Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Sampling
2.3. Experimental Design
2.4. Free Fatty Acidity Determination—Regulated Method
2.5. Peroxides Number Determination—Regulated Method
2.6. Anisidine Number Determination—Regulated Method
2.7. Statistical Analysis
3. Results and Discussions
3.1. Free Fatty Acidity
3.2. Peroxides Number
3.3. Anisidine Number
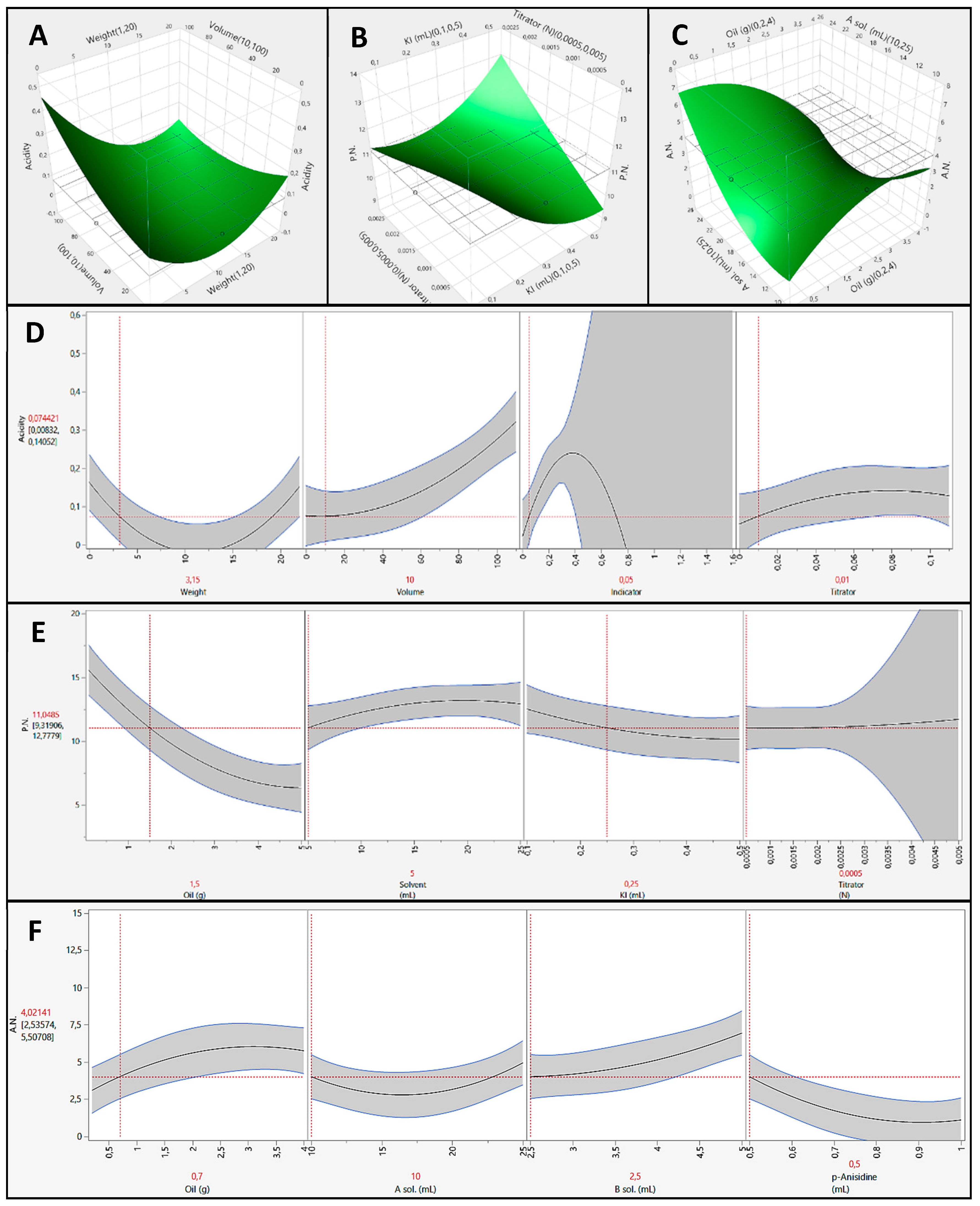
3.4. Optimized Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rijavec, T.; Janjic, S.; Acko, D.K. Revitalization of Industrial Hemp Cannabis sativa L. var, sativa in Slovenia: A Study of Green Hemp Fibres. Text. Tilec 2017, 60, 36–48. [Google Scholar]
- Amaducci, S.; Gusovius, H.-J. Hemp—Cultivation, Extraction and Processing. Ind. Appl. Nat. Fibres 2010, 109–134. [Google Scholar] [CrossRef]
- Karus, M.; Vogt, D. European hemp industry: Cultivation, processing and product lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- EUR-Lex. European Communities Council Regulation (EC) No 1251/1999. 1999. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31999R1251 (accessed on 31 May 2019).
