ford-Lloyd, B.V.; Iriondo, J.M.; Jarvis, A. Gap analysis: A tool for complementary genetic conservation assessment. Divers. Distrib. 2008, 14, 1018–1030. [Google Scholar] [CrossRef]
- Fielder, H.; Brotherton, P.; Hosking, J.; Hopkins, J.J.; Ford-Lloyd, B.; Maxted, N. Enhancing the Conservation of Crop Wild Relatives in England. PLoS ONE 2015, 10, e0130804. [Google Scholar] [CrossRef]
- Fitzgerald, H. The National Crop Wild Relative Strategy Report for Finland MTT; AgriFood Reaseach Finland: Helsinki, Finland, 2013. [Google Scholar]
- Phillips, J.; Kyratzis, A.; Christoudoulou, C.; Kell, S.; Maxted, N. Development of a national crop wild relative conservation strategy for Cyprus. Genet. Resour. Crop Evol. 2014, 61, 817–827. [Google Scholar] [CrossRef]
- Reyers, B.; van Jaarsveld, A.S.; Krüger, M. Complementarity as a biodiversity indicator strategy. Proc. R. Soc. B Biol. Sci. 2000, 267, 505–513. [Google Scholar] [CrossRef]
- Kati, V.; Devillers, P.; Dufrêne, M.; Legakis, A.; Vokou, D.; Lebrun, P. Hotspots, complementarity or representativeness? Designing optimal small-scale reserves for biodiversity conservation. Biol. Conserv. 2004, 120, 471–480. [Google Scholar] [CrossRef]
- Araújo, M.B.; Williams, P.H. The bias of complementarity hotspots toward marginal populations. Conserv. Biol. 2001, 15, 1710–1720. [Google Scholar] [CrossRef]
- Faith, D.P.; Walker, P.A. How do indicator groups provide information about the relative biodiversity of different sets of areas? On hotspots, complementarity and pattern-based approaches. Biodivers. Lett. 1996, 3, 18–25. [Google Scholar] [CrossRef]
- Araújo, M.B.; Williams, P.H. Selecting areas for species persistence using occurrence data. Biol. Conserv. 2000, 96, 331–345. [Google Scholar] [CrossRef]
- Iriondo, J.M.; Maxted, N.; Kell, S.P.; Ford-Lloyd, B.V.; Lara-Romero, C.; Labokas, J.; Magos Brehm, J. Quality standards for genetic reserve conservation of crop wild relatives. In Agrobiodiversity Conservation. Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., Dulloo, E., Ford-Lloyd, B.V., Frese, L., Iriondo, J., Pinheiro de Carvalho, M.A.A., Eds.; CAB International: Oxfordshire, UK, 2012; pp. 72–77. [Google Scholar]
