The Potential Use of Microorganisms as Restorative Agents: An Update
Abstract
1. Introduction
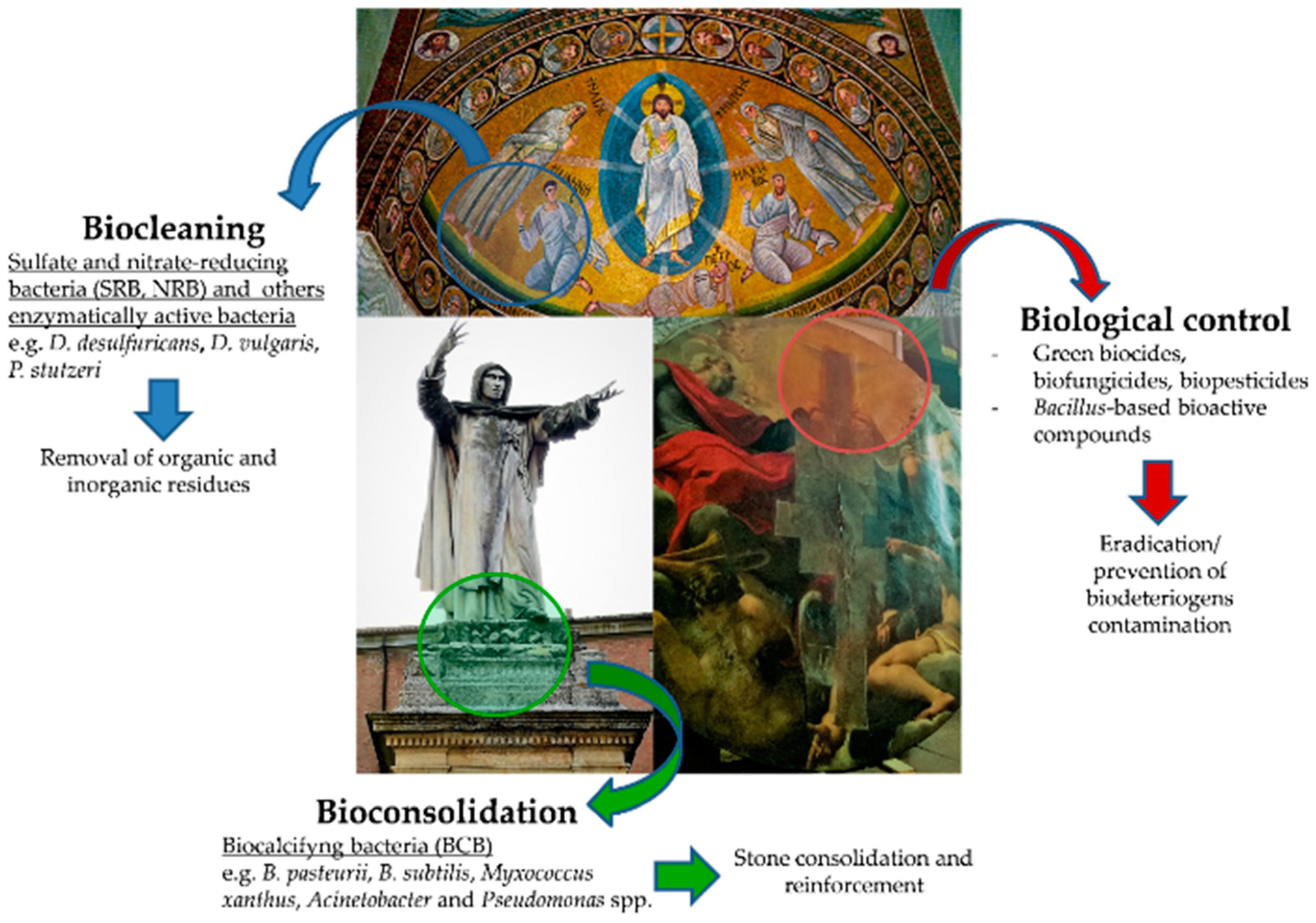
2. Prevention and Control of Biodeterioration
3. Biorestoration Approach: Microorganisms as “Restorative Agents”
3.1. Bioremediation and Bioconsolidation
3.2. Biocleaning
3.3. Biological Methods for the Control of Microbial Contamination
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hueck, H.J. The biodeterioration of materials-An appraisal (Reprinted). Int. Biodeterior. Biodegrad. 2001, 48, 5–11. [Google Scholar] [CrossRef]
- Ranalli, G.; Zanardini, E.; Sorlini, C. Biodeterioration—Including Cultural Heritage. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 191–205. [Google Scholar]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Environmental factors in biodeterioration. In Biology in the Conservation of Works of Art; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; ICCROM: Rome, Italy, 1991; pp. 3–24. [Google Scholar]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The accelerating effects of the microorganisms on biodeterioration of stone monuments under air pollution and continental-cold climatic conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Ciferri, O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [PubMed]
- Leonardi, R. Nuclear physics and painting: Sub-topic of the wide and fascinating field of science and art. Nucl. Phys. A 2005, 752, 659c–674c. [Google Scholar] [CrossRef]
- Stulik, D. Paint. The Science of Paintings; Taft, W.S.J., Mayer, J.W., Eds.; Springer: New York, NY, USA, 2000; pp. 12–25. [Google Scholar]
- Taft, W.S.; Mayer, J.W. The Structure and Analysis of Paintings. In The Science of Paintings; Taft, W.S.J., Mayer, J.W., Eds.; Springer: New York, NY, USA, 2000; pp. 1–11. [Google Scholar]
- Matteini, M.; Mazzeo, R. Structure of panel and canvas paintings. In Scientific Examination for the Investigation of Paintings, a Handbook for Conservator-Restorers; Pinna, D., Galeotti, M., Mazzeo, R., Eds.; Centro Di: Firenze, Italy, 2009; pp. 11–20. [Google Scholar]
- Strzelczyk, A.B. Paintings and sculptures. Econ. Microbiol. 1981, 6, 203–234. [Google Scholar]
- Pinna, D.; Salvadori, O. Processes of biodeterioration: General mechanisms. In Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 15–34. [Google Scholar]
- Capodicasa, S.; Fedi, S.; Porcelli, A.M.; Zannoni, D. The microbial community dwelling on a biodeteriorated 16th century painting. Int. Biodeterior. Biodegrad. 2010, 64, 727–733. [Google Scholar] [CrossRef]
- Lopez-Miras, M.D.; Martin-Sanchez, I.; Yebra-Rodriguez, A.; Romero-Noguera, J.; Bolivar-Galiano, F.; Ettenauer, J.; Sterflinger, K.; Pinar, G. Contribution of the Microbial Communities Detected on an Oil Painting on Canvas to Its Biodeterioration. PLoS ONE 2013, 8, e80198. [Google Scholar] [CrossRef] [PubMed]
- Pavic, A.; Ilic-Tomic, T.; Pacevski, A.; Nedeljkovic, T.; Vasiljevic, B.; Moric, I. Diversity and biodeteriorative potential of bacterial isolates from deteriorated modern combined-technique canvas painting. Int. Biodeterior. Biodegrad. 2015, 97, 40–50. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Heyrman, J.; Dornieden, T.; Gonzalez-Delvalle, M.; Krumbein, W.E.; Laiz, L.; Petersen, K.; Saiz-Jimenez, C.; Swings, J. Bacterial and fungal diversity and biodeterioration problems in mural painting environments of St. Martins church (Greene–Kreiensen, Germany). Int. Biodeterior. Biodegrad. 2004, 53, 13–24. [Google Scholar] [CrossRef]
