The Edge Effect on Plant Diversity and Soil Properties in Abandoned Fields Targeted for Ecological Restoration
Abstract
1. Introduction
2. Materials and Methods
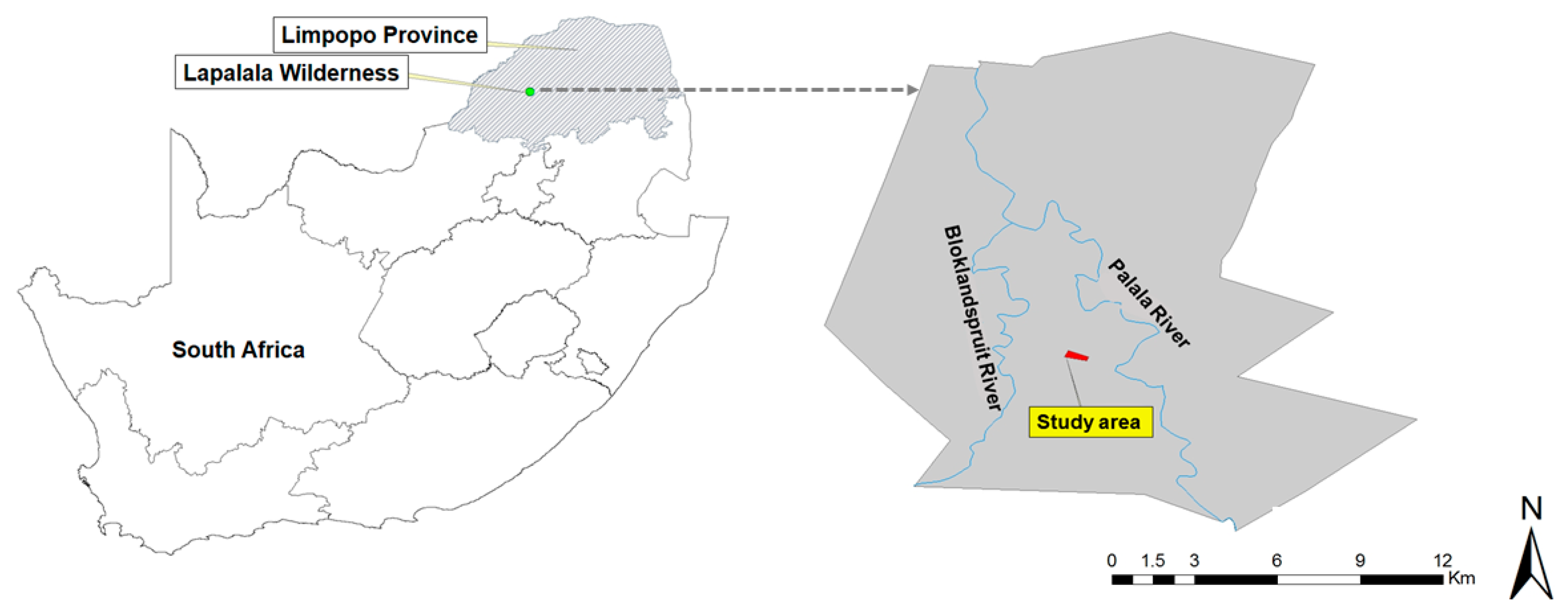
2.1. Study Area
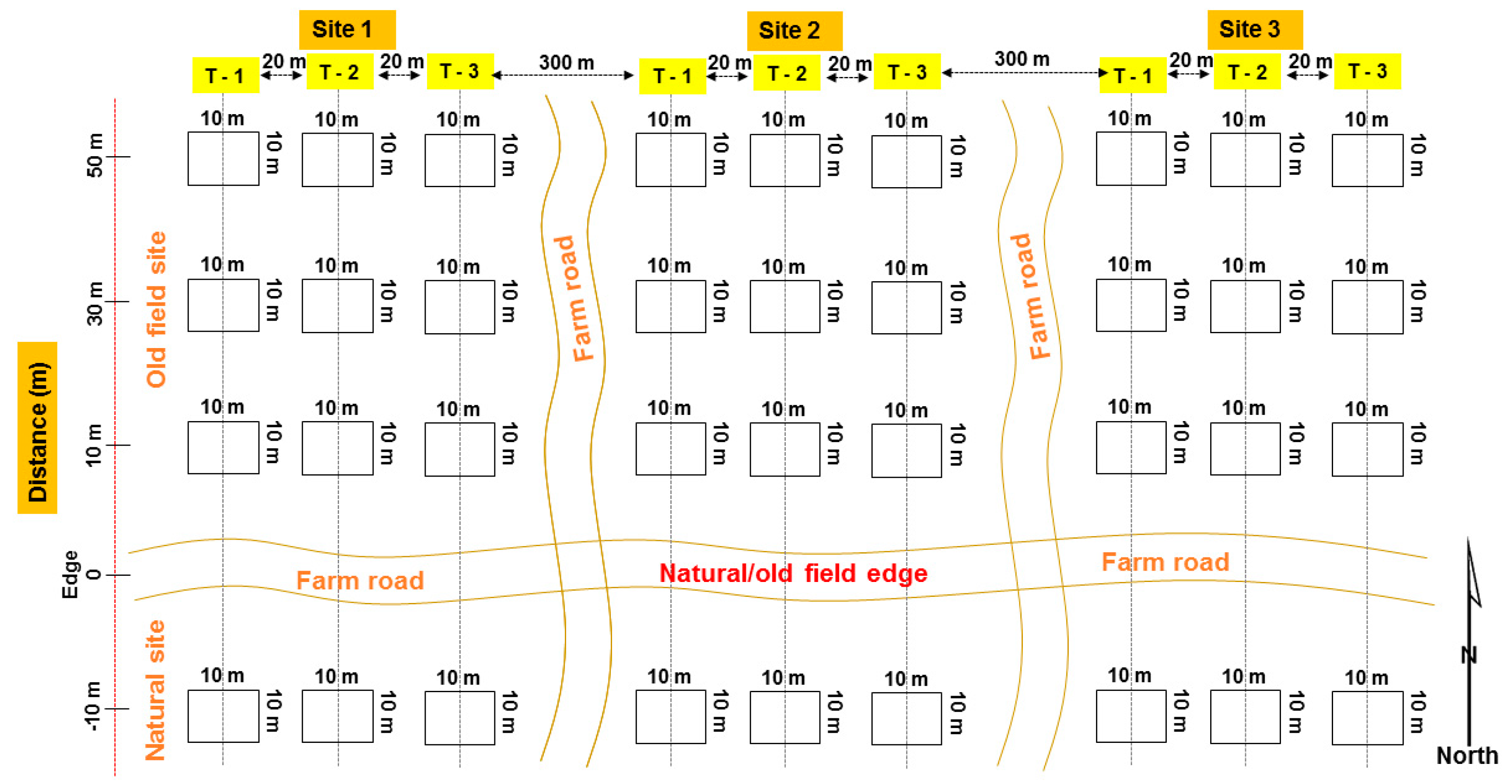
2.2. Sampling Design
2.3. Vegetation Surveys
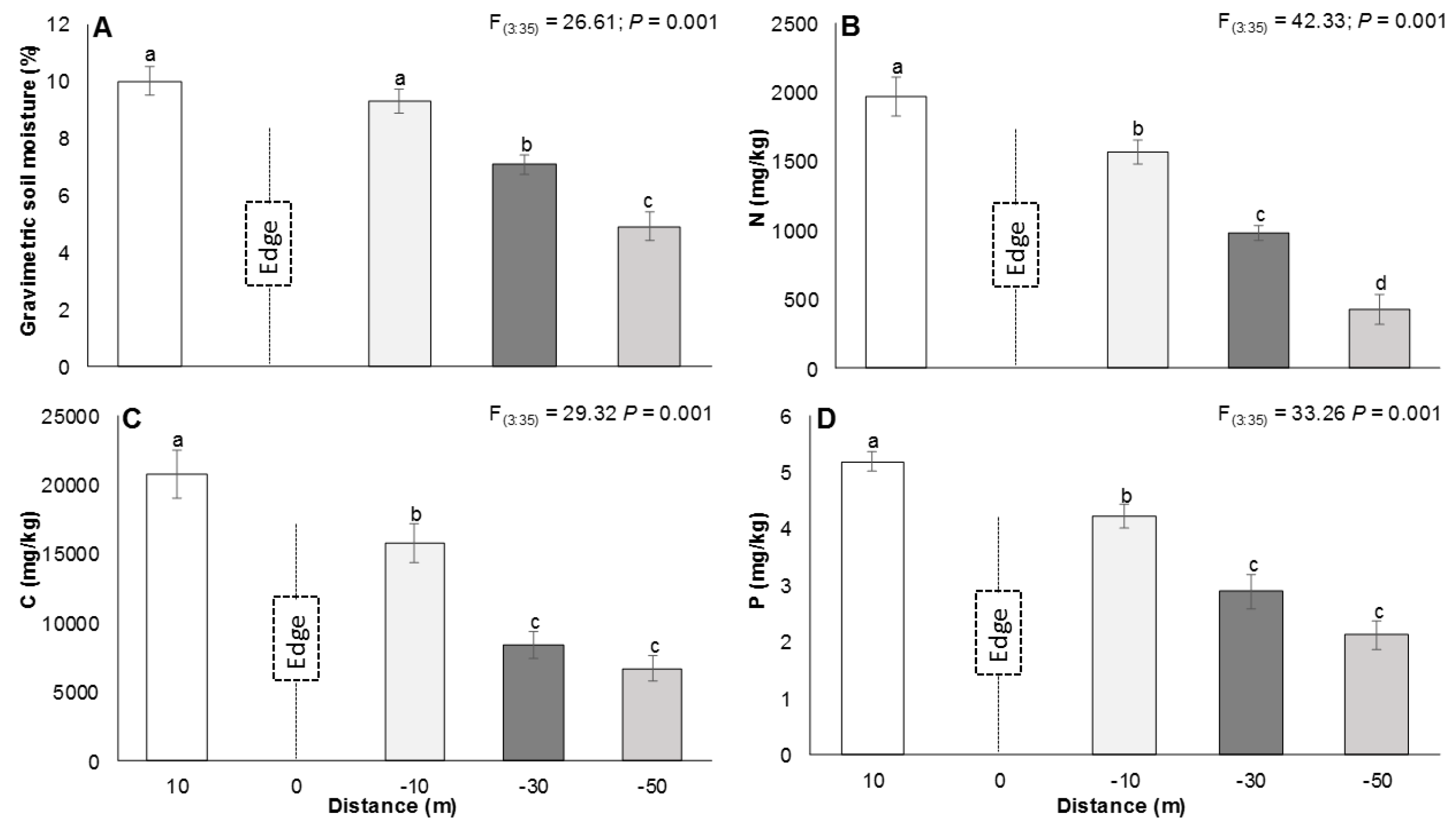
2.4. Soil Sampling
2.5. Statistical Analysis
3. Results
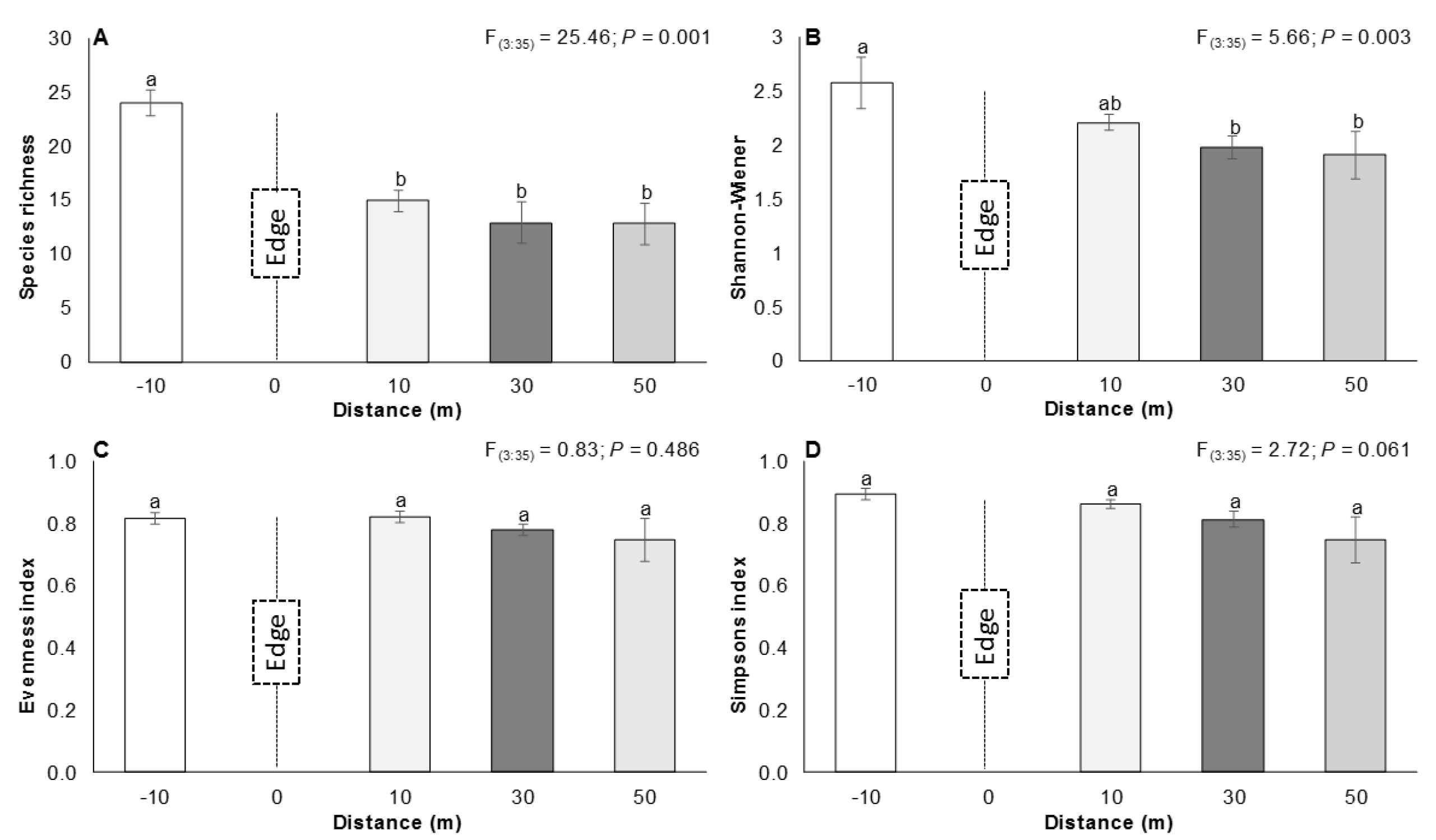
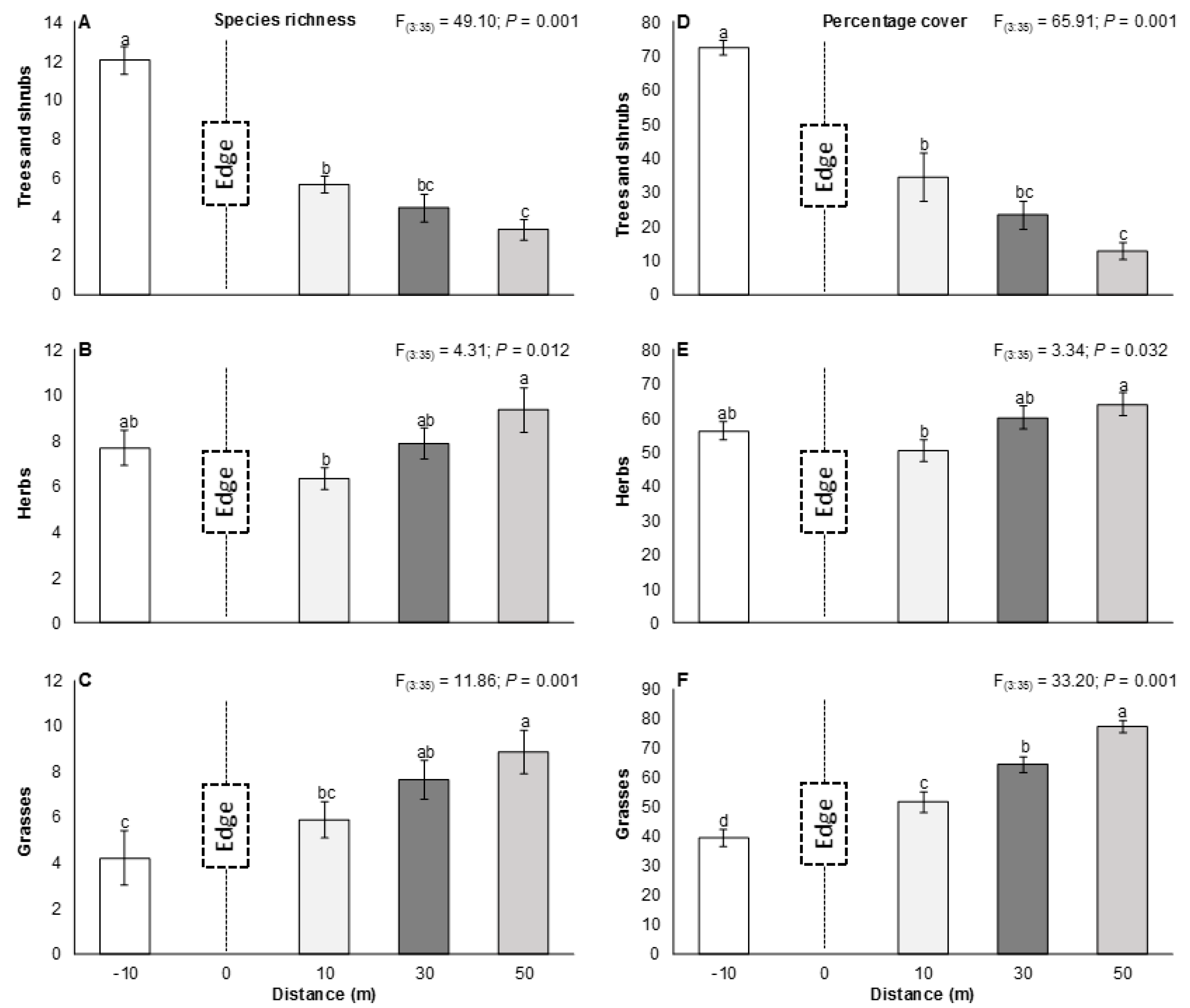
3.1. The Edge Effect on Vegetation
3.2. The Edge Effect on Soils
4. Discussion
4.1. The Edge Effect on Vegetation
4.2. The Edge Effect on Soil
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Distance from Edge (in m) | |||
|---|---|---|---|---|
| −10 | 10 | 30 | 50 | |
| Trees and Shrubs | ||||
| Dombeya rotundifolia | *** | - | - | - |
| Euclea undulata | *** | - | - | - |
| Grewia monticola | *** | - | - | - |
| Gymnosporia buxifolia | *** | - | - | - |
| Kirkia acuminata | *** | - | - | - |
| Mimusops zeyheri | *** | - | - | - |
| Pterocarpus rotundifolius | *** | - | - | - |
| Senegalia caffra | *** | - | - | - |
| Peltophorum africanum | *** | ** | ** | * |
| Senegalia nigrescens | **** | *** | * | * |
| Senegalia senegal | *** | ** | * | * |
| Vachellia tortilis | *** | *** | ** | ** |
| Vachellia nilotica | *** | *** | ** | ** |
| Vachellia karroo | *** | ** | ** | * |
| Combretum molle | **** | ** | - | - |
| Ziziphus mucronata | *** | ** | - | - |
| Grewia bicolor | *** | ** | - | - |
| Grewia flava | *** | * | - | - |
| Lippia javanica | **** | * | * | * |