- Finnan, J.; Styles, D. Hemp: A more sustainable annual energy crop for climate and energy policy. Energy Policy 2013, 58, 152–162. [Google Scholar] [CrossRef]
- Zanetti, F.; Monti, A.; Berti, M.T. Challenges and opportunities for new industrial oilseed crops in EU-27: A review. Ind. Crops Prod. 2013, 50, 580–595. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten- free bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Potential oil yield, fatty acid composition, and oxidation stability of the hempseed oil from four Cannabis sativa L. cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Vyviurska, O.; Jánošková, N.; Jakubík, T.; Špánik, I. Comprehensive Two-Dimensional Gas Chromatography–Mass Spectrometry Analysis of Different Types of Vegetable Oils. J. Am. Oil Chem. Soc. 2015, 92, 783–790. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016, 1307, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Delegated Regulation (EU) 2015/1830 of 8 July 2015 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. 2015. Vol. L266. pp. 9–13. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32015R1830 (accessed on 31 May 2019).
- British Standards Institution. Animal and Vegetable Fats and Oils. Determination of Anisidine Value. Int. Stand. 2006. Available online: https://www.iso.org/standard/40052.html (accessed on 31 May 2019).
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC—Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Sanz-Landaluze, J.; Bocanegra-Salazar, M.; Ortiz-Pérez, D.; Cámara, C. Miniaturisated method for the analysis of polycyclic aromatic hydrocarbons in leaf samples. J. Chromatogr. A 2010, 1217, 3567–3574. [Google Scholar] [CrossRef]
- Daneshvand, B.; Ara, K.M.; Raofie, F. Comparison of supercritical fluid extraction and ultrasound-assisted extraction of fatty acids from quince (Cydonia oblonga Miller) seed using response surface methodology and central composite design. J. Chromatogr. A 2012, 1252, 1–7. [Google Scholar] [CrossRef]
- Ghaedi, M.; Mazaheri, H.; Khodadoust, S.; Hajati, S.; Purkait, M.K. Application of central composite design for simultaneous removal of methylene blue and Pb2+ ions by walnut wood activated carbon. Spectrochim. Acta—Part. A Mol. Biomol. Spectrosc. 2015, 135, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, K.; Giacomazza, D.; Dahmoune, F.; Mangione, M.R.; Bulone, D.; San Biagio, P.L.; Passantino, R.; Costa, M.A.; Guarrasi, V.; Madani, K. Pectin from Opuntia ficus indica: Optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017, 221, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Rapa, M.; Testa, G.; Venditti, I.; Scaramuzzo, F.A.; Vinci, G. Response surface methodology for the optimization of phenolic compounds extraction from extra virgin olive oil with functionalized gold nanoparticles. Microchem. J. 2018, 138, 430–437. [Google Scholar] [CrossRef]
- Gerasimenko, E.O.; Tur, Y.I. Automated flow pH-method for the determination of total free fatty acids content in edible oils. Food Chem. 2012, 132, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Maria, V.; Marchand, S.; De Revel, G.; Bordignon-luiz, M.T. Development and validation of method for heterocyclic compounds in wine: Optimization of HS-SPME conditions applying a response surface methodology. Talanta 2013, 117, 87–93. [Google Scholar]
