Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia
Abstract
1. Introduction
2. Materials and Methods
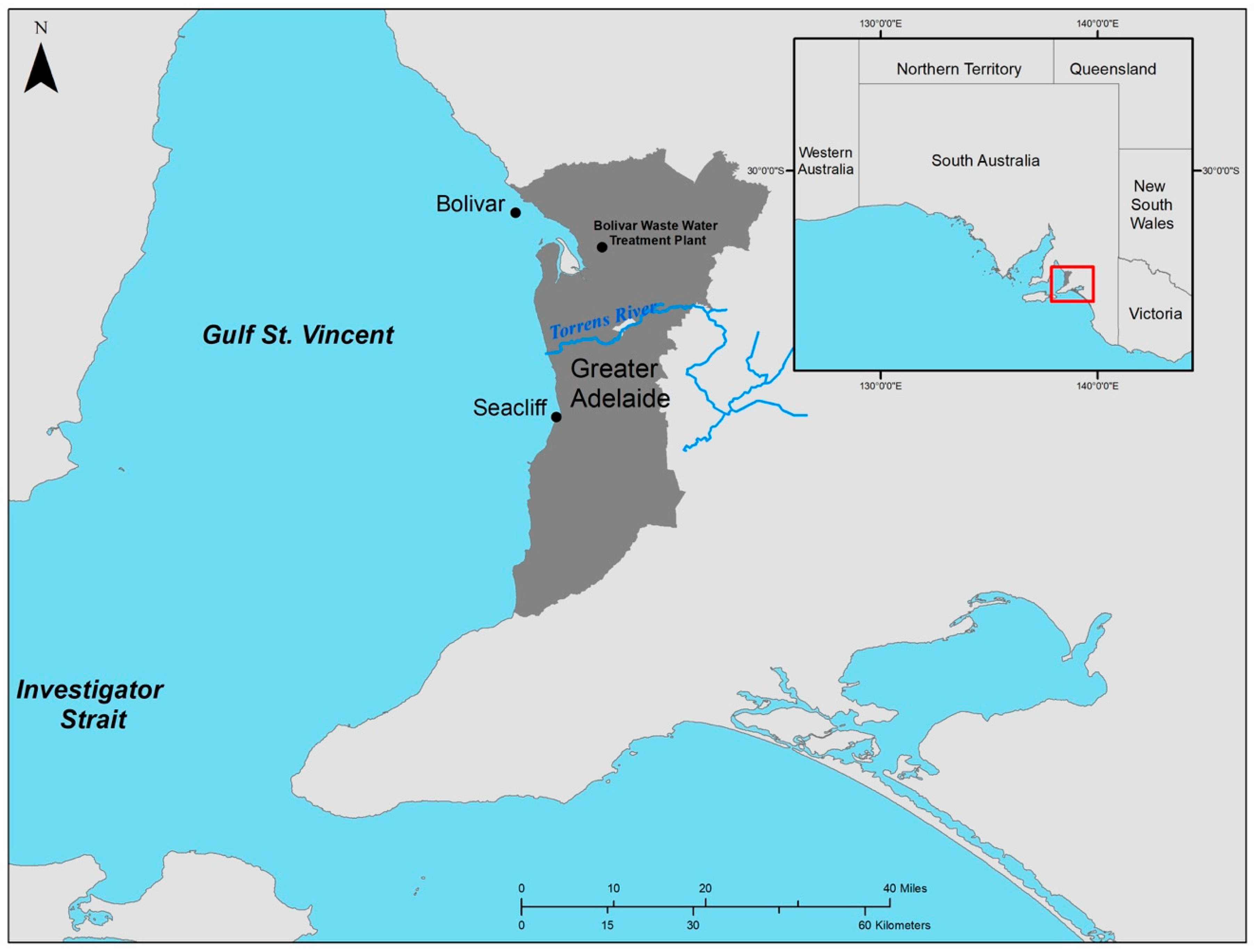
2.1. Study Area
2.2. Field Sampling