| Terminalia sericea | **** | * | - | - |
| Herbs | ||||
| Gomphrena celosioides | - | *** | *** | *** |
| Xerochrysum bracteatum | - | *** | *** | *** |
| Solanum incanum | ** | **** | *** | *** |
| Datura stramonium | - | *** | **** | *** |
| Solanum panduriforme | - | *** | - | - |
| Emilia transvaalensis | ** | *** | - | - |
| Felicia sp | ** | *** | - | - |
| Pseudognaphalium sp | ** | *** | - | - |
| Grasses | ||||
| Arundinella nepalensis | * | *** | **** | **** |
| Brachiaria deflexa | * | **** | *** | **** |
| Cynodon dactylon | * | **** | *** | **** |
| Panicum maximum | * | **** | *** | **** |
| Eragrostis lehmanniana | * | **** | *** | **** |
| Aristida congesta | - | *** | - | - |
| Digitaria eriantha | - | **** | - | - |
| Eragrostis inamoena | - | **** | - | - |
References
- De la Hey, M.; Beinart, W. Why have South African smallholders largely abandoned arable production in fields? A case study. J. S. Afr. Stud. 2017, 43, 753–770. [Google Scholar] [CrossRef]
- Blair, D.; Shackleton, C.M.; Mograbi, P.J. Cropland abandonment in South African smallholder communal lands: Land cover change (1950–2010) and farmer perceptions of contributing factors. Land 2018, 7, 121. [Google Scholar] [CrossRef]
- Myster, R.W. Post-agricultural invasion, establishment, and growth of Neotropical trees. Bot. Rev. 2004, 70, 381–402. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.; Llamb, L.D. Facilitation and edge effects influence vegetation regeneration in old-fields at the tropical Andean forest line. Appl. Veg. Sci. 2015, 18, 613–623. [Google Scholar] [CrossRef]
