The Effects of Pressure and Temperature on the Process of Auto-Ignition and Combustion of Rape Oil and Its Mixtures
Abstract
1. Introduction
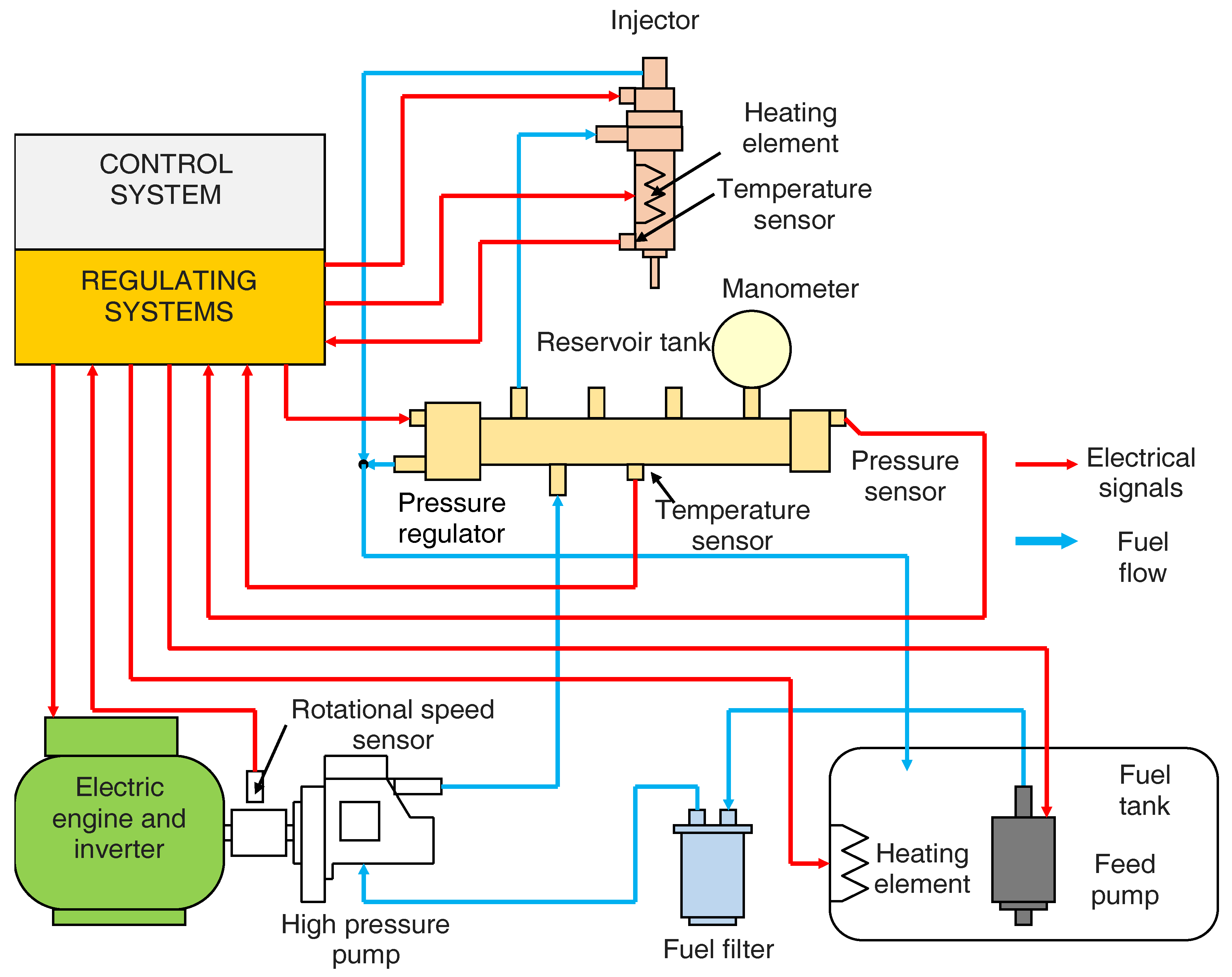
2. Materials and Methods
Description of the Program
3. Calculation Results
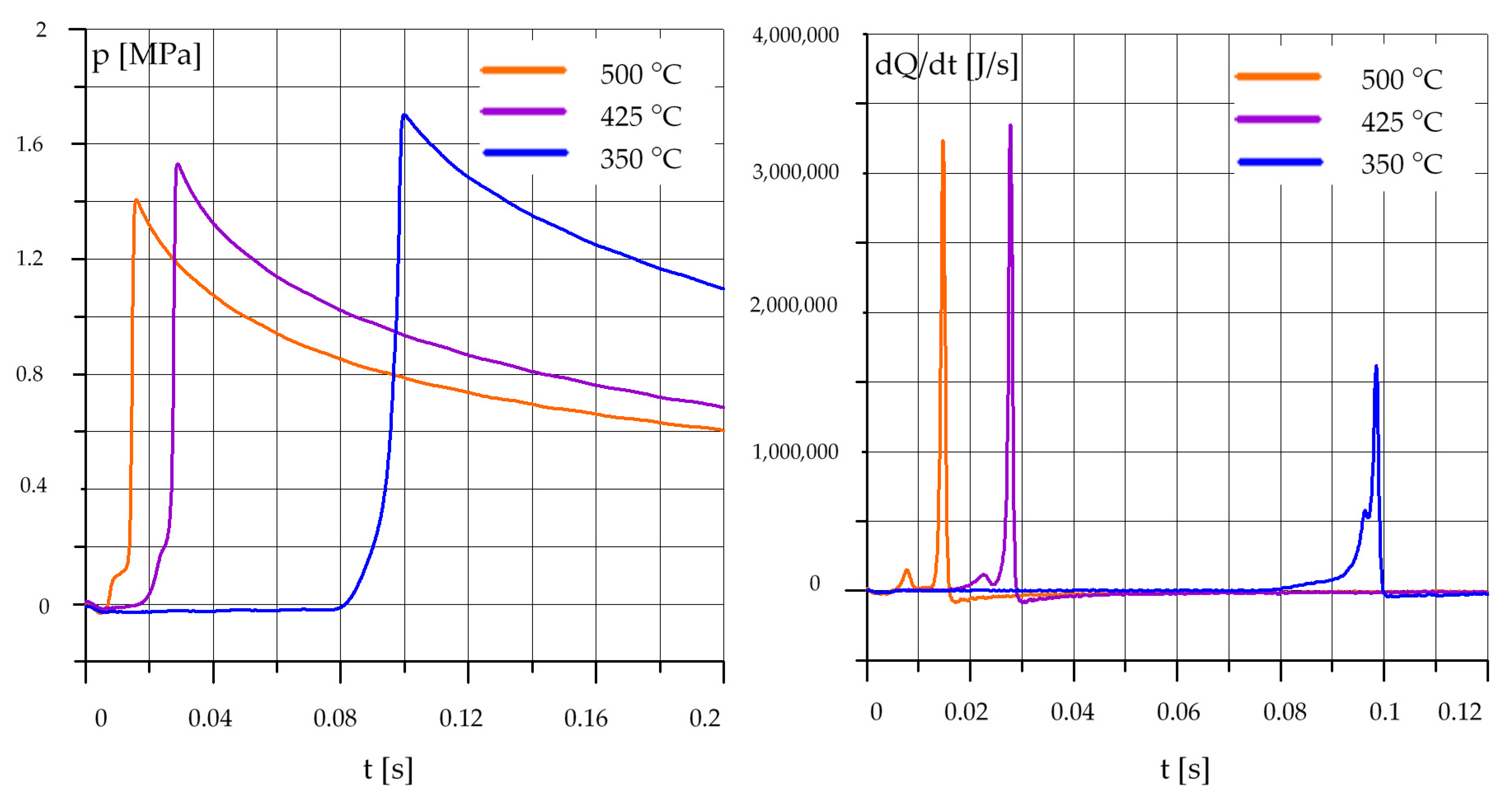