2.3. Measuring Reflectance Spectra of Benthic Types
2.4. Deconvolution Analyses
2.5. Statistical Analyses
3. Results and Discussion
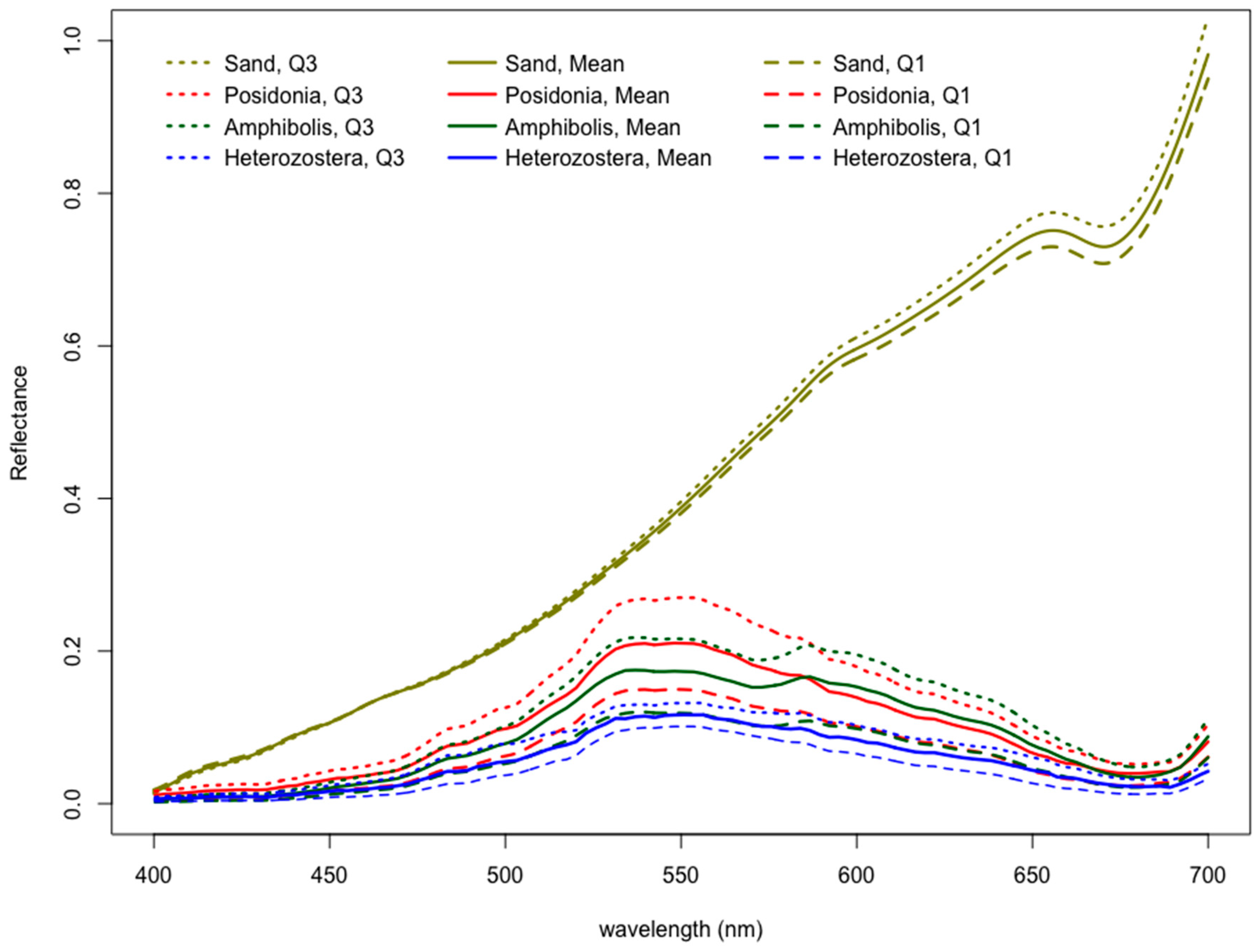
3.1. Separating between Benthic Bottom Types