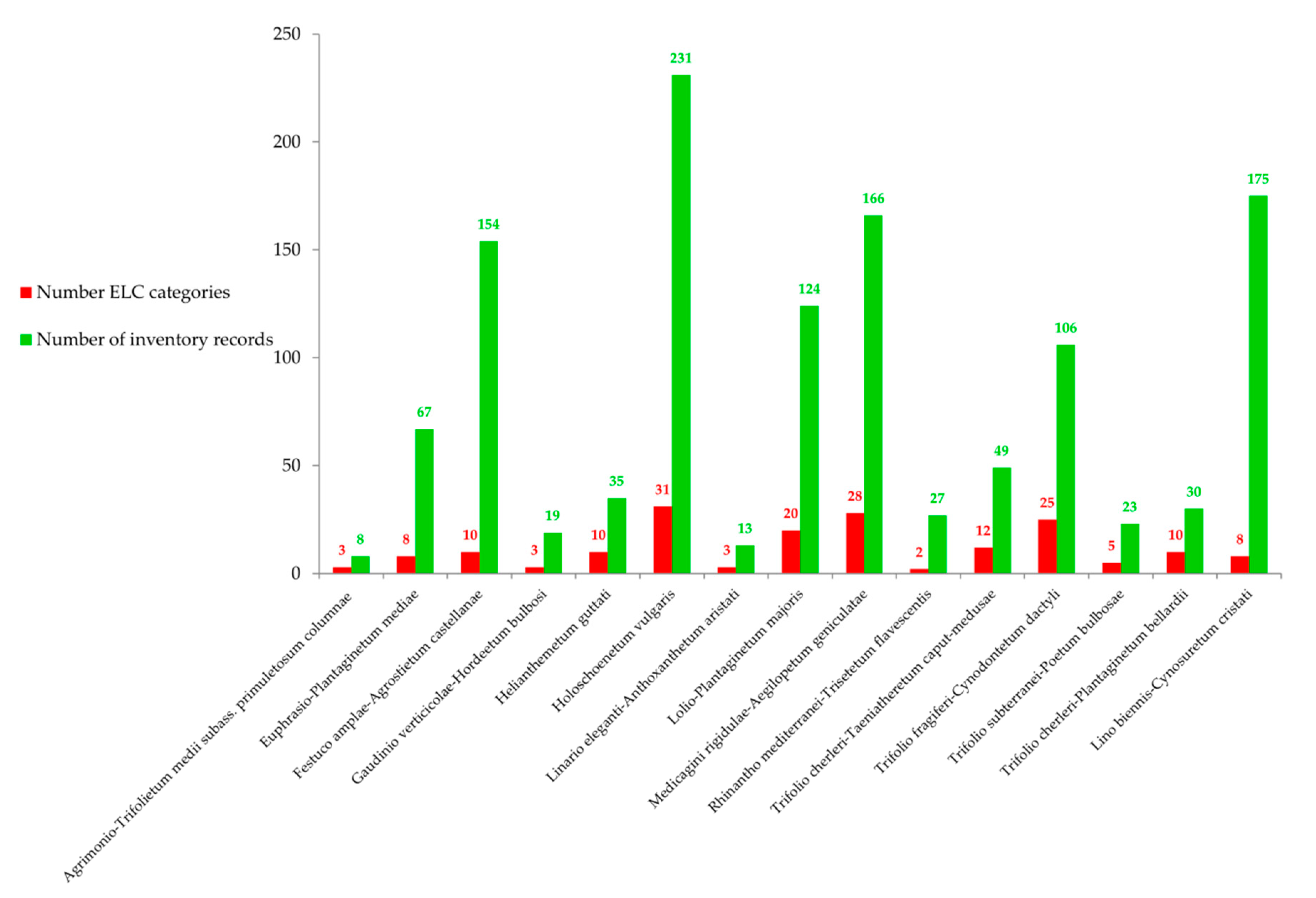


| Target Associations | Priority CWR Species | Inventories | Fidelity (%) | |
|---|---|---|---|---|
| 1 | Agrimonio-Trifolietum medii subass. primuletosum columnae | Astragalus glycyphyllos L. | 10 | 19.6 |
| Trifolium medium L. | 44 | 86.3 | ||
| 2 | Euphrasio-Plantaginetum mediae | Poa alpina L. | 32 | 11.9 |
| Poa compressa L. | 12 | 4.5 | ||
| Agrostis capillaris L. | 121 | 45.0 | ||
| 3 | Festuco amplae-Agrostietum castellanae | Deschampsia cespitosa (L.) P. Beauv. | 6 | 1.9 |
| Trifolium dubium Sibth. | 87 | 27.9 | ||
| 4 | Gaudinio verticicolae-Hordeetum bulbosi | Hedysarum coronarium L. | 21 | 95.5 |
| Trifolium lappaceum L. | 1 | 4.5 | ||
| Trifolium squamosum L. | 9 | 40.9 | ||
| Trifolium squarrosum L. | 2 | 9.1 | ||
| 5 | Helianthemetum guttati | Medicago truncatula Gaertn. | 9 | 8.7 |
| Trifolium nigrescens Viv. | 9 | 8.7 | ||
| 6 | Holoschoenetum vulgaris | Agrostis stolonifera L. | 214 | 41.2 |
| 7 | Linario eleganti-Anthoxanthetum aristati | Lupinus luteus L. | 7 | 14.3 |
| Medicago arabica (L.) Huds. | 1 | 2.0 | ||
| Ornithopus sativus Brot. | 5 | 10.2 | ||
| Lupinus angustifolius L. | 8 | 16.3 | ||
| 8 | Lolio-Plantaginetum majoris | Lolium perenne L. | 308 | 88.3 |
| 9 | Medicagini rigidulae-Aegilopetum geniculatae | Medicago rigidula (L.) All. | 150 | 38.4 |
| 10 | Rhinantho mediterranei-Trisetetum flavescentis | Dactylis glomerata L. | 117 | 95.9 |
| Medicago lupulina L. | 70 | 57.4 | ||
| Trifolium incarnatum L. | 2 | 1.6 | ||
| Trifolium pratense L. | 113 | 92.6 | ||
| Festuca arundinacea Schreb. | 16 | 13.1 | ||
| Festuca pratensis Huds. | 39 | 32.0 | ||
| Medicago sativa L. | 23 | 18.9 | ||
| Poa pratensis L. | 70 | 57.4 | ||
| Trifolium repens L. | 78 | 63.9 | ||
| 11 | Trifolio cherleri-Taeniatheretum caput-medusae | Trifolium arvense L. | 87 | 52.9 |