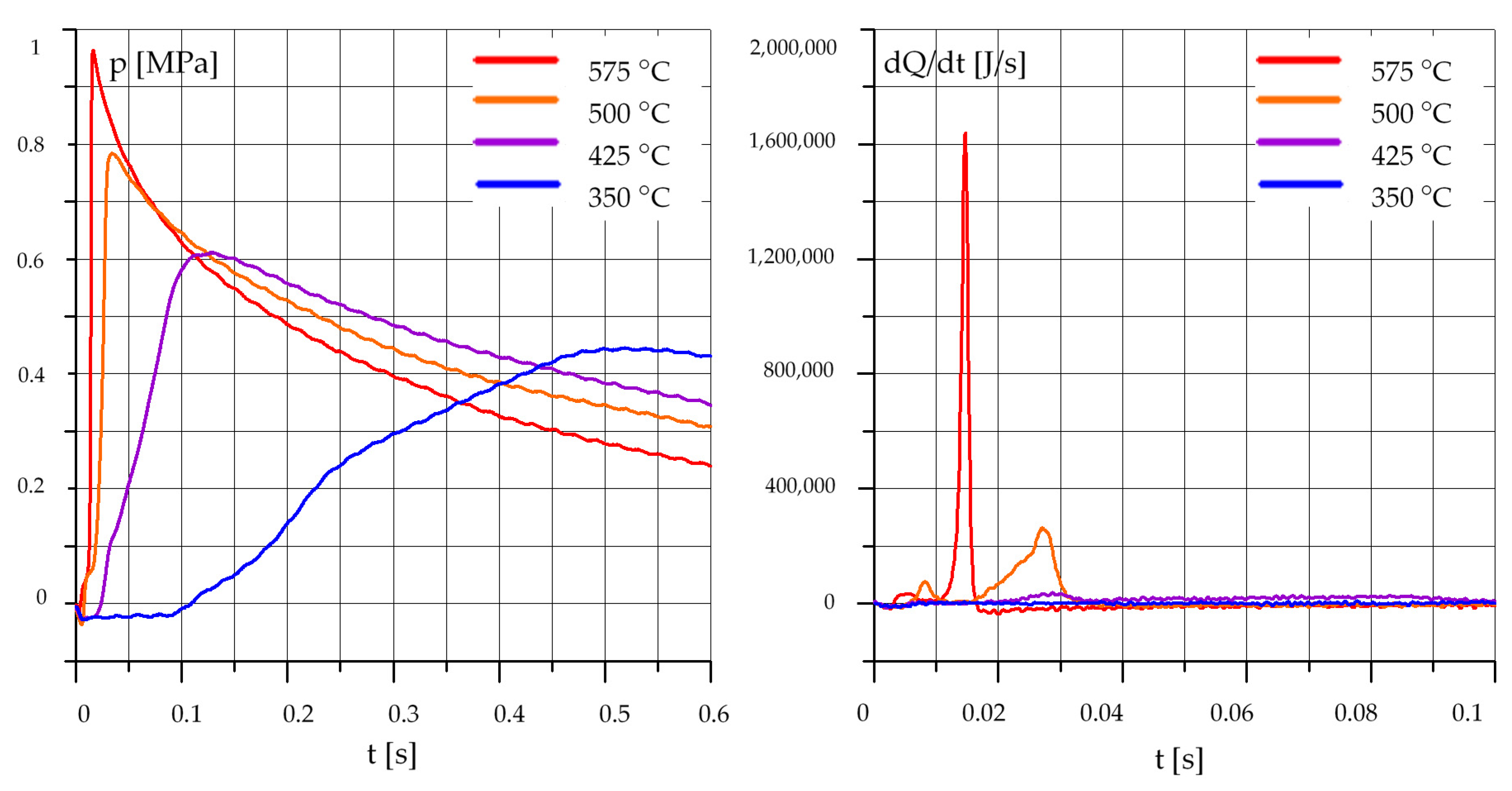
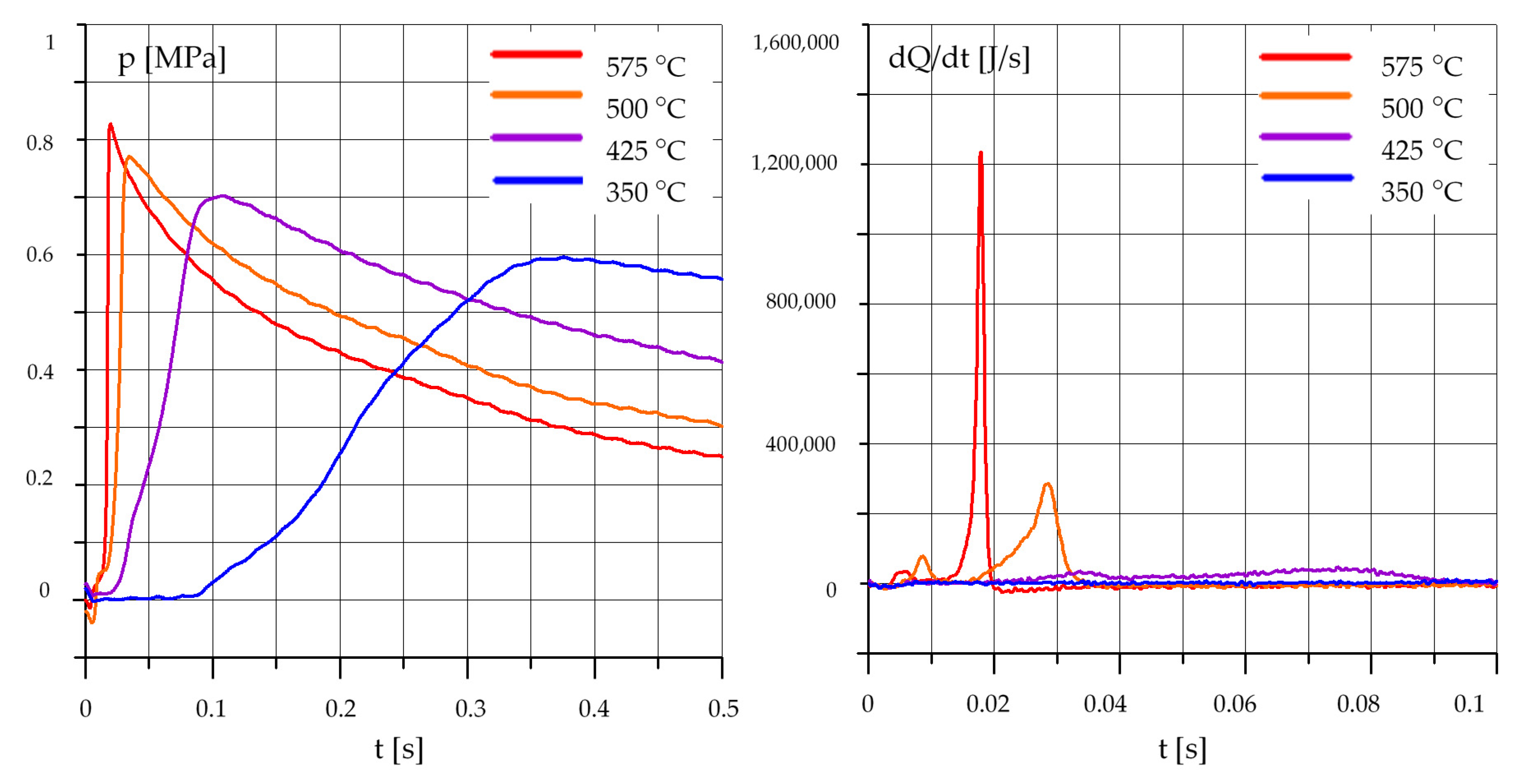
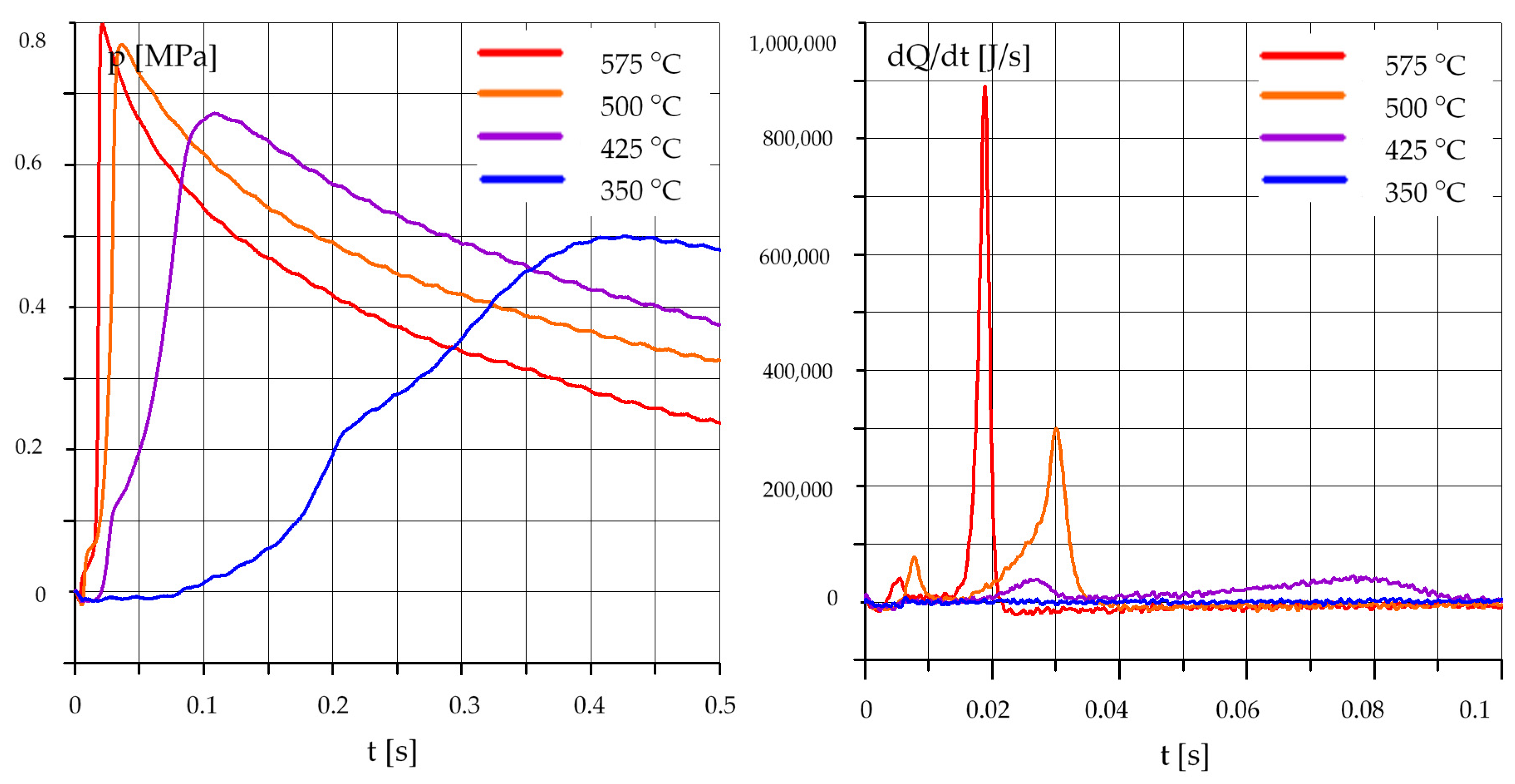
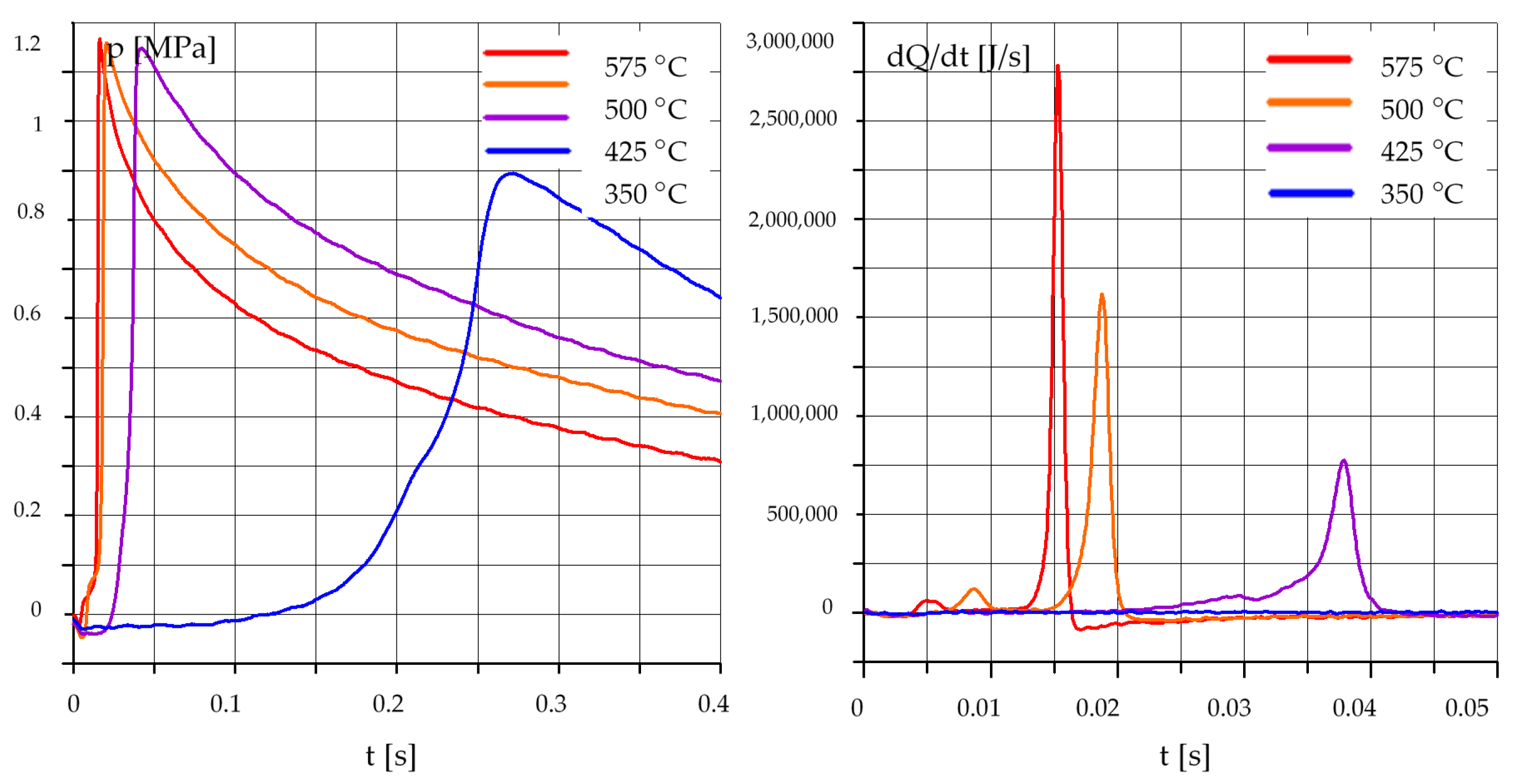
3.1. The Effect of Temperature
3.1.1. Diesel Oil (O)
3.1.2. Rapeseed Oil (R)
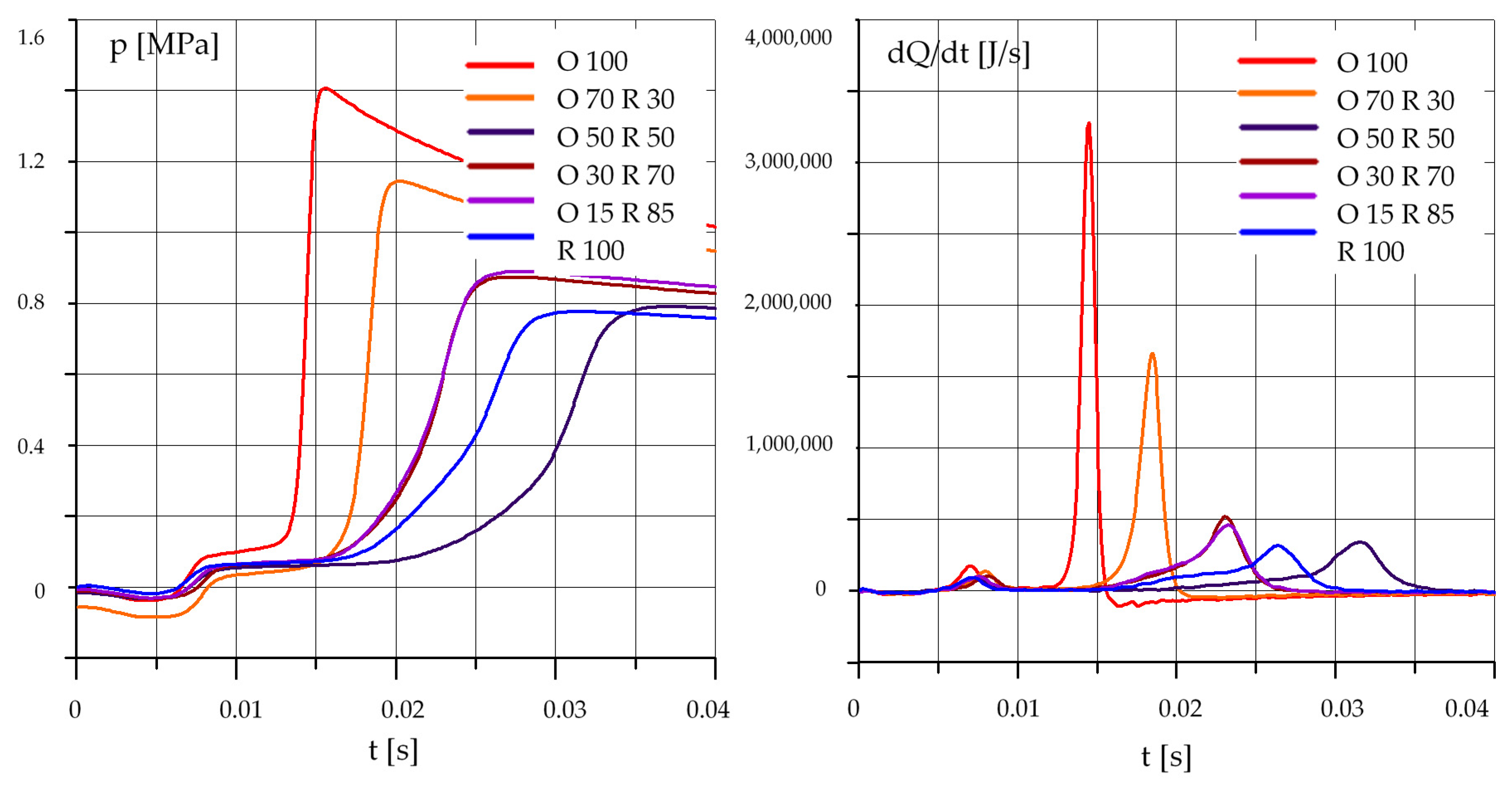
