Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different pH Values
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Reagents and Stock Solution Preparation
2.2. Coagulation and Dissolution
2.3. Additional Characterization of CuO NPs
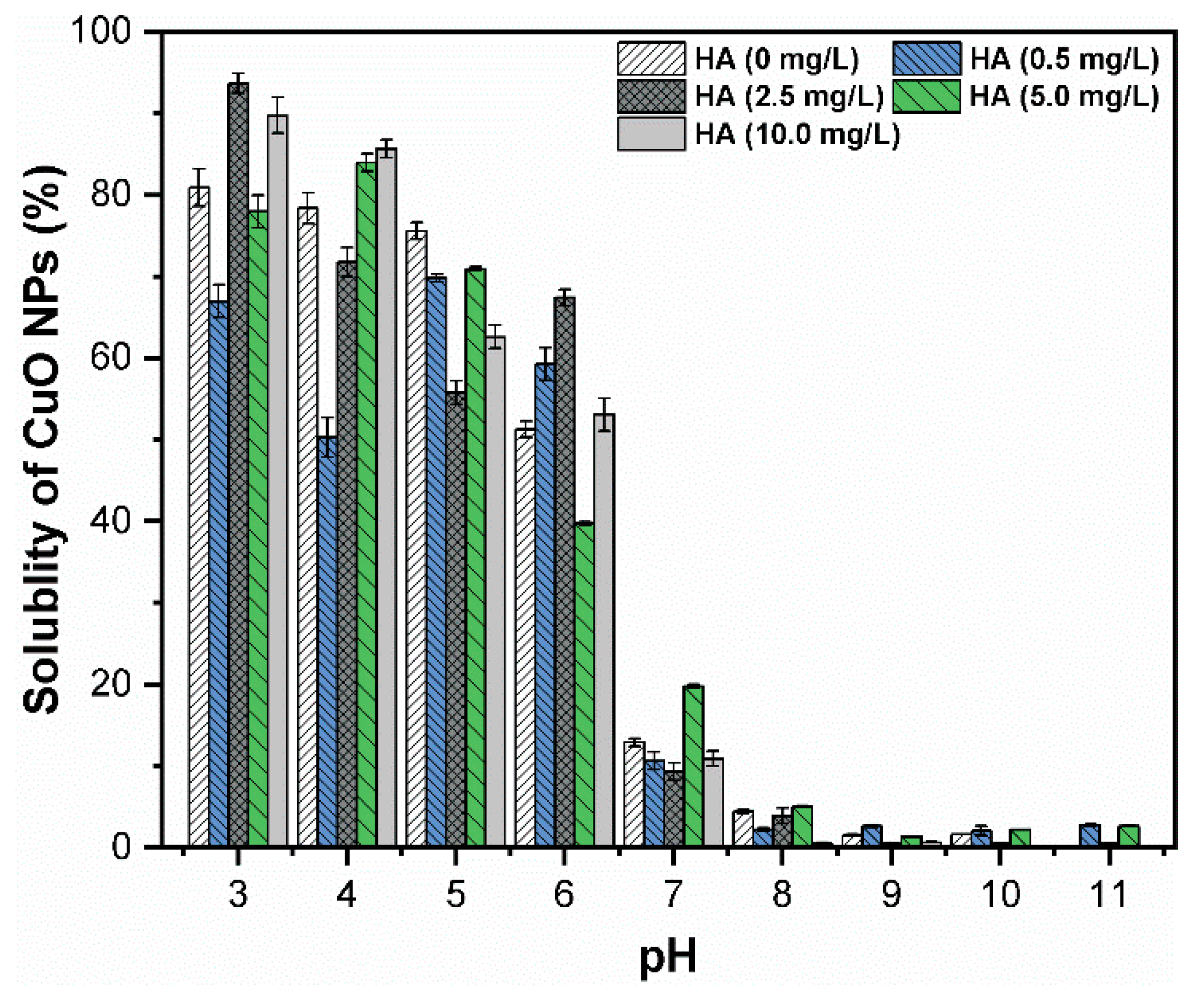
3. Results and Discussion
3.1. Characteristics of CuO NPs
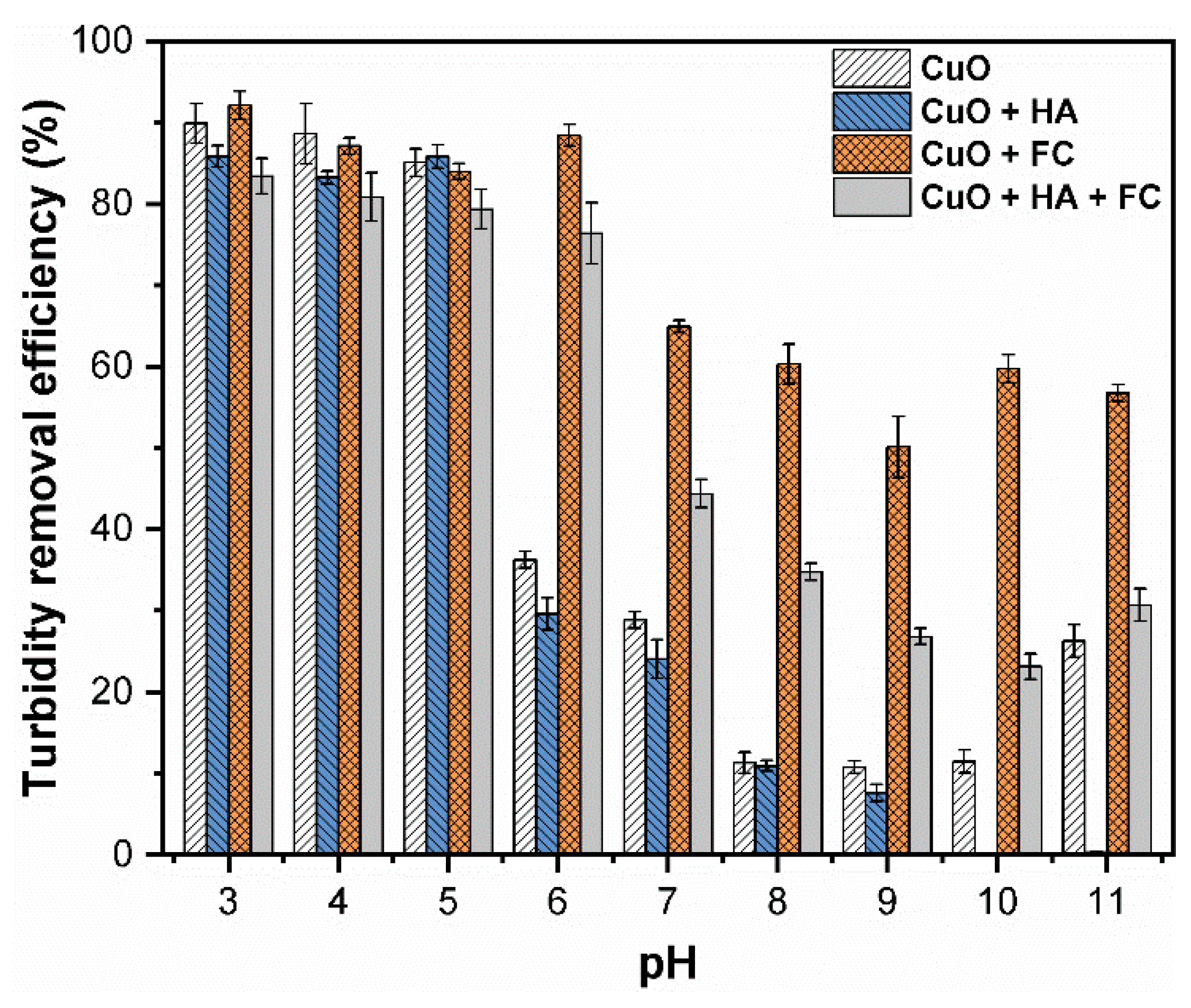
3.2. Effect of Coagulant Dose on CuO NPs Removal
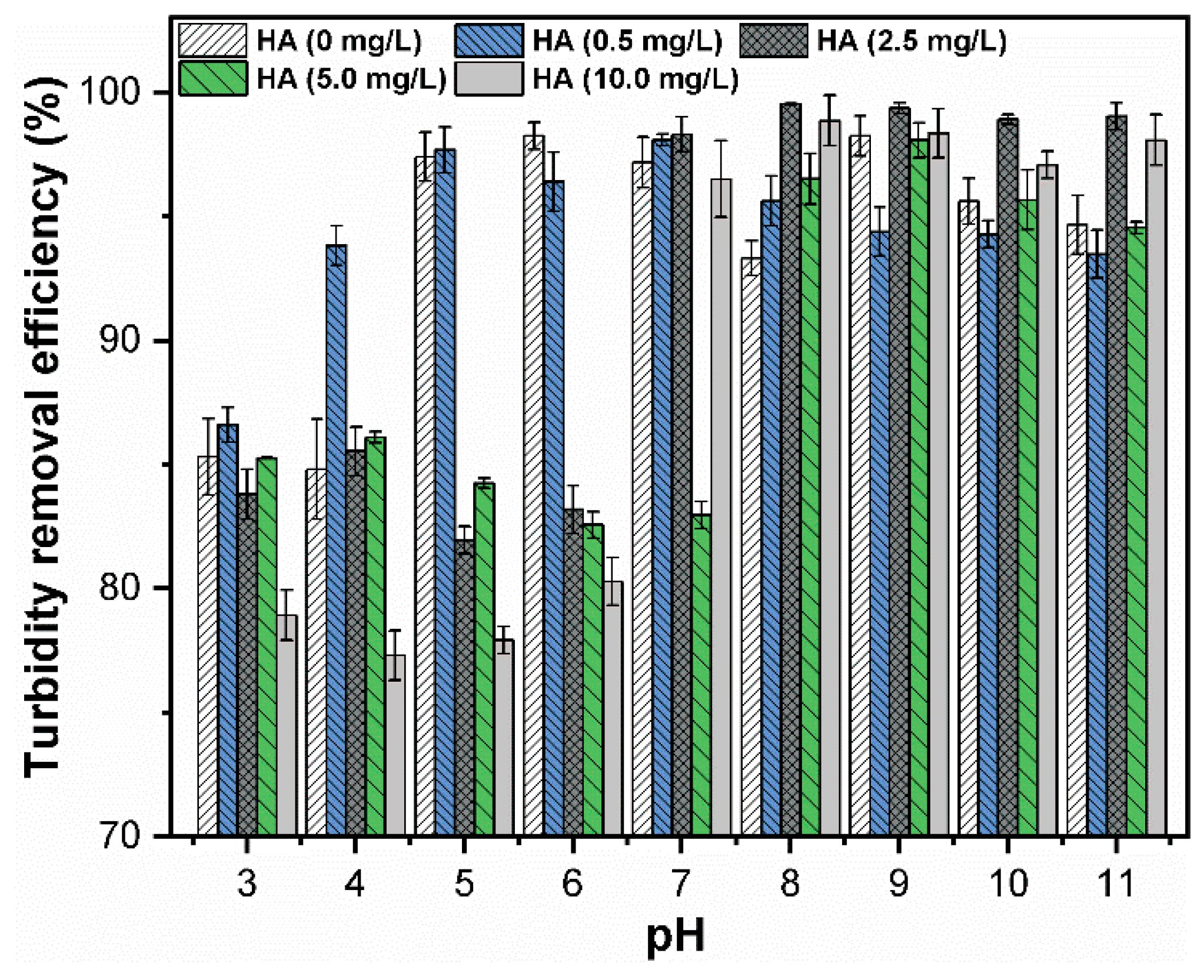
3.3. Effect of HA Concentration on CuO NPs Removal
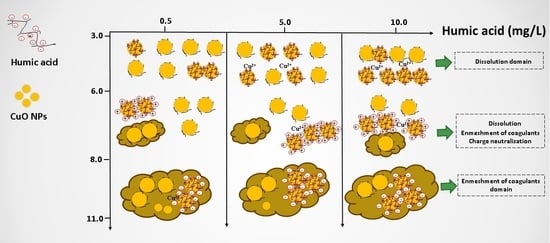
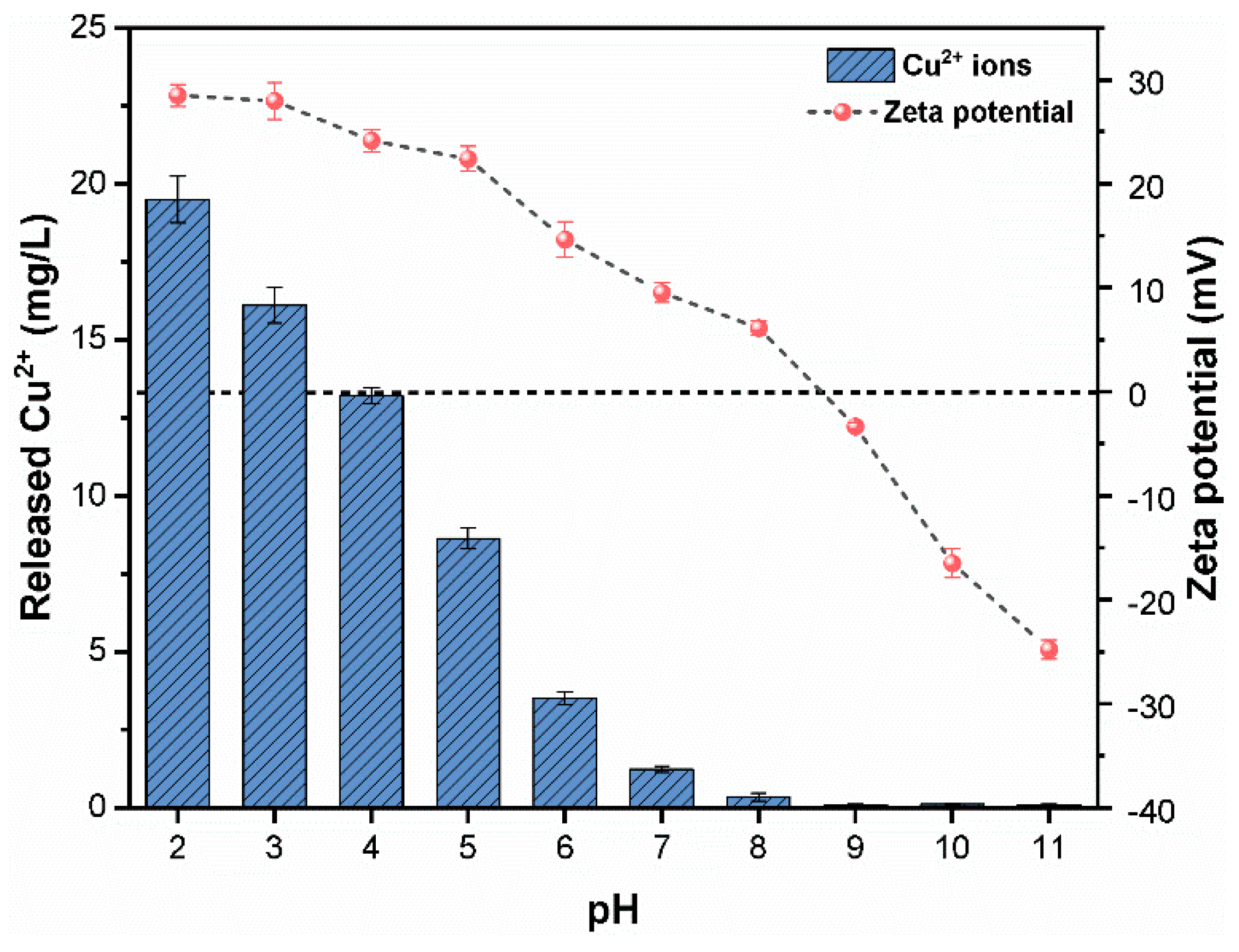
3.4. Effect of pH and HA on the Dissolution of CuO NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella Jr, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Vosti, W.; Wang, H.; Lazareva, A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanopart. Res. 2014, 16, 2489. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.-C.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, R.; Chen, Y. Effects of ZnO nanoparticles on wastewater biological nitrogen and phosphorus removal. Environ. Sci. Technol. 2011, 45, 2826–2832. [Google Scholar] [CrossRef]