- Almeida, M.; Erthal, R.; Padua, E.; Silveira, L.; Am, L. Response surface methodology ( RSM ) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crops Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Isnen, M.; Nasution, T.I.; Perangin-angin, B. Design of Edible Oil Degradation Tool by Using Electromagnetic Field Absorbtion Principle which was Characterized to Peroxide Number. J. Phys. Conf. Ser. 2016, 739, 012076. [Google Scholar] [CrossRef]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K. Omega-3 Long-Chain Polyunsaturated Fatty Acid Content and Oxidation State of Fish Oil Supplements in New Zealand. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Quality Parameters | Variables | Levels |
|---|---|---|
| Free Fatty Acidity | Hempseed oil | 1—10.5—20* g |
| Solvent Volume | 10—55—100* mL | |
| Indicator Volume | 0.05—0.175—0.3* mL | |
| KOH concentration | 0.01—0.055—0.1* N | |
| Peroxides Number | Hempseed oil | 0.1—2.55—5* g |
| Solvent Volume | 5—15—25* mL | |
| KI Volume | 0.1—0.3—0.5* mL | |
| Na2SO4 concentration | 0.0005—0.00275—0.005* N | |
| Anisidine Number | Hempseed oil | 0.1—2.05—4* g |
| A solution Volume | 10—17.5—25* mL | |
| B solution Volume | 2.5—3.75—5* mL | |
| p-Anisidine Volume | 0.5—0.75—1* mL |
| Parameter | Estimated Coefficient | S.D. | Sum of Square | Log of Valence | F-Value | p > F |
|---|---|---|---|---|---|---|
| MODEL | - | - | 0.58 | - | 27.38 | <0.0001 * |
| Intercept | ||||||
| Q0 | −0.79 | 1.05 | - | - | - | - |
| Linear | ||||||
| Weight(1,20) | −0.31 | 0.065 | 0.034 | 3.341 | 22.75 | 0.0005 * |
| Volume(10,100) | −0.03 | 0.065 | 0.0003 | 0.193 | 0.23 | 0.6415 |
| Indicator(0,05,0,3) | −1.16 | 1.28 | 0.0012 | 0.417 | 0.82 | 0.3826 |
| Titrator(0.01.0.1) | −0.13 | 0.065 | 0.0065 | 1.220 | 4.30 | 0.0602 |
| Interaction | ||||||
| Weight*Volume | −0.06 | 0.0097 | 0.012 | 4.660 | 44.93 | <0.0001 * |
| Weight*Indicator | −0.11 | 0.039 | 0.003 | 1.828 | 8.07 | 0.0149 |
| Volume*Indicator | −0.01 | 0.039 | 0.0037 | 1.484 | 1.99 | 0.1828 |
| Weight*Titrator | −0.55 | 0.0097 | 0.0088 | 0.840 | 2.43 | 0.1446 |
| Volume*Titrator | −0.02 | 0.0097 | 0.0087 | 0.738 | 5.82 | 0.0328 |
| Indicator*Titrator | −0.09 | 0.039 | 0.012 | 1.466 | 5.71 | 0.0342 |
| Quadratic | ||||||
| Weight*Weight | 0.13 | 0.024 | 0.045 | 3.834 | 29.75 | 0.0001 * |
| Volume*Volume | 0.05 | 0.024 | 0.0059 | 1.148 | 3.92 | 0.0711 |
| Indicator*Indicator | −0.37 | 0.39 | 0.0014 | 0.453 | 0.94 | 0.3524 |
| Titrator*Titrator | −0.03 | 0.024 | 0.0021 | 0.577 | 1.37 | 0.2648 |
| STATISTICS | ||||||
| R2 | 0.97 | - | - | - | - | - |
| R2adj | 0.93 | - | - | - | - | - |
| RMSE | 0.0039 | - | - | - | - | - |
| CV % | 2.14 | - | - | - | - | - |
| Parameter | Estimated Coefficient | S.D. | Sum of Square | Log of Valence | F-Value | p > F |
|---|---|---|---|---|---|---|
| Model | - | - | 19.9526 | - | 15.66 | 0.0002* |
| Intercept | ||||||
| Q0 | 10.26 | 1.11 | - | - | - | - |
| Linear | ||||||
| Weight(1.20) | −4.13 | 0.44 | 113.22 | 4.880 | 88.85 | <0.0001 * |
| Volume(5.25) | 1.25 | 0.44 | 10.43 | 1.675 | 8.18 | 0.0211 * |
| KI(0.1.0.5) | 0.73 | 0.44 | 3.54 | 0.872 | 2.78 | 0.1342 |
| Titrator(0.0005.0.005) | −2.16 | 3.53 | 0.48 | 0.254 | 0.37 | 0.5569 |
| Interaction | ||||||
| Weight*Volume | 0.33 | 0.28 | 1.78 | 0.567 | 1.39 | 0.2709 |
| Weight*KI | 0.23 | 0.28 | 0.85 | 0.359 | 0.67 | 0.4378 |
| Volume*KI | −0.082 | 0.28 | 0.10 | 0.107 | 0.083 | 0.7811 |
| Weight*Titrator | −0.063 | 0.59 | 0.015 | 0.038 | 0.012 | 0.9166 |
| Volume*Titrator | 0.21 | 0.58 | 0.16 | 0.138 | 0.13 | 0.7284 |
| KI*Titrator | 1.85 | 0.58 | 12.98 | 1.894 | 10.18 | 0.0128 * |
| Quadratic | ||||||
| Weight*Weight | 2.65 | 0.72 | 17.31 | 2.210 | 13.59 | 0.0062 * |
