1. Introduction
Cassava is a staple food crop for more than half a billion people in the tropical and subtropical regions of the world and mainly used as food, feed and industrial raw material [
1,
2,
3,
4]. It is the sixth most important commercially cultivated food crop after wheat, maize, potato, rice and barley [
2]. Cassava is a highly resilient crop that can withstand stress from drought and dry poor soils while still giving acceptable yields [
2]. In addition, cassava is easy to cultivate and its roots can stay reserved in the soil for several months [
2] when the farmer’s storage space is limited, thereby creating opportunity for extended harvest and sustained availability. It therefore fits description as a
survival crop that can potentially secure food supply [
4] and sustain livelihoods of large populations in difficult times.
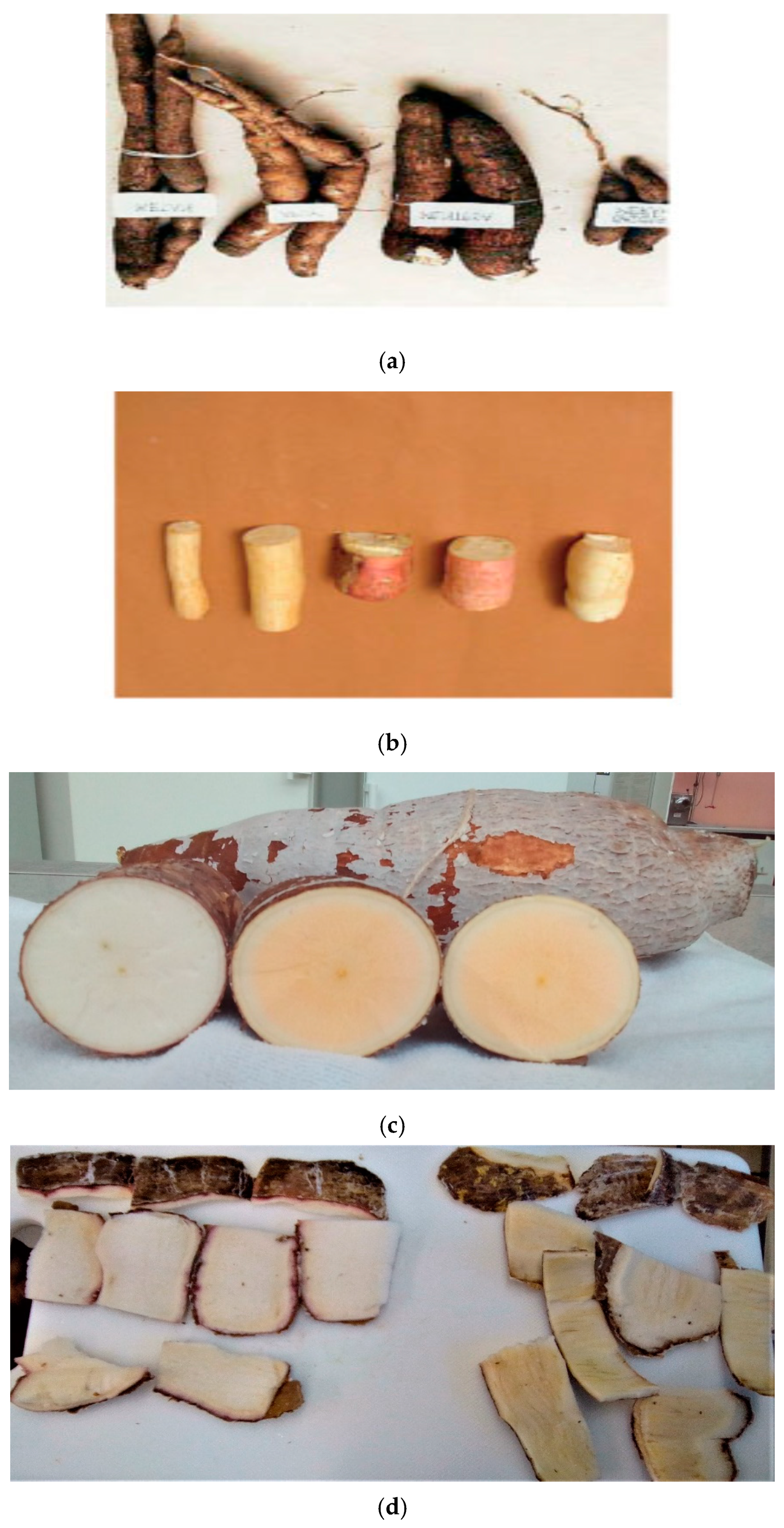
In the regions where cassava is cultivated, the flesh color is traditionally white [
1]. This is conventionally important to produce “white” starch or high quality flour. However, other colored-flesh cassava variants (yellow, orange, cream, and red) have emerged to challenge this supposition. In this article, the term “variant” generally refers to phenotypes, genotypes, progenies, accessions, varieties, clones, or cultivars—as the case may be in the referred literature—while yellow-flesh cassava and biofortified yellow-flesh cassava all refer to same thing. Flesh color and culinary quality have therefore become vital in the selection of cassava for food [
1]. White-flesh (and other dull colored) variants have negligible carotenoid content compared to yellow-flesh variants [
2,
3,
4,
5]. Variants with deeper color intensity have a higher carotene content [
6]. Visual differences also exist between root and flour of white-flesh and yellow-flesh variants, but not for the starch.