- Caselli, E.; Pancaldi, S.; Baldisserotto, C.; Petrucci, F.; Impallaria, A.; Volpe, L.; D’Accolti, M.; Soffritti, I.; Coccagna, M.; Sassu, G.; et al. Characterization of biodegradation in a 17th century easel painting and potential for a biological approach. PLoS ONE 2018, 13, e0207630. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.J.; Mitchell, R. Microbial deterioration of historic stone. Front. Ecol. Environ. 2005, 3, 445–451. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappinscott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Warscheid, T.; Oelting, M.; Krumbein, W.E. Physicochemical Aspects of Biodeterioration Processes on Rocks with Special Regard to Organic Pollutants. Int. Biodeterior. 1991, 28, 37–48. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Laiz, L. Occurrence of halotolerant/halophilic bacterial communities in deteriorated monuments. Int. Biodeterior. Biodegrad. 2000, 46, 319–326. [Google Scholar] [CrossRef]
- McNamara, C.J.; Perry, T.D.; Zinn, M.; Breuker, M.; Mitchell, R. Microbial processes in the deterioration of Mayan archaeological buildings in southern Mexico. In Art, Biology, and Conservation: Biodeterioration of Works of Art; Koestler, R.J., Koestler, V., Charola, A.E., Nieto-Fernandez, F.E., Eds.; The Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 248–265. [Google Scholar]
- Schabereiter-Gurtner, C.; Pinar, G.; Lubitz, W.; Rolleke, S. An advanced molecular strategy to identify bacterial communities on art objects. J. Microbiol. Methods 2001, 45, 77–87. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Pinar, G.; Lubitz, W.; Rolleke, S. Phylogenetic diversity of bacteria associated with Paleolithic paintings and surrounding rock walls in two Spanish caves (Llonin and La Garma). FEMS Microbiol. Ecol. 2004, 47, 235–247. [Google Scholar] [CrossRef]
- Milanesi, C.; Baldi, F.; Vignani, R.; Ciampolini, F.; Faleri, C.; Cresti, M. Fungal deterioration of medieval wall fresco determined by analysing small fragments containing copper. Int. Biodeterior. Biodegrad. 2006, 57, 7–13. [Google Scholar] [CrossRef]
- Wang, S.; Levin, R.E. Discrimination of viable Vibrio vulnificus cells from dead cells in real-time PCR. J. Microbiol. Methods 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Mustapha, A. Detection of viable Escherichia coli O157:H7 by ethidium monoazide real-time PCR. J. Appl. Microbiol. 2009, 107, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Mustapha, A. EMA-Real-Time PCR as a Reliable Method for Detection of Viable Salmonella in Chicken and Eggs. J. Food Sci. 2010, 75, M134–M139. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, O. The control of biodeterioration. Coalition 2003, 6, 16–20. [Google Scholar]
- Cappitelli, F.; Sorlini, C. From papyrus to compact disc: The microbial deterioration of documentary heritage. Crit. Rev. Microbiol. 2005, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.V. Biodeterioration of Stone in Tropical Environments: An Overview; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Fernandes, P. Applied microbiology and biotechnology in the conservation of stone cultural heritage materials. Appl. Microbiol. Biotechnol. 2006, 73, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Roig, P.; Ranalli, G. The safety of biocleaning technologies for cultural heritage. Front. Microbiol. 2014, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Zanardini, E.; Rampazzi, L.; Corti, C.; Andreotti, A.; Colombini, M.P.; Bosch-Roig, P.; Lustrato, G.; Giantomassi, C.; Zari, D.; et al. Onsite advanced biocleaning system on historical wall paintings using new agar-gauze bacteria gel. J. Appl. Microbiol. 2019, 126, 1785–1796. [Google Scholar] [CrossRef]