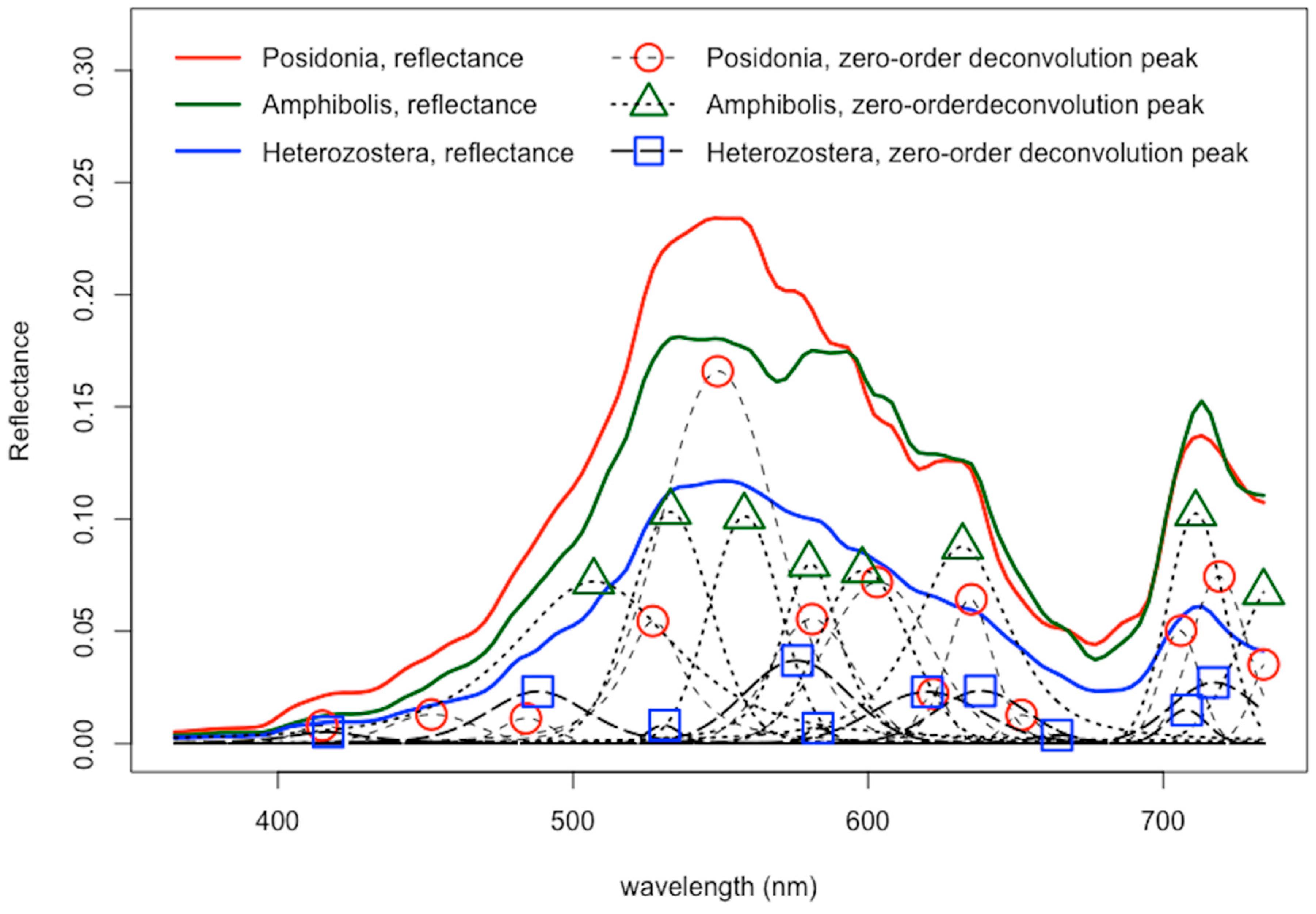
3.2. Spectral Deconvolution of Seagrass Spectra
3.3. Influence of Site Location
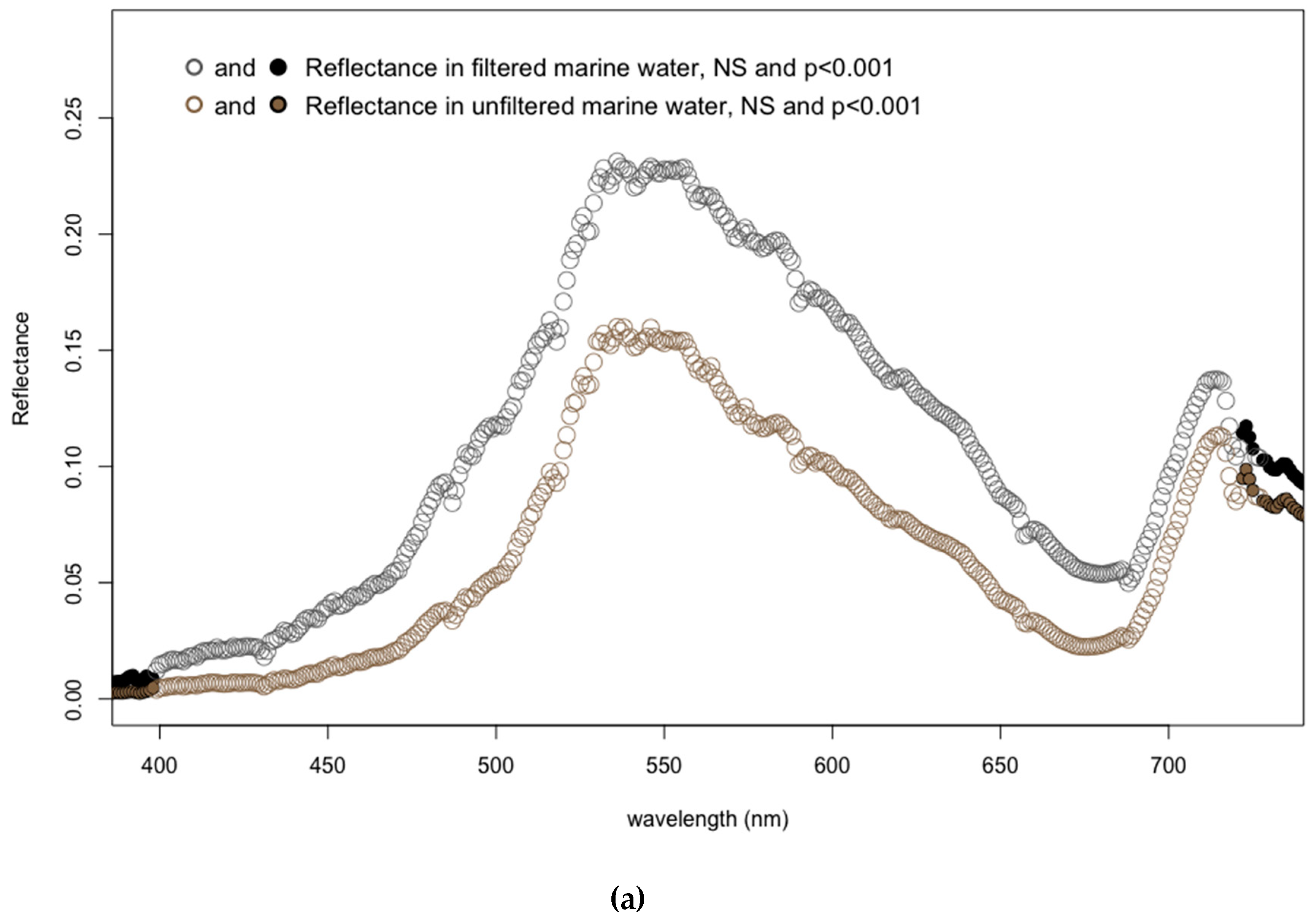
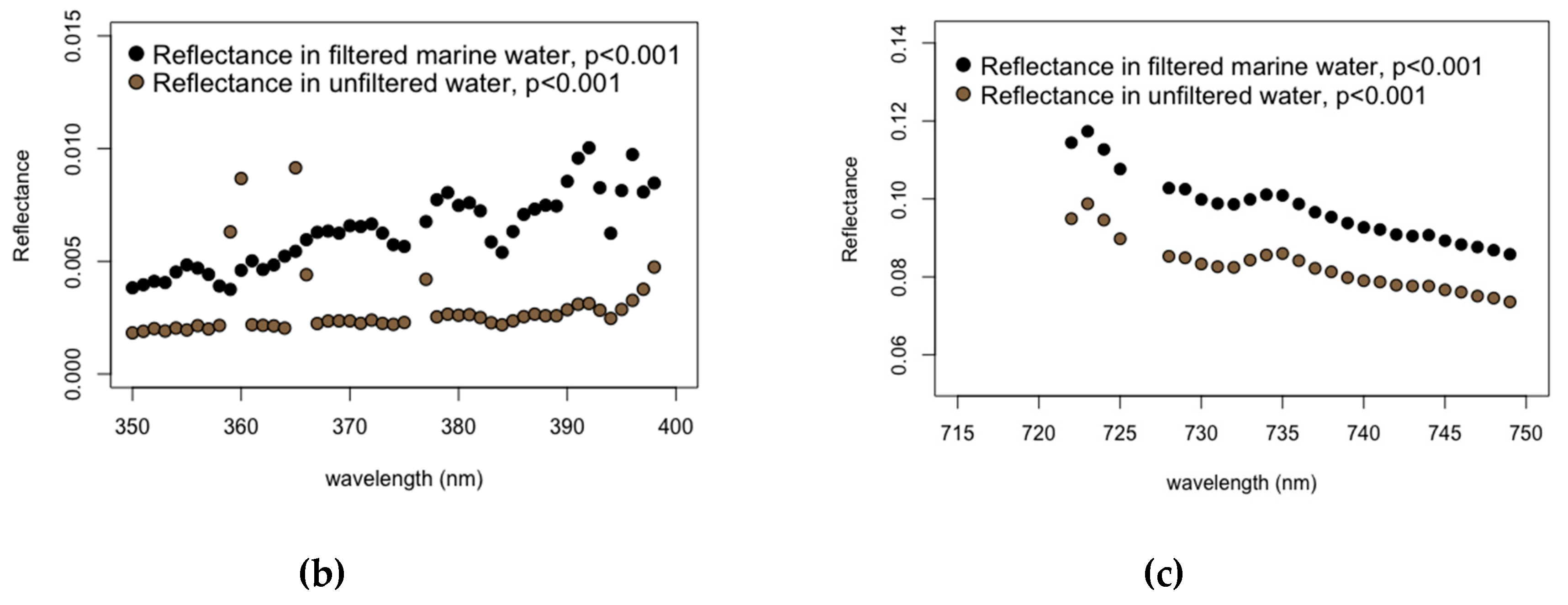
3.4. Effects of Marine Filtered Water
4. Conclusions
- The bandwidth ratio of 566:689 helps distinguish seagrasses from sand, and the bandwidth ratio 566:600 may help distinguish seagrasses from algae and detritus.