- Blignaut, J.; Aronson, J.; de Wit, M. The economics of restoration: Looking back and leaping forward. Ann. N. Y. Acad. Sci. 2014, 1322, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.P.; Rey Benayas, J.M.; Meli, P.; Maceira, N.O. Quantifying the impacts of ecological restoration on biodiversity and ecosystem services in agroecosystems: A global meta-analysis. Agric. Ecosyst. Environ. 2015, 202, 223–231. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Ruwanza, S. Furrows as centers of restoration in old fields of Renosterveld, South Africa. Ecol. Restor. 2017, 35, 289–291. [Google Scholar] [CrossRef]
- Riedel, S.M.; Epstein, H.E. Edge effects on vegetation and soils in a Virginia old-field. Plant Soil 2005, 270, 13–22. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Lin, L.; Cao, M. Edge effects on soil seed banks and understory vegetation in subtropical and tropical forests in Yunnan, SW China. For. Ecol. Manag. 2009, 257, 1344–1352. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Gehlhausen, S.M.; Schwartz, M.W.; Augspurger, C.K. Vegetation and microclimatic edge effects in two mixed-mesophytic forest fragments. Plant Ecol. 2000, 147, 21–35. [Google Scholar] [CrossRef]
- Smit, R.; Olff, H. Woody species colonization in relation to habitat productivity. Plant Ecol. 1998, 139, 203–209. [Google Scholar] [CrossRef]
- Meiners, S.J.; Pickett, S.T.A.; Handel, S.N. Probability of tree seedling establishment changes across a forest–old field edge gradient. Am. J. Bot. 2002, 89, 466–471. [Google Scholar] [CrossRef]