- Junier, P.; Joseph, E. Microbial biotechnology approaches to mitigating the deterioration of construction and heritage materials. Microb. Biotechnol. 2017, 10, 1145–1148. [Google Scholar] [CrossRef]
- Kohli, R. Chapter 15-Application of Microbial Cleaning Technology for Removal of Surface Contamination. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques, 1st ed.; Kohli, R., Mittal, K.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 591–617. [Google Scholar]
- Tiano, P.; Biagiotti, L.; Mastromei, G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: Methods of evaluation. J. Microbiol. Methods 1999, 36, 139–145. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. Biodeterioration vs. biodegradation: The role of microorganisms in the removal of pollutants deposited on historic buildings. Int. Biodeterior. Biodegrad. 1997, 24, 225–232. [Google Scholar] [CrossRef]
- Wolbers, R. Cleaning Painted Surfaces: Aqueous Methods; Archetype Publications: London, UK, 2000. [Google Scholar]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis-the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Jroundi, F.; Gomez-Suaga, P.; Jimenez-Lopez, C.; Gonzalez-Munoz, M.T.; Fernandez-Vivas, M.A. Stone-isolated carbonatogenic bacteria as inoculants in bioconsolidation treatments for historical limestone. Sci. Total Environ. 2012, 425, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Gonzalez-Munoz, M.T. Influence of Substrate Mineralogy on Bacterial Mineralization of Calcium Carbonate: Implications for Stone Conservation. Appl. Environ. Microb. 2012, 78, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, K.; Ramakrishnan, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using microorganisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Reddy, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Micallef, R.; Vella, D.; Sinagra, E.; Zammit, G. Biocalcifying Bacillus subtilis cells effectively consolidate deteriorated Globigerina limestone. J. Ind. Microbiol. Biotechnol. 2016, 43, 941–952. [Google Scholar] [CrossRef]
- Perito, B.; Mastromei, G. Conservation of monumental stones by bacterial biomineralization. Microbiol. Today 2003, 30, 113–114. [Google Scholar]
- Rodriguez-Navarro, C.; Rodriguez-Gallego, M.; Ben Chekroun, K.; Gonzalez-Munoz, M.T. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microb. 2003, 69, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Rodriguez-Navarro, C.; Pinar, G.; Carrillo-Rosua, F.J.; Rodriguez-Gallego, M.; Gonzalez-Munoz, M.T. Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the microbiota inhabiting the stone. Chemosphere 2007, 68, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, M.I.; Magoulas, A.; Kotoulas, G.; Catsikis, I.; Bakolas, A.; Karageorgis, A.P.; Mavridou, A.; Doulia, D.; Rigas, F. Pseudomonas, Pantoea and Cupriavidus isolates induce calcium carbonate precipitation for biorestoration of ornamental stone. J. Appl. Microbiol. 2013, 115, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Perito, B.; Marvasi, M.; Barabesi, C.; Mastromei, G.; Bracci, S.; Vendrell, M.; Tiano, P. A Bacillus subtilis cell fraction (BCF) inducing calcium carbonate precipitation: Biotechnological perspectives for monumental stone reinforcement. J. Cult. Herit. 2014, 15, 345–351. [Google Scholar] [CrossRef]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martin-Sanchez, I.; Gonzalez-Munoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Sorlini, C. Bioremediation. In Plant Biology for Cultural Heritage. Bioremediation and Conservation; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 340–346. [Google Scholar]