- Deconvolution analyses proved useful in the reduction of dimensions by identifying overlapping bandwidths of different seagrass genera and decreasing the number of wavelengths that need to be considered. Specifically, first-derivative deconvolution spectral peak analyses reveal to be the most efficient derivative-based method in isolating crucial, non-contiguous bandwidths throughout the visible light spectrum that can be used to distinguish seagrass genera.
- Variations between local regions appear to have no effect on spectral endmembers, thereby making spectral reflectance values suitable markers for identifying submerged benthic bottom types throughout the world, not just within a particular region.
- Fluctuations in marine water composition appear to have no significant effect on endmember selection for the detection of submerged aquatic vegetation.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2008; p. 312. [Google Scholar]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping seagrass species, cover and biomass in shallow waters: An assessment of satellite multi-spectral and airborne hyper-spectral imaging systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Williams, S.L.; Heck, K.L., Jr. Seagrass Community Ecology. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2011; pp. 317–337. [Google Scholar]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef]
- Fyfe, S.K. Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef]
- Wabnitz, C.C.; Andréfouët, S.; Torres-Pulliza, D.; Müller-Karger, F.E.; Kramer, P.A. Regional-scale seagrass habitat mapping in the Wider Caribbean region using Landsat sensors: Applications to conservation and ecology. Remote Sens. Environ. 2008, 112, 3455–3467. [Google Scholar] [CrossRef]
- Borfecchia, F.; De Cecco, L.; Martini, S.; Ceriola, G.; Bollanos, S.; Vlachopoulos, G.; Belmonte, A.; Micheli, C. Posidonia oceanica genetic and biometry mapping through high-resolution satellite spectral vegetation indices and sea-truth calibration. Int. J. Remote Sens. 2013, 34, 4680–4701. [Google Scholar] [CrossRef]
- Cunha, A.H.; Assis, J.F.; Serrão, E.A. Reprint of Seagrasses in Portugal: A most endangered marine habitat. Aquat. Bot. 2014, 115, 3–13. [Google Scholar] [CrossRef]
- Ralph, P.J.; Durako, M.J.; Enríquez, S.; Collier, C.J.; Doblin, M.A. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Kimura, T.; Fujiwara, S.; Shibuno, T.; Mito, K.; Nakai, T.; Sasaki, Y.; Chang-Feng, D.; Gang, C. Status of Coral Reefs in East and North Asia (China, Hong Kong, Taiwan, South Korea and Japan). In Status of Coral Reefs of the World 2008; Wilkinson, C., Ed.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Center: Townsville, Australia, 2008; pp. 145–158. [Google Scholar]
- Duarte, C.; Marba, N.; Santos, R. What May Cause Loss of Seagrasses? In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; EU Monitoring and Managing of European Seagrasses (M&MS) EVK3-CT-2000-00044: Brussels, Belgium, 2004; pp. 24–32. [Google Scholar]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Blackburn, D.T.; Dekker, A.G. Remote Sensing Study of Marine and Coastal Features and Interpretation of Changes in Relation to Natural and Anthropogenic Processes: Final Technical Report; ACWS Technical Report No. 6. Prepared for the Adelaide Coastal Waters Study Steering Committee; South Australian Environment Protection Authority (SA EPA): Adelaide, Australia, 2006.
- Nayar, S.; Collings, G.; Pfennig, P.; Royal, M. Managing nitrogen inputs into seagrass meadows near a coastal city: Flow-on from research to environmental improvement plans. Mar. Pollut. Bull. 2012, 64, 932–940. [Google Scholar] [CrossRef]
- Tanner, J.E.; Irving, A.D.; Fernandes, M.; Fotheringham, D.; McArdle, A.; Murray-Jones, S. Seagrass rehabilitation off metropolitan Adelaide: A case study of loss, action, failure and success. Ecol. Manag. Restor. 2014, 15, 168–179. [Google Scholar] [CrossRef]
- Hart, D. Seagrass Extent Change 2007–2013—Adelaide Coastal Waters; DEWNR Technical Note 2013/07; Government of South Australia. Department of Environment Water and Natural Resources: Adelaide, Australia, 2013; p. 19.
- Tanner, J.E.; Theil, M.; Fotheringham, D.G. Seagrass Condition Monitoring: Encounter Bay and Port Adelaide: Final Report Prepared for the Adelaide and Mount Lofty Ranges Natural Resources Management Board; SARDI Research Report, Series; Theil, M., Ed.; South Australian Research and Development Institution: West Beach, Australia, 2014; pp. 1324–2083.