- Hester, A.J.; Hobbs, R.J. Influence of fire and soil nutrients on native and non-native annuals at remnant vegetation edges in the Western Australian wheatbelt. J. Veg. Sci. 1992, 3, 101–108. [Google Scholar] [CrossRef]
- Goldblum, D.; Beatty, S.W. Influence of an old field/forest edge on a Northeastern United States deciduous forest understory community. J. Torrey Bot. Soc. 1999, 126, 335–343. [Google Scholar] [CrossRef]
- Phillips, O.L.; Rose, S.; Mendoza, A.M.; Vargas, P.N. Resilience of Southwestern Amazon forests to anthropogenic edge effects. Conserv. Biol. 2006, 20, 1698–1710. [Google Scholar] [CrossRef]
- Ramırez, L.; Rada., F.; Llambı, L.D. Linking patterns and processes through ecosystem engineering: Effects of shrubs on microhabitat and water status of associated plants in the high tropical Andes. Plant Ecol. 2015, 216, 213–225. [Google Scholar]
- Duncan, R.S.; Chapman, C.A. Seed dispersal and potential forest succession in abandoned agriculture in tropical Africa. Ecol. Appl. 1999, 9, 998–1008. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Ruwanza, S.; Mulaudzi, D. Soil physico-chemical properties in Lapalala Wilderness old agricultural fields, Limpopo Province of South Africa. Appl. Ecol. Environ. Res. 2018, 16, 2475–2486. [Google Scholar] [CrossRef]
- Midgley, D.C.; Pitman, W.V.; Middleton, B.J. A Guide to Surface Water Resources of South Africa; Water Research Commission: Johannesburg, South Africa, 1983. [Google Scholar]