- Gauri, K.L.; Parks, L.; Jaynes, J.; Atlas, R. Removal of Sulfated-Crust from Marble Using Sulfate-Reducing Bacteria. In Proceedings of the International Conference on Stone Cleaning and the Nature, Soiling and Decay Mechanisms of Stone, Edinburgh, UK, 14–16 April 1992; pp. 160–165. [Google Scholar]
- Atlas, R.M.; Chowdhury, A.N.; Gauri, K.L. Microbial calcification of gypsum-rock and sulfated marble. J. Stud. Conserv. 1988, 33, 149–153. [Google Scholar]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2015, 65, 1227–1241. [Google Scholar] [CrossRef]
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. The use of microorganisms for the removal of sulphates on artistic stoneworks. Int. Biodeterior. Biodegrad. 1997, 40, 255–261. [Google Scholar] [CrossRef]
- Cappitelli, F.; Zanardini, E.; Ranalli, G.; Mello, E.; Daffonchio, D.; Sorlini, C. Improved methodology for bioremoval of black crusts on historical stone artworks by use of sulfate-reducing bacteria. Appl. Environ. Microbiol. 2006, 72, 3733–3737. [Google Scholar] [CrossRef]
- Cappitelli, F.; Toniolo, L.; Sansonetti, A.; Gulotta, D.; Ranalli, G.; Zanardini, E.; Sorlini, C. Advantages of using microbial technology over traditional chemical technology in removal of black crusts from stone surfaces of historical monuments. Appl. Environ. Microbiol. 2007, 73, 5671–5675. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Cappitelli, F.; Brusetti, L.; Principi, P.; Villa, F.; Giacomucci, L.; Ranalli, G.; Sorlini, C. Feasibility of removing surface deposits on stone using biological and chemical remediation methods. Microb. Ecol. 2010, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Zanardini, E.; Toniolo, L.; Abbruscato, P.; Ranalli, G.; Sorlini, C. Bioconservation of the marble base of the Pietà Rondanini by Michelangelo Buonarroti. J. Appl. Microbiol. 2005, 7, 06675. [Google Scholar]
- Gioventù, E.; Lorenzi, P. Bio-removal of black Crust from marble surface: Comparison with traditional methodologies and application on a sculpture from the Florence’s English Cemetery. Procedia Chem. 2013, 8, 123–129. [Google Scholar] [CrossRef]
- Elhagrassy, A.F.; Hakeem, A. Comparative study of biological cleaning and laser techniques for conservation of weathered stone in Failaka Island, Kuwait. Sci. Cult. 2018, 4, 43–50. [Google Scholar]
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research; The Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. The use of microorganisms for the removal of nitrates and organic substances on artistic stoneworks. In Proceedings of the 8th International Congress on Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996. [Google Scholar]
- May, E.; Webster, A.M.; Inkpen, R.; Zamarreno, D.; Kuever, J.; Rudolph, C.; Warscheid, T.; Sorlini, C.; Cappitelli, F.; Zanardini, E.; et al. The Biobrush Project for Bioremediation of Heritage Stone. In Heritage Microbiology and Science: Microbes, Monuments and Maritime Materials; May, E., Jones, M., Mitchell, J., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 76–93. [Google Scholar]
- Bosch-Roig, P.; Regidor-Ros, J.L.; Montes-Estellés, R. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef]
- Romano, I.; Abbate, M.; Poli, A.; D’Orazio, L. Bio-cleaning of nitrate salt efflorescence on stone samples using extremophilic bacteria. Sci. Rep. 2019, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Gioventù, E.; Lorenzi, P.F. Valutazione dell’efficacia nell’utilizzo di microrganismi per la biorimozione delle croste nere dai materiali lapidei ed approfondimento delle metodologie applicative. OPD Restauro 2010, 22, 127–138. [Google Scholar]