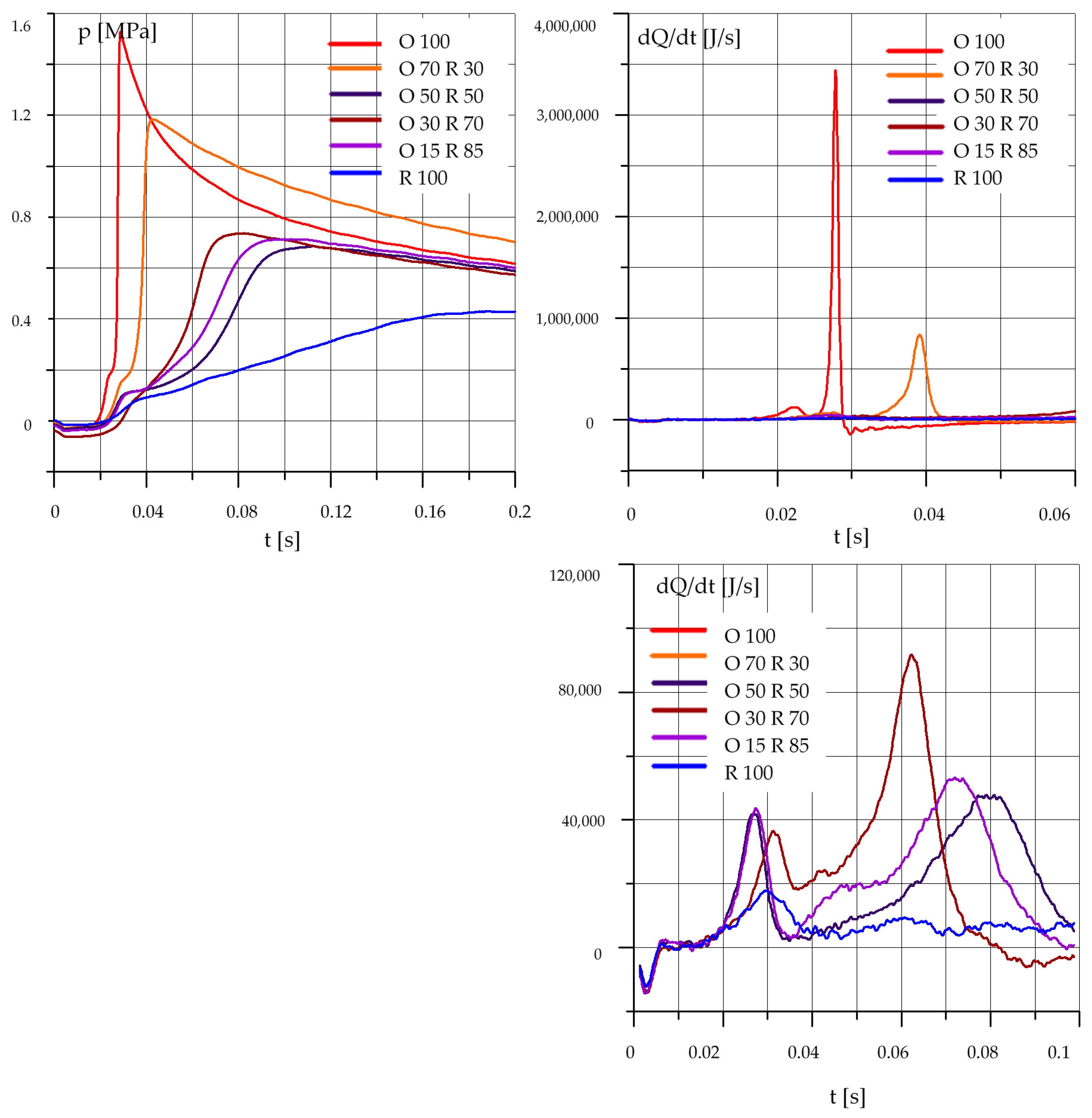
3.1.3. Mixtures of Rapeseed Oil (R) and Diesel Oil (O)
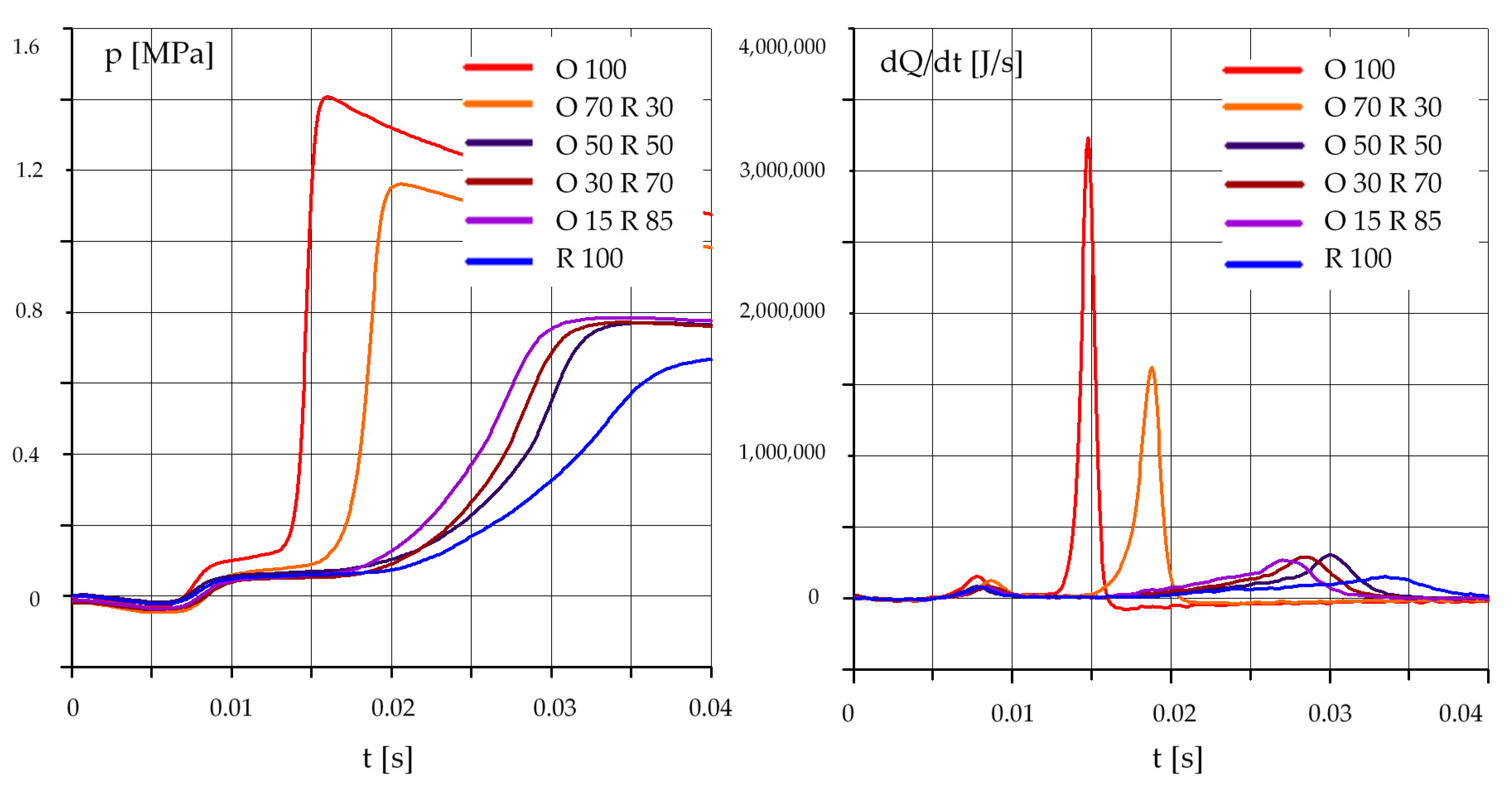
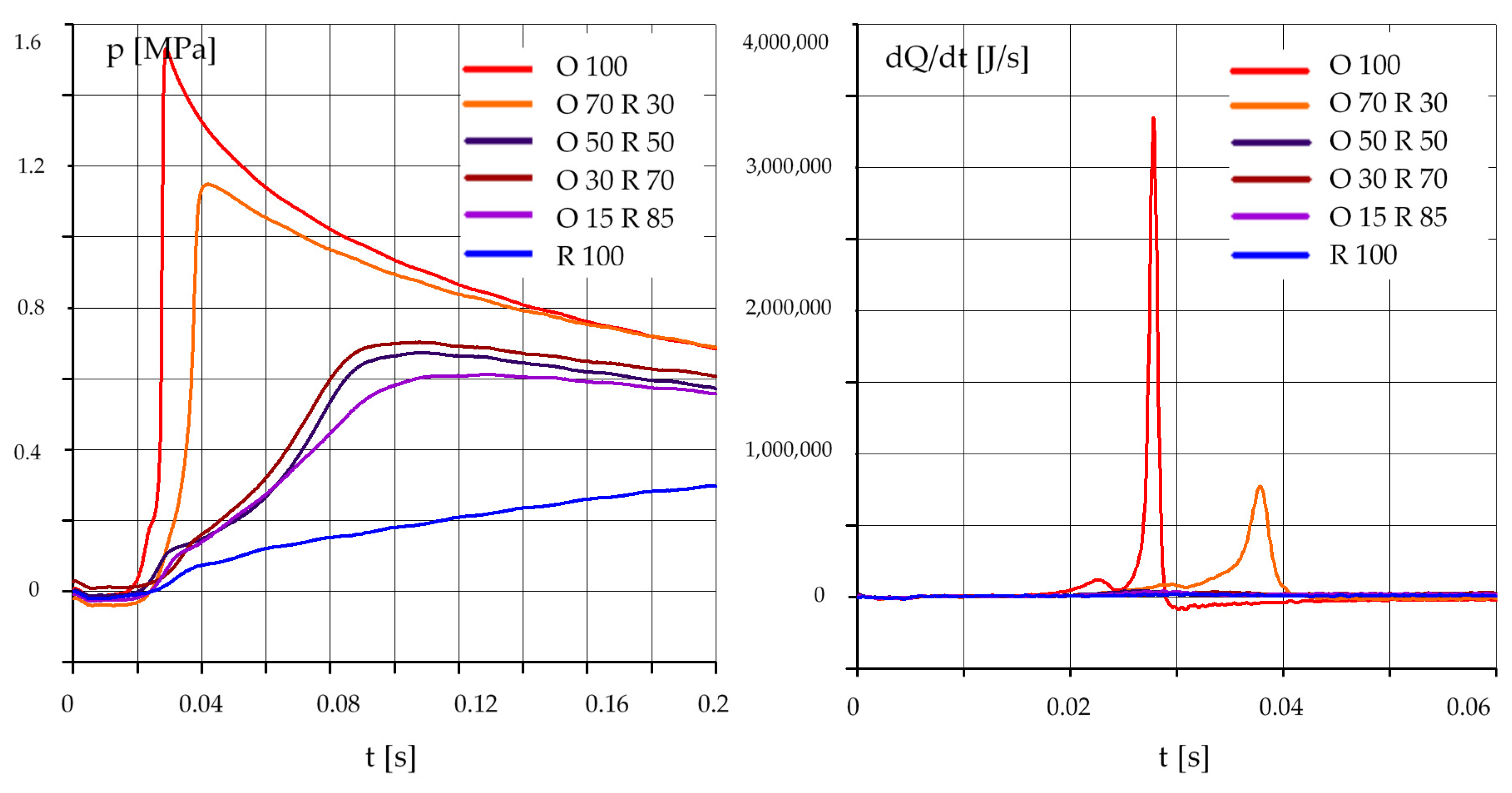
3.2. The Effect of the Composition of the Mixture
4. Conclusions
- an increase in the rapeseed oil content in the mixture causes an increase in the self-ignition delay and a decrease in the maximum pressure, especially at lower initial temperature values in the test chamber;
- in the case of mixtures of rapeseed oil and diesel oil, the best results were obtained for a mixture containing 70% by mass of diesel oil and 30% of rapeseed oil;
- results obtained for a mixture containing 50% diesel oil and 50% rapeseed oil require further testing and confirmation.
Author Contributions
Funding
Conflicts of Interest
References
- Forin, S.; Radebach, A.; Steckel, J.C.; Ward, H. The effect of industry delocalization on global energy use: A global sectoral perspective. Energy Econ. 2018, 70, 233–243. [Google Scholar] [CrossRef]
- Energy and Economy. Electric Power Research Institute and Galvin Electricity Initiative. Available online: http://www.iiasa.ac.at/ (accessed on 28 April 2019).