- Galatowitsch, S.; Richardson, D.M. Riparian scrub recovery after clearing of invasive alien trees in headwater streams of the Western Cape, South Africa. Biol. Conserv. 2005, 122, 509–521. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis: Part I Physical and Mineralogical Properties; American Society of Agronomy: Madison, WA, USA, 1965. [Google Scholar]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci. Soc. Am. J. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Bray, R.H.; Krutz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: London, UK, 1996. [Google Scholar]
- Hurlbert, S. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- StatSoft, Inc. Statistica (Data Analysis Software System). Version 13.1. 2017. Available online: http://www. statsoft.com (accessed on 10 July 2018).
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Yao, J.; Holt, R.D.; Rich, P.M.; Marshall, W.S. Woody plant colonization in an experimentally fragmented landscape. Ecography 1999, 22, 715–728. [Google Scholar] [CrossRef]
- Horn, A.; Krug, C.B.; Newton, I.P.; Esler, K.J. Specific edge effects in highly endangered Swartland Shale Renosterveld in the Cape Region. Ecol. Mediterr. 2011, 37, 63–74. [Google Scholar]
- Cramer, V.A.; Hobbs, R.J. Old Fields: Dynamics and Restoration of Abandoned Farmland; Island Press: Washington, DC, USA; London, UK, 2007. [Google Scholar]