- Troiano, F.; Guiotta, D.; Balloi, A.; Polo, A.; Toniolo, L.; Lombardi, E.; Daffonchio, D.; Sorlini, C.; Cappitelli, F. Successful combination of chemical and biological treatments for the cleaning of stone artworks. Int. Biodeterior. Biodegrad. 2013, 85, 294–304. [Google Scholar] [CrossRef]
- Alfano, G.; Lustrato, G.; Belli, C.; Zanardini, E.; Cappitelli, F.; Mello, E.; Sorlini, C.; Ranalli, G. Biodegradation, The bioremoval of nitrate and sulfate alterations on artistic stonework: The case-study of Matera Cathedral after six years from the treatment. Int. Biodeterior. Biodegrad. 2011, 65, 1004–1011. [Google Scholar] [CrossRef]
- Mazzoni, M.; Alisi, C.; Tasso, F.; Cecchini, A.; Marconi, P.; Sprocati, A.R. Laponite micro-packs for the selective cleaning of multiple coherent deposits on wall paintings: The case study of Casina Famese on the Palatine Hill (Rome-Italy). Int. Biodeterior. Biodegrad. 2014, 94, 1–11. [Google Scholar] [CrossRef]
- Antonioli, P.; Zapparoli, G.; Abbruscato, P.; Sorlini, C.; Ranalli, G.; Righetti, P.G. Art-loving bugs: The resurrection of Spinello Aretino from Pisa’s cemetery. Proteomics 2005, 5, 2453–2459. [Google Scholar] [CrossRef]
- Ranalli, G.; Belli, C.; Baracchini, C.; Caponi, G.; Pacini, P.; Zanardini, E.; Sorlini, C. Deterioration and bioremediation of frescoes: A case-study. In Molecular Biology and Cultural Heritage; Saiz-Jimenez, C., Ed.; Balkema Publishers: Lisse, The Netherlands, 2003; pp. 243–246. [Google Scholar]
- Lustrato, G.; Alfano, G.; Andreotti, A.; Colombini, M.P.; Ranalli, G. Fast biocleaning of mediaeval frescoes using viable bacterial cells. Int. Biodeterior. Biodegrad. 2012, 69, 51–61. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Regidor-Ros, J.L.; Soriano-Sancho, P.; Montes-Estelles, R.M. Biocleaning of animal glue on wall paintings by Pseudomonas stutzeri. Chim. Oggi-Chem. Today 2013, 31, 50–53. [Google Scholar]
- Ranalli, G.; Zanardini, E.; Andreotti, A.; Colombini, M.P.; Corti, C.; Bosch-Roig, P.; De Nuntiis, P.; Lustrato, G.; Mandrioli, P.; Rampazzi, L.; et al. Hi-tech restoration by two-steps biocleaning process of Triumph of Death fresco at the Camposanto Monumental Cemetery (Pisa, Italy). J. Appl. Microbiol. 2018, 125, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, L.; Toja, F.; Sanmartín, P.; Toniolo, L.; Prieto, B.; Villa, F.; Cappitelli, F. Degradation of nitrocellulose-based paint by Ddesulfuricans ATCC 13541. Biodegradation 2012, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A.; Mitchell, R. Feasibility study involving the search for natural strains of microorganisms capable of degrading graffiti from heritage materials. Int. Biodeterior. Biodegrad. 2015, 103, 186–190. [Google Scholar] [CrossRef]
- Sanmartín, P.; Bosch-Roig, P. Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action. Coatings 2019, 9, 104. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Santana, M.A.; Galindo-Castro, I.; Gonzalez, A. The role of biotechnology in art preservation. Trends Biotechnol. 2005, 23, 584–588. [Google Scholar] [CrossRef]
- Szewczyk, B.; Hoyos-Carvajal, L.; Paluszek, M.; Skrzecz, W.; de Souza, M.L. Baculoviruses-re-emerging biopesticides. Biotechnol. Adv. 2006, 24, 143–160. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Mikkola, R.; Andersson, M.A.; Grigoriev, P.; Teplova, V.V.; Saris, N.E.; Rainey, F.A.; Salkinoja-Salonen, M.S. Bacillus amyloliquefaciens strains isolated from moisture-damaged buildings produced surfactin and a substance toxic to mammalian cells. Arch. Microbiol. 2004, 181, 314–323. [Google Scholar]