| Volume*Volume | −1.13 | 0.72 | 3.16 | 0.813 | 2.48 | 0.1537 |
| Indicator*Indicator | 0.48 | 0.72 | 0.57 | 0.284 | 0.45 | 0.5205 |
| Titrator*Titrator | −2.41 | 2.91 | 0.88 | 0.365 | 0.69 | 0.4312 |
| STATISTICS | ||||||
| R2 | 0.97 | - | - | - | - | - |
| R2adj | 0.91 | - | - | - | - | - |
| RMSE | 0.36 | - | - | - | - | - |
| CV % | 3.02 | - | - | - | - | - |
| Parameter | Estimated Coefficient | S.D. | Sum of Square | Log of Valence | F-Value | p > F | ||
|---|---|---|---|---|---|---|---|---|
| Model | - | - | 118.36 | - | 17.71 | <0.0001 * | ||
| Intercept | ||||||||
| Q0 | 2.37 | 0.32 | - | - | - | - | ||
| Linear | ||||||||
| Weight(0.2.4) | −0.73 | 0.20 | 9.68 | 2.415 | 12.75 | 0.0038 * | ||
| A sol. (mL)(10.25) | 0.81 | 0.20 | 11.78 | 2.706 | 15.51 | 0.0020 * | ||
| B sol. (mL)(2.5.5) | 1.37 | 0.20 | 33.67 | 4.632 | 44.33 | <0.0001 * | ||
| p-Anisidine (mL)(0.5.1) | −0.80 | 0.20 | 11.58 | 2.680 | 15.25 | 0.0021 * | ||
| Interaction | ||||||||
| Weight (g)*A sol. (mL) | −1.58 | 0.22 | 39.93 | 4.994 | 52.58 | <0.0001 * | ||
| Weight (g)*B sol. (mL) | −1.13 | 0.22 | 20.69 | 3.668 | 27.25 | 0.0002 * | ||
| A sol. (mL)*B sol. (mL) | 1.04 | 0.22 | 17.30 | 3.343 | 22.78 | 0.0005 * | ||
| Weight (g)*Anisidine (mL) | 0.65 | 0.22 | 6.79 | 1.948 | 8.94 | 0.0113 * | ||
| A sol. (mL)*Anisidine (mL) | 0.47 | 0.22 | 3.57 | 1.292 | 4.70 | 0.0510 | ||
| B sol. (mL)*Anisidine (mL) | −0.29 | 0.22 | 1.40 | 0.702 | 1.85 | 0.1985 | ||
| Quadratic | ||||||||
| Weight*Weight | −1.25 | 0.54 | 4.06 | 1.406 | 5.35 | 0.0393 * | ||
| A sol. (mL)*A sol. (mL) | 1.65 | 0.54 | 7.07 | 1.998 | 9.31 | 0.0101 * | ||
| B sol. (mL)*B sol. (mL) | 0.66 | 0.54 | 1.11 | 0.604 | 1.47 | 0.2491 | ||
| Anisidine (mL)*Anisidine (mL) | 1.16 | 0.54 | 3.46 | 1.267 | 4.56 | 0.0540 | ||
| STATISTICS | ||||||||
| R2 | 0.95 | - | - | - | - | - | ||
| R2adj | 0.90 | - | - | - | - | - | ||
| RMSE | 0.26 | - | - | - | - | - | ||
| CV % | 6.86 | - | - | - | - | - | ||
| Parameter | Variables | Standard Method | Optimized Method | Saving |
|---|---|---|---|---|
| Free Fatty Acidity | Weight (g) | 20 | 3.15 | 84% |
| Solvent Volume (mL) | 100 | 10 | 90% | |
| Titrator Concentration (N) | 0.1 | 0.05 | 50% | |
| Indicator Volume (mL) | 0.3 | 0.01 | 97% | |
| Peroxides Number | Weight (g) | 5 | 1.5 | 70% |
| Solvent Volume (mL) | 25 | 5 | 80% | |
| KI Volume (mL) | 0.5 | 0.25 | 50% | |
| Titrator Concentration (N) | 0.002 | 0.0005 | 75% | |
| Anisidine Number | Weight (g) | 4 | 0.7 | 82% |
| Isooctane Volume (mL) | 25 | 10 | 60% | |
| Solvent Volume (mL) | 5 | 2.5 | 50% | |
| Anisidine Volume (mL) | 1 | 0.5 | 50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapa, M.; Ciano, S.; Rocchi, A.; D’Ascenzo, F.; Ruggieri, R.; Vinci, G. Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization. Sustainability 2019, 11, 3104. https://doi.org/10.3390/su11113104
Rapa M, Ciano S, Rocchi A, D’Ascenzo F, Ruggieri R, Vinci G. Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization. Sustainability. 2019; 11(11):3104. https://doi.org/10.3390/su11113104
Chicago/Turabian StyleRapa, Mattia, Salvatore Ciano, Andrea Rocchi, Fabrizio D’Ascenzo, Roberto Ruggieri, and Giuliana Vinci. 2019. "Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization" Sustainability 11, no. 11: 3104. https://doi.org/10.3390/su11113104
APA StyleRapa, M., Ciano, S., Rocchi, A., D’Ascenzo, F., Ruggieri, R., & Vinci, G. (2019). Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization. Sustainability, 11(11), 3104. https://doi.org/10.3390/su11113104