- Samuelsson, K.; Giusti, M.; Peterson, G.D.; Legeby, A.; Brandt, S.A.; Barthel, S. Impact of environment on people’s everyday experiences in Stockholm. Landsc. Urban Plan. 2018, 171, 7–17. [Google Scholar] [CrossRef]
- Liu, D.; Guo, X.; Xiao, B. What causes growth of global greenhouse gas emissions? Evidence from 40 countries. Sci. Total Environ. 2019, 661, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Tucki, K.; Orynycz, O.; Wasiak, A.; Świć, A.; Wichłacz, J. The Impact of Fuel Type on the Output Parameters of a New Biofuel Burner. Energies 2019, 12, 1383. [Google Scholar] [CrossRef]
- Tucki, K.; Mruk, M.; Orynycz, O.; Botwińska, K.; Gola, A. Toxicity of Exhaust Fumes (CO, NOx) of the Compression-Ignition (Diesel) Engine with the Use of Simulation. Sustainability 2019, 11, 2188. [Google Scholar] [CrossRef]
- Air Pollution: Our Health Still Insufficiently Protected. Available online: http://publications.europa.eu (accessed on 20 March 2019).
- Kuklinska, K.; Wolska, L.; Namiesnik, J. Air quality policy in the US and the EU—A review. Atmos. Pollut. Res. 2015, 6, 129–137. [Google Scholar] [CrossRef]
- Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and Repealing Directive 2001/81/EC. Available online: https://eur-lex.europa.eu (accessed on 28 April 2019).
- National Environmental Protection Program until 2020 (with a prospect until 2030). Available online: https://www.gov.pl/web/srodowisko (accessed on 28 April 2019).
- Pisoni, E.; Christidis, P.; Thunis, P.; Trombetti, M. Evaluating the impact of “Sustainable Urban Mobility Plans” on urban background air quality. J. Environ. Manag. 2019, 231, 249–255. [Google Scholar] [CrossRef]
- Santos, F.M.; Gómez-Losada, A.; Pires, J.C.M. Impact of the implementation of Lisbon low emission zone on air quality. J. Hazard. Mater. 2019, 365, 632–641. [Google Scholar] [CrossRef]
- Kupczyk, A.; Mączyńska, J.; Redlarski, G.; Tucki, K.; Bączyk, A.; Rutkowski, D. Selected Aspects of Biofuels Market and the Electromobility Development in Poland: Current Trends and Forecasting Changes. Appl. Sci. 2019, 9, 254. [Google Scholar] [CrossRef]
- Mączyńska, J.; Krzywonos, M.; Kupczyk, A.; Tucki, K.; Sikora, M.; Pińkowska, H.; Bączyk, A.; Wielewska, I. Production and use of biofuels for transport in Poland and Brazil—The case of bioethanol. Fuel 2019, 241, 989–996. [Google Scholar] [CrossRef]
- Wang, T.; Lin, B. Fuel consumption in road transport: A comparative study of China and OECD countries. J. Clean. Prod. 2019, 206, 156–170. [Google Scholar] [CrossRef]
- Mattioli, G.; Lucas, K.; Marsden, G. Reprint of Transport poverty and fuel poverty in the UK: From analogy to comparison. Transp. Policy 2018, 65, 114–125. [Google Scholar] [CrossRef]
- Rietmann, N.; Lieven, T. How policy measures succeeded to promote electric mobility—Worldwide review and outlook. J. Clean. Prod. 2019, 206, 66–75. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Shen, L.; Schakel, W.; Ramirez, A.; Kramer, G.J. Potential and challenges of low-carbon energy options: Comparative assessment of alternative fuels for the transport sector. Appl. Energy 2019, 236, 590–606. [Google Scholar] [CrossRef]
- Clean Transport, Urban Transport. Alternative Fuels for Sustainable Mobility in Europe. Available online: https://ec.europa.eu/transport/themes/urban/cpt_en (accessed on 28 April 2019).
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodiesel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Samson, I.; Sikora, M.; Bączyk, A.; Mączyńska, J.; Tucki, K. Technologies used to enhance the biogas and biomethane yield: A review. Przemysł Chem. 2017, 96, 1605–1611. [Google Scholar]
- Arnold, M.; Tainter, J.A.; Strumsky, D. Productivity of innovation in biofuel technologies. Energy Policy 2019, 124, 54–62. [Google Scholar] [CrossRef]
- Mustapha, W.F.; Kirkerud, J.G.; Bolkesjø, T.F.; Trømborg, E. Large-scale forest-based biofuels production: Impacts on the Nordic energy sector. Energy Convers. Manag. 2019, 187, 93–102. [Google Scholar] [CrossRef]
- Leong, W.H.; Lim, J.W.; Lam, M.K.; Uemura, Y.; Ho, Y.C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Krzywonos, M.; Tucki, K.; Wojdalski, J.; Kupczyk, A.; Sikora, M. Analysis of Properties of Synthetic Hydrocarbons Produced Using the ETG Method and Selected Conventional Biofuels Made in Poland in the Context of Environmental Effects Achieved. Rocz. Ochr. Śr. 2017, 19, 394–410. [Google Scholar]
- Sharma, N.; Patel, C.; Tiwari, N.; Agarwal, A.K. Experimental investigations of noise and vibration characteristics of gasoline-methanol blend fuelled gasoline direct injection engine and their relationship with combustion characteristics. Appl. Therm. Eng. 2019, 158, 113754. [Google Scholar] [CrossRef]
- Küüt, A.; Ilves, R.; Küüt, K.; Raide, V.; Ritslaid, K.; Olt, J. Influence of European Union Directives on the Use of Liquid Biofuel in the Transport Sector. Procedia Eng. 2017, 187, 30–39. [Google Scholar] [CrossRef]
- Ballesteros, M.; Manzanares, P. Chapter Three—Liquid Biofuels. In The Role of Bioenergy in the Bioeconomy, 1st ed.; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Waltham, MA, USA, 2019; pp. 113–144. [Google Scholar]
- Alptekin, E.; Sanli, H.; Canakci, M. Combustion and performance evaluation of a common rail DI diesel engine fueled with ethyl and methyl esters. Appl. Therm. Eng. 2019, 149, 180–191. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Krzywonos, M.; Skudlarski, J.; Kupczyk, A.; Wojdalski, J.; Tucki, K. Forecast for transport biofuels in Poland in 2020–2030. Przemysł Chem. 2015, 94, 2218–2222. [Google Scholar]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A.M. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Viccaro, M.; Cozzi, M.; Rocchi, B.; Romano, S. Conservation agriculture to promote inland biofuel production in Italy: An economic assessment of rapeseed straight vegetable oil as a self-supply agricultural biofuel. J. Clean. Prod. 2019, 217, 153–161. [Google Scholar] [CrossRef]