- Ben Slimene, I.; Tabbene, O.; Djebali, N.; Cosette, P.; Schmitter, J.M.; Jouenne, T.; Urdaci, M.C.; Limam, F. Putative use of a Bacillus subtilis L194 strain for biocontrol of Phoma medicaginis in Medicago truncatula seedlings. Res. Microbiol. 2012, 163, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, A.T.; Feio, S.S.; Arteiro, J.M.S.; Roseiro, J.C. Bacillus amyloliquefaciens CCMI 1051 in vitro activity against wood contaminant fungi. Ann. Microbiol. 2007, 57, 29–33. [Google Scholar] [CrossRef]
- Tsuge, K.; Akiyama, T.; Shoda, M. Cloning, sequencing, and characterization of the iturin A operon. J. Bacteriol. 2001, 183, 6265–6273. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Rosado, T.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Production of Green Biocides for Cultural Heritage. Novel Biotechnological Solutions. Int. J. Conserv. Sci. 2015, 6, 519–530. [Google Scholar]
- Silva, M.; Pereira, A.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Combined Use of NMR, LC-ESI-MS and Antifungal Tests for Rapid Detection of Bioactive Lipopeptides Produced by Bacillus. Adv. Microbiol. 2016, 6, 788–796. [Google Scholar] [CrossRef]
- Silva, M.; Rosado, T.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Green mitigation strategy for cultural heritage: Bacterial potential for biocide production. Environ. Sci. Pollut. Res. Int. 2017, 24, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Sasso, S.; Scrano, L.; Ventrella, E.; Bonomo, M.G.; Crescenzi, A.; Salzano, G.; Bufo, S.A. Natural biocides to prevent the microbial growth on cultural heritage. In Proceedings of the Conference Built Heritage 2013 Monitoring Conservation Management, Milan, Italy, 18–20 November 2013; pp. 1035–1042. [Google Scholar]
- Scrano, L.; Milella, G.; Napolitano, G.; Milan, S.; Lelario, F.; Bonomo, M.G.; Bufo, S.A. Utilizzo virtuoso di biocidi naturali nella conservazione dei manufatti d’interesse storico-culturale. In Proceedings of the XXXII° Convegno Nazionale della Società Italiana di Chimica Agraria, Libera Università di Bolzano, Bolzano, Italy, 7–9 September 2014. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Racioppi, R.; Scrano, L.; Iacobellis, N.S.; Bufo, S.A. In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 2012, 13, 16291–16302. [Google Scholar] [CrossRef]
- Marin, E.; Vaccaro, C.; Leis, M. Biotechnology Applied to Historic Stoneworks Conservation: Testing the Potential Harmfulness of Two Biological Biocides. Int. J. Conserv. Sci. 2016, 7, 227–238. [Google Scholar]
- Goel, A.K. Anthrax: A disease of biowarfare and public health importance. World J. Clin. Cases 2015, 3, 20–33. [Google Scholar] [CrossRef]
- Granum, P.E.; Lund, T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997, 157, 223–228. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Bressollier, P.; Verneuil, B.; Fenet, B.; Sorokulova, I.B.; Megraud, F.; Urdaci, M.C. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 2001, 45, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.B.; Kirik, D.L.; Pinchuk, I.I. Probiotics against Campylobacter Pathogens. J. Travel Med. 1997, 4, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation-A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Vaseeharan, B.; Ramasamy, P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett. Appl Microbiol. 2003, 36, 83–87. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Mazzacane, S.; Finzi, G.; Aparo, L.; Balboni, P.G.; Vandini, A.; Lanzoni, L.; Camerada, M.T.; Coccagna, M.; Antonioli, P.; Branchini, A.; et al. The Sanitation of Hospital Stays: New Strategies for The Reduction of HAIs. Health Manag. 2014, 14, 1–12. [Google Scholar]