| Trifolium angustifolium L. | 70 | 45.8 | ||
| Trifolium campestre Schreb. | 89 | 58.2 | ||
| Trifolium striatum L. | 56 | 36.6 | ||
| 12 | Trifolio fragiferi-Cynodontetum dactyli | Medicago scutellata (L.) Mill. | 1 | 0.3 |
| 13 | Trifolio subterranei-Poetum bulbosae | Trifolium resupinatum L. | 4 | 7.4 |
| Poa bulbosa L. | 54 | 100.0 | ||
| Trifolium subterraneum L. | 31 | 57.4 | ||
| 14 | Trifolio cherleri-Plantaginetum bellardii | Ornithopus compressus L. | 81 | 53.6 |
| 15 | Lino biennis-Cynosuretum cristati | Lolium multiflorum Lam. | 22 | 5.9 |
| Name of SCI Area | Site Code | Autonomous Community | Number of New Associations Added | Number of Asso-EcoUs Present |
|---|---|---|---|---|
| Cuenca del río Lozoya y Sierra Norte | ES3110002 | Comunidad de Madrid | 5 | 9 |
| Aiguamolls de l’Alt Empordà | ES0000019 | Cataluña | 3 | 3 |
| Ordesa y Monte Perdido | ES0000016 | Aragón | 3 | 3 |
| Los Alcornocales | ES0000049 | Andalucía | 1 | 4 |
| Montaña Oriental | ES1300002 | Cantabria | 1 | 1 |
| Baixa Limia | ES1130001 | Galicia | 1 | 1 |
| Montes Aquilanos y Sierra de Teleno | ES4130117 | Castilla y León | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio Teso, M.L.; Iriondo, J.M. In situ Conservation Assessment of Forage and Fodder CWR in Spain Using Phytosociological Associations. Sustainability 2019, 11, 5882. https://doi.org/10.3390/su11215882
Rubio Teso ML, Iriondo JM. In situ Conservation Assessment of Forage and Fodder CWR in Spain Using Phytosociological Associations. Sustainability. 2019; 11(21):5882. https://doi.org/10.3390/su11215882
Chicago/Turabian StyleRubio Teso, María Luisa, and José M. Iriondo. 2019. "In situ Conservation Assessment of Forage and Fodder CWR in Spain Using Phytosociological Associations" Sustainability 11, no. 21: 5882. https://doi.org/10.3390/su11215882
APA StyleRubio Teso, M. L., & Iriondo, J. M. (2019). In situ Conservation Assessment of Forage and Fodder CWR in Spain Using Phytosociological Associations. Sustainability, 11(21), 5882. https://doi.org/10.3390/su11215882