- Clarke, K.; Hennessy, A.; Lewis, M. Adelaide Metropolitan Coastline benthic Habitat Mapping from Hyperspectral Imagery; Product A: Bare-substrate vs. non-bare Substrate; Prepared for SA Water, SA Department for Environment and Water, and SA Environmental Protection Authority; School of Biological Sciences, The University of Adelaide: Adelaide, Australia, 2018. [Google Scholar]
- Dekker, A.G.; Brando, V.E.; Anstee, J.M. Retrospective seagrass change detection in a shallow coastal tidal Australian lake. Remote Sens. Environ. 2005, 97, 415–433. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Costa, M.; Sharma, T. Remote Sensing of Shallow Coastal Benthic Substrates: In situ Spectra and Mapping of Eelgrass (Zostera marina) in the Gulf Islands National Park Reserve of Canada. Remote Sens. 2011, 3, 975–1005. [Google Scholar] [CrossRef]
- Dekker, A.; Rando, V.; Anstee, J.; Fyfe, S.; Malthus, T.; Karpouzli, E. Remote Sensing of Seagrass Ecosystems: Use of Spaceborne and Airborne Sensors. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 347–359. [Google Scholar]
- Dunk, I.; Lewis, M. Seagrass and shallow water feature discrimination using HyMap imagery. In Proceedings of the 10th Australasian Remote Sensing Photogrammetry Conference, Adelaide, Australia, 21–25 August 2000. [Google Scholar]
- Brando, V.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Costa, M. Mapping eelgrass (Zostera marina) in the Gulf Islands National Park Reserve of Canada using high spatial resolution satellite and airborne imagery. Remote Sens. Environ. 2013, 133, 152–167. [Google Scholar] [CrossRef]
- Kakuta, S.; Takeuchi, W.; Prathep, A. Seaweed and seagrass mapping in Thailand measured by using Landsat 8 optical and texture properties. In Proceedings of the International Symposium on Remote Sensing (ISRS), Tainan, Taiwan, 22–24 April 2015. [Google Scholar]
- Medjahed, S.A.; Ouali, M. Band selection based on optimization approach for hyperspectral image classification. Egypt. J. Remote Sens. Space Sci. 2018, 21, 413–418. [Google Scholar] [CrossRef]
- Veys, C.; Hibbert, J.; Davis, P.; Grieve, B. An ultra-low-cost active multispectral crop diagnostics device. In Proceedings of the 2017 IEEE SENSORS, Glasgow, Scotland, 30 October–1 November 2017. [Google Scholar]
- Transon, J.; d’Andrimont, R.; Maugnard, A.; Defourny, P. Survey of Hyperspectral Earth Observation Applications from Space in the Sentinel-2 Context. Remote Sens. 2018, 10, 157. [Google Scholar] [CrossRef]
- Vahtmäe, E.; Kutser, T.; Martin, G.; Kotta, J. Feasibility of hyperspectral remote sensing for mapping benthic macroalgal cover in turbid coastal waters—A Baltic Sea case study. Remote Sens. Environ. 2006, 101, 342–351. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, C.C.; Chung, H.W.; Lee, L.J.; Yang, W.C. Development and evaluation of a genetic algorithm-based ocean color inversion model for simultaneously retrieving optical properties and bottom types in coral reef regions. Appl. Opt. 2014, 53, 605–617. [Google Scholar] [CrossRef]
- Mumby, P.J.; Skirving, W.; Strong, A.E.; Hardy, J.T.; LeDrew, E.F.; Hochberg, E.J.; Stumpf, R.P.; David, L.T. Remote sensing of coral reefs and their physical environment. Mar. Pollut. Bull. 2004, 48, 219–228. [Google Scholar] [CrossRef]
- Patten, B.C. Systems approach to the concept of environment. Ohio J. Sci. 1978, 78, 206–222. [Google Scholar]
- Gallopin, G.C. Environmental and sustainability indicators and the concept of situational indicators. A systems approach. Environ. Model. Assess. 1996, 1, 101–117. [Google Scholar] [CrossRef]
- Hedley, J.D.; Mumby, P.J. A remote sensing method for resolving depth and subpixel composition of aquatic benthos. Limnol. Oceanogr. 2003, 48, 480–488. [Google Scholar] [CrossRef]
- Hornberger, G.M.; Spear, R.C. Approach to the preliminary analysis of environmental systems. J. Environ. Mgmt. 1981, 12, 7–18. [Google Scholar]
- Karpouzli, E.; Malthus, T.J.; Place, C.J. Hyperspectral discrimination of coral reef benthic communities in the western Caribbean. Coral Reefs 2004, 23, 141–151. [Google Scholar] [CrossRef]
- Miglani, A. Hyperspectral Remote Sensing—An Overview. 7 December 2010. Available online: https://www.geospatialworld.net/article/hyperspectral-remote-sensing-an-overview/ (accessed on 13 April 2019).