- Mucina, L.; Bustamante-Sánchez, M.A.; Duguy, B.; Holmes, P.; Keeler-Wolf, T.; Armesto, J.J.; Dobrowolski, M.; Gaertner, M.; Smith-Ramírez, C.; Vilagrosa, A. Ecological restoration in mediterranean-type shrublands and woodlands. In Routledge Handbook of Ecological and Environmental Restoration; Allison, S., Murphy, S., Eds.; Taylor & Francis: Abingdon, UK, 2017; pp. 173–196. [Google Scholar]
- Cubina, A.; Aide, T.M. The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Krug, C.B.; Krug, R.M. Restoration of old fields in Renosterveld: A case study in a Mediterranean type shrubland of South Africa. In Old Fields: Dynamics and Restoration of Abandoned Farmland; Cramer, V.A., Hobbs, R.J., Eds.; Island Press: Washington, DC, USA, 2007; pp. 265–285. [Google Scholar]
- Donaldson, J.; Nänni, I.; Zachariades, C.; Kemper, J. Effects of fragmentation on pollinator diversity and plant reproductive success in renosterveld shrublands of South Africa. Conserv. Biol. 2002, 16, 1267–1276. [Google Scholar] [CrossRef]
- Statton, J.; Gustin-Craig, S.; Dixon, K.W.; Kendrick, G.A. Edge effects along a seagrass margin result in an increased grazing risk on Posidonia australis transplants. PLoS ONE 2015, 10, e0137778. [Google Scholar] [CrossRef] [PubMed]
- Hovel, K.A.; Regan, H.M. Using an individual-based model to examine the roles of habitat fragmentation and behavior on predator-prey relationships in seagrass landscapes. Landsc. Ecol. 2008, 23, 75–89. [Google Scholar] [CrossRef]
- Sousa, W.P. The role of disturbance in natural communities. Annu. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar] [CrossRef]
- Dohn, J.; Dembélé, F.; Karembé, M.; Moustakas, A.; Amévor, K.A.; Hanan, N.P. Tree effects on grass growth in savannas: Competition, facilitation and the stress-gradient hypothesis. J. Ecol. 2013, 101, 202–209. [Google Scholar] [CrossRef]