- Dreher, K.L. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol. Sci. 2004, 77, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Vavra, J.; Forbes, V.E. Effects of water quality parameters on agglomeration and dissolution of copper oxide nanoparticles (CuO-NPs) using a central composite circumscribed design. Sci. Total Environ. 2015, 521, 183–190. [Google Scholar] [CrossRef]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Banfield, J.F. Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876–14884. [Google Scholar] [CrossRef]
- Wall, N.A.; Choppin, G.R. Humic acids coagulation: influence of divalent cations. Appl. Geochem. 2003, 18, 1573–1582. [Google Scholar] [CrossRef]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of fulvic acid by carbon nanotubes from water. Environ. Pollut. 2009, 157, 1095–1100. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, W.; Xing, W.; Xu, N. Crossflow filtration of nanosized catalysts suspension using ceramic membranes. Sep. Purif. Technol. 2011, 76, 223–230. [Google Scholar] [CrossRef]
- Springer, F.; Laborie, S.; Guigui, C. Removal of SiO2 nanoparticles from industry wastewaters and subsurface waters by ultrafiltration: Investigation of process efficiency, deposit properties and fouling mechanism. Sep. Purif. Technol. 2013, 108, 6–14. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Wang, X.; Gore, D.; Tiede, D.; Baxter, M.; David, H.; Tear, S.P.; Lewis, J. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J. Anal. At. Spectrom. 2010, 25, 1149–1154. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Stability and removal of water soluble CdTe quantum dots in water. Environ. Sci. Technol. 2007, 42, 321–325. [Google Scholar] [CrossRef]
- Hyung, H.; Kim, J.-H.H. Dispersion of C60in natural water and removal by conventional drinking water treatment processes. Water Res. 2009, 43, 2463–2470. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Kline, C.N.; Filliben, J.J. Impact of source water quality on multiwall carbon nanotube coagulation. Environ. Sci. Technol. 2010, 44, 1386–1391. [Google Scholar] [CrossRef]
- Abbott Chalew, T.E.; Ajmani, G.S.; Huang, H.; Schwab, K.J. Evaluating nanoparticle breakthrough during drinking water treatment. Environ. Health Perspect. 2013, 121, 1161–1166. [Google Scholar] [CrossRef]
- Wang, H.T.; Ye, Y.Y.; Qi, J.; Li, F.T.; Tang, Y.L. Removal of titanium dioxide nanoparticles by coagulation: effects of coagulants, typical ions, alkalinity and natural organic matters. Water Sci. Technol. 