- Takebe, M.; Yoneyama, T.; Inada, K.; Murakami, T. Spectral reflectance ratio of rice canopy for estimating crop nitrogen status. Plant Soil 1990, 122, 295–297. [Google Scholar] [CrossRef]
- Christensen, S.; Goudriaan, J. Deriving light interception and biomass from spectral reflectance ratio. Remote Sens. Environ. 1993, 43, 87–95. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Han, L.; Rundquist, D.C. Comparison of NIR/RED ratio and first derivative of reflectance in estimating algal-chlorophyll concentration: A case study in a turbid reservoir. Remote Sens. Environ. 1997, 62, 253–261. [Google Scholar] [CrossRef]
- Demetriades-Shah, T.H.; Steven, M.D.; Clark, J.A. High resolution derivative spectra in remote sensing. Remote Sens. Environ. 1990, 33, 55–64. [Google Scholar] [CrossRef]
- Scheinost, A.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Spectral discrimination of coral reef benthic communities. Coral Reefs 2000, 19, 164–171. [Google Scholar] [CrossRef]
- Han, L. Spectral reflectance of Thalassia testudinum with varying depths. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; pp. 2123–2125. [Google Scholar]
- Bargain, A.; Robin, M.; Le Men, E.; Huete, A.; Barillé, L. Spectral response of the seagrass Zostera noltii with different sediment backgrounds. Aquat. Bot. 2012, 98, 45–56. [Google Scholar] [CrossRef]
- Bargain, A.; Robin, M.; Méléder, V.; Rosa, P.; Le Menn, E.; Harin, N.; Barillé, L. Seasonal spectral variation of Zostera noltii and its influence on pigment-based Vegetation Indices. J. Exp. Mar. Biol. Ecol. 2013, 446, 86–94. [Google Scholar] [CrossRef]
- Fulton, S. An Analysis of the Spectral Reflectance Separability of Amphibolis Griffithii and Posidonia Sinuosa at Different Water Depths, in School of the Environment; The Flinders University of South Australia: Adelaide, Australia, 2014; p. 97. [Google Scholar]
- Dierssen, H.M.; Kudela, R.M.; Ryan, J.P.; Zimmerman, R.C. Red and black tides: Quantitative analysis of water-leaving radiance and perceived color for phytoplankton, colored dissolved organic matter, and suspended sediments. Limnol. Oceanogr. 2006, 51, 2646–2659. [Google Scholar] [CrossRef]
- Tsai, F.; Philpot, W. Derivative analysis of hyperspectral data. Remote Sens. Environ. 1998, 66, 41–51. [Google Scholar] [CrossRef]
- Treilibs, C.; Lewis, M.; Sparrow, B. Seagrass Discrimination and Mapping in Association with Atlantic Salmon Aquaculture, Cape Jaffa, South Australia: Report to the Native Vegetation Council; The University of Adelaide: Adelaide, Australia, 2005; p. 40. [Google Scholar]
- Poulsen, J.; French, A. Discriminant Function Analysis (DA); San Francisco State University: San Francisco, CA, USA, 2001. [Google Scholar]
- Maritorena, S.; Morel, A.; Gentili, B. Diffuse reflectance of oceanic shallow waters: Influence of water depth and bottom albedo. Limnol. Oceanogr. 1994, 39, 1689–1703. [Google Scholar] [CrossRef]
- Holden, H.; LeDrew, E. Hyperspectral identification of coral reef features. Int. J. Remote Sens. 1999, 20, 2545–2563. [Google Scholar] [CrossRef]
- Davies, T. The new automated mass spectrometry deconvolution and identification system (AMDIS). Spectrosc. Eur. 1998, 10, 24–27. [Google Scholar]
- Zhang, Q.; Alfarra, M.R.; Worsnop, D.R.; Allan, J.D.; Coe, H.; Canagaratna, M.R.; Jimenez, J.L. Deconvolution and quantification of hydrocarbon-like and oxygenated organic aerosols based on aerosol mass spectrometry. Environ. Sci. Technol. 2005, 39, 4938–4952. [Google Scholar] [CrossRef]
- Hasler-Sheetal, H.; Fragner, L.; Holmer, M.; Weckwerth, W. Diurnal effects of anoxia on the metabolome of the seagrass Zostera marina. Metabolomics 2015, 11, 1208–1218. [Google Scholar] [CrossRef]
- Bryars, S.; Collings, G.; Nayar, S.; Westphalen, G.; Miller, D.; O’Loughlin, E.; Fernandes, M.; Mount, G.; Tanner, J.; Wear, R.; et al. Assessment of the Effects of Inputs to the Adelaide Coastal Waters on the Meadow Forming Seagrasses, Amphibolis and Posidonia; Task EP 1 Final Technical Report; ACWS Technical Report No. 15. Prepared for the Adelaide Coastal Waters Study Steering Committee (Publication No. RD01/0208-19); South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2006.