- Penido, G.; Ribeiro, V.; Fortunato, D.S. Edge effect on post-dispersal artificial seed predation in the southeastern Amazonia. Braz. J. Biol. 2015, 75, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Normann, C.; Tscharntke, T.; Scherber, C. How forest edge–center transitions in the herb layer interact with beech dominance versus tree diversity. J. Plant Ecol. 2016, 9, 498–507. [Google Scholar] [CrossRef]
- Piessens, K.; Honnay, O.; Devlaeminck, R.; Hermy, M. Biotic and abiotic edge effects in highly fragmented heathlands adjacent to cropland and forest. Agric. Ecosyst. Environ. 2006, 114, 335–342. [Google Scholar] [CrossRef]
- Duncan, R.S.; Duncan, V.E. Forest succession and distance from forest edge in an afro-tropical grassland. Biotropica 2000, 32, 33–41. [Google Scholar] [CrossRef]
- Wright, T.E.; Kasel, S.; Tausz, M.; Bennett, L.T. Edge microclimate of temperate woodlands as affected by adjoining land use. Agric. For. Meteorol. 2010, 150, 1138–1146. [Google Scholar] [CrossRef]
- Ren, H.; Yang, L.; Liu, N. Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog. Nat. Sci. 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Badano, E.I.; Samour-Nieva, O.R.; Flores, J.; Flores-Flores, J.L.; Flores-Cano, J.A.; Rodas-Ortíz, J.P. Facilitation by nurse plants contributes to vegetation recovery in human-disturbed desert ecosystems. J. Plant Ecol. 2016, 9, 485–497. [Google Scholar] [CrossRef]