- Merkisz, J.; Kozak, M. Influence of the blend composition of the biofuel and the conventional fuel on exhaust emissions. Eksploat. I Niezawodn. Maint. Reliab. 2003, 3, 12–18. [Google Scholar]
- Bukrejewski, P.; Skolniak, M.; Kowalski, Ł. Comparison of the environmental effect of M1 category vehicles fed with traditional and alternative fuels. Arch. Automot. Eng. 2017, 75, 5–21. [Google Scholar]
- Czapliński, E.; Idzior, M. The research into the influence of the technical condition of motor vehicles in operation on exhaust pollutant emissions. Combust. Engines 2015, 54, 578–581. [Google Scholar]
- Zhu, G.; Liu, J.; Fu, J.; Xu, Z.; Guo, Q.; Zhao, H. Experimental study on combustion and emission characteristics of turbocharged gasoline direct injection (GDI) engine under cold start new European driving cycle (NEDC). Fuel 2018, 215, 272–284. [Google Scholar] [CrossRef]
- Flekiewicz, M.; Kubica, G. The use of gaseous fuels mixtures for SI engines propulsion. IOP Conf. Ser. Mater. Sci. Eng. 2016, 148, 1–11. [Google Scholar] [CrossRef]
- Jęczmionek, Ł.; Porzycka-Semczuk, K. Triglyceride zeoforming—A method for improving the low-temperature properties of second generation bio-components obtained from natural oils. Fuel 2013, 113, 17–23. [Google Scholar]
- Azadi, P.; Malina, R.; Barrett, S.R.H.; Kraft, M. The evolution of the biofuel science. Renew. Sustain. Energy Rev. 2017, 76, 1479–1484. [Google Scholar] [CrossRef]
- Tucki, K.; Mruk, R.; Orynycz, O.; Wasiak, A.; Botwińska, K.; Gola, A. Simulation of the Operation of a Spark Ignition Engine Fueled with Various Biofuels and Its Contribution to Technology Management. Sustainability 2019, 11, 2799. [Google Scholar] [CrossRef]
- Guo, H.; Cao, D.; Chen, H.; Sun, Z.; Hu, Y. Model predictive path following control for autonomous cars considering a measurable disturbance: Implementation, testing, and verification. Mech. Syst. Signal Process. 2019, 118, 41–60. [Google Scholar] [CrossRef]
- Su, G.; Li, N.; Yildiz, Y.; Girard, A.; Kolmanovsky, I. A Traffic Simulation Model with Interactive Drivers and High-fidelity Car Dynamics. IFAC Pap. Line 2019, 51, 384–389. [Google Scholar] [CrossRef]
- Martynov, S.B.; Mason, D.J.; Heikal, M.R. Numerical Simulation of Cavitation Flows Based on Their Hydrodynamic Similarity. Int. J. Engine Res. 2006, 7, 283–296. [Google Scholar] [CrossRef]
- The Use of Crude Rapeseed Oil as a Fuel for Diesel Engines; Report on the implementation of the development Project No. R1003703; Faculty of Production Engineering, Warsaw University of Life Sciences: Warsaw, Poland, 2011.


| Symbol | Description |
|---|---|
| pressure | |
| volume | |
| gas mass | |
| gas constant | |
| temperature | |
| i-th instant of time | |
| pressure in the chamber at the i-th instant of time | |
| i-1 instant of time | |
| pressure in the chamber in i-1 instant of time | |
| i + 1 instant of time | |
| chamber pressure in i + 1 instant of time | |
| pressure in the chamber at the i-th instant of time after smoothing | |
| mass fraction of oxygen | |
| mass fraction of nitrogen | |
| mass fraction of carbon dioxide | |
| mass fraction of water vapor | |
| specific heat at constant pressure of the gas mixture in the chamber | |
| specific heat at a constant volume of the gas mixture in the chamber | |
| mass heat at constant oxygen pressure | |
| mass heat at constant nitrogen pressure | |
| specific heat at constant carbon dioxide pressure | |
| specific heat at constant water vapor pressure | |
| individual gas constant | |
| coefficients, individual for each gas | |
| mass of burnt fuel or mass of injected fuel | |
| emitted heat | |
| the heat of burning fuel | |
| mass fraction of oxygen at the instant i-1 | |
| mass fraction of nitrogen at the instant i-1 | |
| mass fraction of carbon dioxide at the instant i-1 | |
| mass fraction of water vapor at the instant i-1 | |
| mass fraction of oxygen at the i-th instant of time | |
| mass fraction of nitrogen at the i-th instant of time | |
| mass fraction of carbon dioxide at the i-th instant of time | |
| mass fraction of water vapor at the i-th instant of time | |
| mass share of hydrogen in the fuel | |
| mass fraction of carbon in fuel | |
| mass fraction of oxygen in fuel | |
| mass of gas at i-1 instant of time | |
| the mass of gas at the instant i | |
| mass fraction of rapeseed oil in fuel | |
| mass fraction of diesel oil in fuel | |
| maximum pressure in the chamber | |
| chamber volume | |
| calorific value of fuel |
| Mixture | Diesel Oil [% m/m] | Rapeseed Oil [% m/m] |
|---|---|---|
| Case 1 | 100 | - |
| Case 2 | - | 100 |
| Case 3 | 15 | 85 |
| Case 4 | 30 | 70 |
| Case 5 | 50 | 50 |
| Case 6 | 70 | 30 |
| Fuel | Diesel Oil | Methyl Esters of Rapeseed Oil | Rapeseed Oil |
|---|---|---|---|
| Calorific value [MJ/kg] | 42.5 | 38.0 | 37.5 |
| Mass fraction of hydrogen [%] | 12.6 | 12.1 | 11.4 |
| Mass fraction of carbon [%] | 87.4 | 77.5 | 77.4 |
| Mass fraction of oxygen [%] | - | 10.4 | 11.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucki, K.; Mruk, R.; Orynycz, O.; Gola, A. The Effects of Pressure and Temperature on the Process of Auto-Ignition and Combustion of Rape Oil and Its Mixtures. Sustainability 2019, 11, 3451. https://doi.org/10.3390/su11123451
Tucki K, Mruk R, Orynycz O, Gola A. The Effects of Pressure and Temperature on the Process of Auto-Ignition and Combustion of Rape Oil and Its Mixtures. Sustainability. 2019; 11(12):3451. https://doi.org/10.3390/su11123451
Chicago/Turabian StyleTucki, Karol, Remigiusz Mruk, Olga Orynycz, and Arkadiusz Gola. 2019. "The Effects of Pressure and Temperature on the Process of Auto-Ignition and Combustion of Rape Oil and Its Mixtures" Sustainability 11, no. 12: 3451. https://doi.org/10.3390/su11123451
APA StyleTucki, K., Mruk, R., Orynycz, O., & Gola, A. (2019). The Effects of Pressure and Temperature on the Process of Auto-Ignition and Combustion of Rape Oil and Its Mixtures. Sustainability, 11(12), 3451. https://doi.org/10.3390/su11123451