- Bryars, S.; Rowling, K. Benthic habitats of eastern Gulf St Vincent: Major changes in benthic cover and composition following European settlement of Adelaide. Trans. R. Soc. S. Aust. 2009, 133, 318–338. [Google Scholar]
- Westphalen, G.; Collings, G.; Wear, R.; Fernandes, M.; Bryars, S.; Cheshire, A. A review of Seagrass Loss on the Adelaide Metropolitan Coastline; ACWS Technical Report No. 2. Prepared for the Adelaide Coastal Waters Study Steering Committee (Publication No. RD04/0073); South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2005.
- Bryars, S.; Miller, D.; Collings, G.; Fernandes, M.; Mount, G.; Wear, R. Field Surveys 2003–2005: Assessment of the Quality of Adelaide’s Coastal Waters, Sediments and Seagrasses; ACWS Technical Report No. 14. Prepared for the Adelaide Coastal Waters Study Steering Committee. Publication No. RD01/0208-15; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2006.
- Bryars, S.; Rowling, K. Chapter 1. Benthic habitats of eastern Gulf St Vincent: Major changes in seagrass distribution and composition since European settlement of Adelaide. In Restoration of Coastal Seagrass Ecosystems: Amphibolis Antarctica in Gulf St Vincent, South Australia; Bryars, S., Ed.; SARDI Aquatic Sciences Publication No. F2008/000078, SARDI Research Report Series No. 277; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2008; pp. 5–27. [Google Scholar]
- Bone, Y.; Deer, L.; Edwards, S.A.; Campbell, E. Adelaide Coastal Waters Study Coastal Sediment Budget; ACWS Technical Report No. 16 prepared for the Adelaide Coastal Waters Study Steering Committee; Adelaide University, Department of Geology: Adelaide, Australia, 2006. [Google Scholar]
- Joyce, K.E.; Phinn, S.R. Bi-directional reflectance of corals. Int. J. Remote Sens. 2002, 23, 389–394. [Google Scholar] [CrossRef]
- Ocean Optics. OceanView Installation and Operation Manual; Document Number 000-20000-310-02-201503; Ocean Optics: Winter Park, FL, USA, 2013. [Google Scholar]
- Ocean Optics. JAZ Installation and Operation Manual; Document Number 013-RD000-000-02-201502; Ocean Optics: Winter Park, FL, USA, 2010. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Han, L.; Rundquist, D.C. The spectral responses of Ceratophyllum demersum at varying depths in an experimental tank. Int. J. Remote Sens. 2003, 24, 859–864. [Google Scholar] [CrossRef]
- Barillé, L.; Robin, M.; Harin, N.; Bargain, A.; Launeau, P. Increase in seagrass distribution at Bourgneuf Bay (France) detected by spatial remote sensing. Aquat. Bot. 2010, 92, 185–194. [Google Scholar] [CrossRef]
- Dekker, A.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of shallow water bathymetry, hydro-optics, and benthos mapping techniques in Australian and Caribbean coastal environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Wilkinson, J.; Pearce, M.; Cromar, N.; Fallowfield, H. Audit of the Quality and Quantity of Treated Wastewater Discharging from Wastewater Treatment Plants (WWTPs) to the Marine Environment; ACWS Technical Report No. 1. Prepared for the Adelaide Coastal Waters Study Steering Committee; Flinders University of South Australia, Department of Environmental Health: Adelaide, Australia, 2003. [Google Scholar]
- Hochberg, E.J.; Atkinson, M.J.; Andréfouët, S. Spectral reflectance of coral reef bottom-types worldwide and implications for coral reef remote sensing. Remote Sens. Environ. 2003, 85, 159–173. [Google Scholar] [CrossRef]
- Greve, T.M.; Binzer, T. Which Factors Regulate Seagrass Growth and Distribution? In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; EU Monitoring and Managing of European Seagrasses (M&MS) EVK3-CT-2000-00044: Brussels, Belgium, 2004; pp. 19–23. [Google Scholar]

| Slope Ratio at | ||
|---|---|---|
| Bottom Type | (566:600) | (566:689) |
| Posidonia | 1.38 | 4.52 |
| Amphibolis | 1.03 | 3.77 |
| Heterozostera | 1.29 | 4.93 |
| Sand | 0.77 | 0.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.; Chang, C.-H.; Burch, M.; Fernandes, M.; Kildea, T. Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia. Sustainability 2019, 11, 3695. https://doi.org/10.3390/su11133695
Hwang C, Chang C-H, Burch M, Fernandes M, Kildea T. Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia. Sustainability. 2019; 11(13):3695. https://doi.org/10.3390/su11133695
Chicago/Turabian StyleHwang, Charnsmorn, Chih-Hua Chang, Michael Burch, Milena Fernandes, and Tim Kildea. 2019. "Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia" Sustainability 11, no. 13: 3695. https://doi.org/10.3390/su11133695
APA StyleHwang, C., Chang, C.-H., Burch, M., Fernandes, M., & Kildea, T. (2019). Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia. Sustainability, 11(13), 3695. https://doi.org/10.3390/su11133695