- Bever, J.D. Feedback between plants and their soil communities in an old field community. Ecology 1994, 75, 1965–1977. [Google Scholar] [CrossRef]
- Dybzinski, R.; Fargione, J.E.; Zak, D.R.; Fornara, D.; Tilman, D. Soil fertility increases with plant species diversity in a long-term biodiversity experiment. Oecologia 2008, 158, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Meyer, S.T.; Leal, I.R.; Tabarelli, M. Plant herbivore interactions at the forest edge. Prog. Bot. 2008, 69, 423–448. [Google Scholar]
- Zahawi, R.A.; Augspurger, C.K. Tropical forest restoration: Tree islands as recruitment foci in degraded lands of Honduras. Ecol. Appl. 2006, 16, 464–478. [Google Scholar] [CrossRef]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruwanza, S. The Edge Effect on Plant Diversity and Soil Properties in Abandoned Fields Targeted for Ecological Restoration. Sustainability 2019, 11, 140. https://doi.org/10.3390/su11010140
Ruwanza S. The Edge Effect on Plant Diversity and Soil Properties in Abandoned Fields Targeted for Ecological Restoration. Sustainability. 2019; 11(1):140. https://doi.org/10.3390/su11010140
Chicago/Turabian StyleRuwanza, Sheunesu. 2019. "The Edge Effect on Plant Diversity and Soil Properties in Abandoned Fields Targeted for Ecological Restoration" Sustainability 11, no. 1: 140. https://doi.org/10.3390/su11010140
APA StyleRuwanza, S. (2019). The Edge Effect on Plant Diversity and Soil Properties in Abandoned Fields Targeted for Ecological Restoration. Sustainability, 11(1), 140. https://doi.org/10.3390/su11010140