- Vandini, A.; Temmerman, R.; Frabetti, A.; Caselli, E.; Antonioli, P.; Balboni, P.G.; Platano, D.; Branchini, A.; Mazzacane, S. Hard Surface Biocontrol in Hospitals Using Microbial-Based Cleaning Products. PLoS ONE 2014, 9, e108598. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Vandini, A.; Lanzoni, L.; Camerada, M.T.; Coccagna, M.; Branchini, A.; Antonioli, P.; Balboni, P.G.; Di Luca, D.; et al. Impact of a Probiotic-Based Cleaning Intervention on the Microbiota Ecosystem of the Hospital Surfaces: Focus on the Resistome Remodulation. PLoS ONE 2016, 11, e0148857. [Google Scholar] [CrossRef]
- Caselli, E.; Antonioli, P.; Mazzacane, S. Safety of probiotics used for hospital environmental sanitation. J. Hosp. Infect. 2016, 94, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E. Hygiene: Microbial strategies to reduce pathogens and drug resistance in clinical settings. Microb. Biotechnol. 2017, 10, 1079–1083. [Google Scholar] [CrossRef]
- Caselli, E.; Brusaferro, S.; Coccagna, M.; Arnoldo, L.; Berloco, F.; Antonioli, P.; Tarricone, R.; Pelissero, G.; Nola, S.; La Fauci, V.; et al. Reducing healthcare-associated infections incidence by a probiotic-based sanitation system: A multicentre, prospective, intervention study. PLoS ONE 2018, 13, e0199616. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Arnoldo, L.; Rognoni, C.; D’Accolti, M.; Soffritti, I.; Lanzoni, L.; Bisi, M.; Volta, A.; Tarricone, R.; Brusaferro, S.; et al. Impact of a probiotic-based hospital sanitation on antimicrobial resistance and HAI-associated antimicrobial consumption and costs: A multicenter study. Infect. Drug Resist. 2019, 12, 501–510. [Google Scholar] [CrossRef]
- Perito, B.; Cavalieri, D. Innovative metagenomic approaches for detection of microbial communities involved in biodeterioration of cultural heritage. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 012074. [Google Scholar]
| Year | Microorganism/s | Artwork | Application | Mechanism/Findings | References [n°] |
|---|---|---|---|---|---|
| 1988 | D. desulfuricans1 | Stonework | Immersion | Removal of sulphates from marble (in vitro tests) | [59] |
| 1992 | D. desulfuricans | Stonework | Immersion | Removal of sulphates from marble (in vitro tests) | [58] |
| 1996 | P. stutzeri2 | Stonework | Sepiolite as delivery system | Removal of nitrates from brickwords/calcareous stones (in vitro tests) | [69] |
| 1997 | Desulfovibrio spp. | Stonework | Sepiolite as delivery system | Removal of sulphates from marble (in vitro tests) | [61] |
| 2001 | B. pasteurii3 | Stonework | Cells immobilized in polyurethane foam | Bioconsolidation of concrete (in vitro tests) | [49] |
| 2003 | M. xanthus4 | Stonework | Immersion | Bioconsolidation of ornamental limestone (in vitro tests) | [52] |
| 2005 | D. vulgaris subsp. vulgaris | Stonework | Carbogel carrier | Removal of black crusts from marble sculpture | [65] |
| 2005 | P. stutzeri | Fresco | Application with cotton wool layer (enzymes added) | Removal of animal glue from frescoes | [36] |
| 2006 | D. vulgaris subsp. vulgaris | Stonework | Sepiolite/Hydrobiogel-97/carbogel carrier | Removal of black crusts from marble surfaces | [62] |
| 2007 | D. vulgaris subsp. vulgaris | Stonework | Carbogel carrier | Removal of black crusts from marble surfaces | [63] |
| 2008 | D. vulgaris subsp. vulgaris, P. pseudoalcaligenes 5, P. stutzeri | Stonework | Carbogel/Mortar and alginate beads | Removal of nitrates and sulphates from sandstone walls | [70] |
| 2010 | D. vulgaris subsp. vulgaris | Stonework | Carbogel (mechanical treatment added) | Removal of black crusts from colored lithotypes | [73] |
| 2010 | D. vulgaris subsp. vulgaris | Stonework | Carbogel carrier (biocide treatment added) | Removal of black crusts from limestone sculpture | [64] |