2013, 68, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Inam, M.; Park, D.; Zam Zam, S.; Shin, S.; Khan, S.; Akram, M.; Yeom, I. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes 2018, 6, 170. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Park, D.R.; Khan, S.; Akram, M.; Yeom, I.T. The Removal of CuO Nanoparticles from Water by Conventional Treatment C/F/S: The Effect of pH and Natural Organic Matter. Molecules 2019, 24, 914. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Zhaoxia, J.I. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Lv, B.; Yang, Y.; You, G.; Xu, Y. Effect of alginate on the aggregation kinetics of copper oxide nanoparticles (CuO NPs): bridging interaction and hetero-aggregation induced by Ca2+. Environ. Sci. Pollut. Res. 2016, 23, 11611–11619. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.; Zhao, Q.; He, S.; Ma, J. Effect of AlCl3 concentration on nanoparticle removal by coagulation. J. Environ. Sci. 2015, 38, 103–109. [Google Scholar] [CrossRef]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.X. Inorganic polymer flocculation theory and flocculants. China Build. Ind. Press. Peking 2006. [Google Scholar]
- Zirino, A.; Yamamoto, S. A pH-dependent model for the chemical speciation of copper, zinc, cadmium, and lead in seawater. Limnol. Oceanogr. 1972, 17, 661–671. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Effect of pH, concentration and temperature on copper and zinc hydroxide formation/precipitation in solution. In Proceedings of the Chemeca 2011: Engineering a Better World, Sydney Hilton Hotel, NSW, Australia, 18–21 September 2011; pp. 2100–2110. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Inam, M.A.; Zam Zam, S.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different pH Values. Sustainability 2019, 11, 2825. https://doi.org/10.3390/su11102825
Khan R, Inam MA, Zam Zam S, Akram M, Shin S, Yeom IT. Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different pH Values. Sustainability. 2019; 11(10):2825. https://doi.org/10.3390/su11102825
Chicago/Turabian StyleKhan, Rizwan, Muhammad Ali Inam, Saba Zam Zam, Muhammad Akram, Sookyo Shin, and Ick Tae Yeom. 2019. "Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different pH Values" Sustainability 11, no. 10: 2825. https://doi.org/10.3390/su11102825
APA StyleKhan, R., Inam, M. A., Zam Zam, S., Akram, M., Shin, S., & Yeom, I. T. (2019). Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different pH Values. Sustainability, 11(10), 2825. https://doi.org/10.3390/su11102825