| 2011 | D. vulgaris subsp. vulgaris P. pseudoalcaligenes | Stonework | Carbogel with multilayer biosystem | Removal of nitrates and sulphates from sandstone walls | [75] |
| 2012 | P. stutzeri | Fresco | Application with cotton wool layer | Removal of animal glue from frescoes | [79] |
| 2013 | P. stutzeri | Wall painting | Agar carrier | Removal of animal glue and nitrates from wall paintings | [71,80] |
| 2013 | Pseudomonas, Pantoea, Cupriavidus | Stonework | Immersion | Bioconsolidation of stone by resident-carbonatogenic microorganisms (in vitro tests) | [54] |
| 2013 | D. vulgaris subsp. vulgaris | Stonework | Carbogel carrier | Removal of black crusts from marble surfaces | [66] |
| 2013 | B. gladioli pv. agaricicola6 | Stonework | In vitro tests (only bacterial metabolites) | Decontaminating activity against biodeteriogenic bacteria/fungi (in vitro tests) | [95] |
| 2013 | D. vulgaris subsp. vulgaris | Stonework | Arbocel (nonionic detergent treatment added) | Removal of black crusts from marble surfaces | [74] |
| 2014 | C. cellulans7, S. maltophilia8, P. koreensis9 | Wall painting | Laponite micro-packs | Biocleaning of organic/inorganic residues from wall painting | [76] |
| 2015 | Bacillus spp. | Wall painting | Immersion (only bacterial metabolites) | Decontaminating activity against biodeteriogenic fungi (in vitro tests) | [92] |
| 2016 | Bacillus spp. | Stonework | Spraying | Biocleaning of biological patina from historical bricks (in vitro tests) | [115] |
| 2016 | B. subtilis10 | Stonework | Spraying | Bioconsolidation of historical limestone (in vitro tests) | [50] |
| 2017 | Indigenous carbonatogenic bacteria | Stonework | Self-inoculation of stone’s bacteria | Bioconsolidation of stone by self-inoculation of resident- carbonatogenic microorganisms | [56] |
| 2018 | Bacillus spp. | Easel painting | In vitro tests | Decontaminating activity against biodeteriogenic bacteria/fungi (in vitro tests) | [17] |
| 2018 | D. vulvaris | Stonework | Hydrobiogel-97 | Removal of black crust from stone surfaces | [67] |
| 2018 | P. stutzeri | Fresco | Brush and cotton layers | Removal of animal glue from frescoes | [81] |
| 2019 | P. stutzeri | Wall painting | Agar-gauze biogel | Removal of organic residues from wall paintings | [37] |
| 2019 | H.campaniensis11 | Stonework | Bacterial layer spread with spatula and agar disc | Removal of nitrate crusts from stone artwork | [72] |
| 2019 | P. stutzeri, A. aerogenes12, Comamonas spp. | Stonework | Application with cotton wool layer/agar carrier | Biocleaning of graffiti paints from granite and concrete surfaces (in vitro tests) | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soffritti, I.; D’Accolti, M.; Lanzoni, L.; Volta, A.; Bisi, M.; Mazzacane, S.; Caselli, E. The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability 2019, 11, 3853. https://doi.org/10.3390/su11143853
Soffritti I, D’Accolti M, Lanzoni L, Volta A, Bisi M, Mazzacane S, Caselli E. The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability. 2019; 11(14):3853. https://doi.org/10.3390/su11143853
Chicago/Turabian StyleSoffritti, Irene, Maria D’Accolti, Luca Lanzoni, Antonella Volta, Matteo Bisi, Sante Mazzacane, and Elisabetta Caselli. 2019. "The Potential Use of Microorganisms as Restorative Agents: An Update" Sustainability 11, no. 14: 3853. https://doi.org/10.3390/su11143853
APA StyleSoffritti, I., D’Accolti, M., Lanzoni, L., Volta, A., Bisi, M., Mazzacane, S., & Caselli, E. (2019). The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability, 11(14), 3853. https://doi.org/10.3390/su11143853