Sub-Saharan Africa, Asia, and South America are the largest cassava producers in the world. Yellow-flesh cassava, and other biofortified cassava have considerable potential in alleviating food insecurity in developing countries [
4]. Currently, issues remain about widespread acceptance, commercial cultivation, and consumption of the yellow-flesh cassava variants despite their immediate nutritional advantage over white-flesh cassava variants. Some of the challenges have arisen from poor understanding of nutritional benefits of the colored-flesh cassava, misinformation about the nature of development of the variants as genetically modified crops, unwillingness of farmers to change cultivation pattern, and weak governmental commitment to propagation and dissemination for public awareness. These challenges could be stumbling blocks in the sustenance of cultivating yellow-flesh and other colored-flesh cassava. This is particularly unexpected since crops such as maize, potato and cocoyam have recorded relatively huge successes in this regard in the past.
Cassava generally has location-specific properties which have been discussed in the literature. At locations in Southern China, variants that are now commonly cultivated for starch include five white-flesh variants and two yellow-flesh variants (
Table 1) [
7]. Indonesia also has different variants cultivated for food [
8]. Among those are yellow-flesh (Mentega) and white-flesh (Adira 2 and Darul Hidayah) variants (
Table 1). A notable difference between these Indonesian variants is that all yellow-flesh variants are sweet, while white-flesh ones are either bitter or sweet. The bitter taste may not only be due to the high content of toxic cyanogenic glucosides (HCN > 100 mg/kg) in the variants, but also due to high levels of antioxidants. Hence, the yellow-flesh variant is preferred for local food preparation in Indonesia [
8]. In a bid to sustain production of improved cassava variants in Nigeria, six yellow-flesh variants were released under the IITA-HarvestPlus Project between 2011 and 2014 [
9,
10]. The first set of released variants had a β-carotene content of 6–8 µg/g on fresh weight basis, while the second set of variants introduced had an average β-carotene content of about 10 µg/g on fresh weight basis. Several clones of yellow-flesh variants have been under investigation to select the most suitable traits for release. A total carotenoid content of almost 25 µg/g has been attained in South American variants [
11,
12]. Sustained efforts are ongoing to develop variants with up to 15 µg/g of β-carotene [
13]. The acceptability of yellow-flesh variants is promising among farmers and consumers, as they are more stable after harvest than their white-flesh counterparts [
14]. The increasing marketability of yellow-fleshed varieties, e.g. Narayanakappa in India [
1,
15] and NR 07/0326 in Nigeria [
16], is documented due to the culinary value and the quality of flour and baked goods that they can yield. From a nutritional point of view, yellow-flesh variants have been used to partially meet the recommended vitamin A requirements of cassava-consuming populations. For example, in Kenya [
17], where every year 23,500 children die due to a deficiency of micronutrients, and where many school-aged children suffer from sub-clinical vitamin A deficiency [
18], yellow-flesh cassava was used to combat these deficiencies. Adding biofortified yellow-flesh cassava to the lunch of the schoolchildren successfully improved nutrient adequacy, but additional nutritional supplement was recommended. In addition, majority of the respondents in the organoleptics aspect of the study preferred the taste, color, and texture of yellow-flesh cassava to the white-flesh one [
17].
Cassava flour is a valuable product obtained from cassava roots after processing. Generally, to produce the flour, cassava root is peeled, washed, chipped, milled, pressed to expel most of the toxic liquor, dried, fine-milled, and sieved. It is relatively cheap to produce traditionally. Industry-grade high quality cassava flour, however, requires improved processing inputs, which may add to the costs. Due to its special properties such as its clear appearance, low off-flavor tendency and ideal viscosity, it is regarded as a vital ingredient in the food industry [
21].
Cassava starch is a highly suitable material for food and industrial use. It is edible, non-toxic, and functionally important in the food and non-food sectors of industry. Briefly, cassava starch is produced in sequence by peeling, chipping, wet milling, sieving, sedimenting, decanting, drying and pulverizing. Cassava has a high proportion (65–80%) of starch [
24,
25], which is low in contaminants compared to other botanical starches [
26,
27]. Cassava starch embodies positive characteristics such as high paste clarity, relatively good stability to retrogradation, low protein clog/complex, swelling capacity, and good texture [
27], which makes it suitable for use in many foods. For instance, better quality bread has been made when cassava starch is included in baking [
28]. Therefore, it is highly desirable to select certain variants of cassava as industrial starch source, depending on their inherent characteristics [
7].
It is worth mentioning that, while cassava flour consists mostly of starch, the presence of relatively higher fiber, protein, minerals and vitamins contents in the flour compared to the starch could confer certain differences in their properties.
Several review articles have been written on the properties of cassava root starches (in native and modified forms) especially from white-flesh variants [
26,
29,
30]. In comparison, there has been little focus on the properties of root, flour, and starch of yellow-flesh cassava variants. It is therefore necessary to compile more information on yellow-flesh (and other colored-flesh) variants, since there is an increasing interest in the value-added, biofortified cassava variants and products derived from them. Due to issues with relatively restricted acceptance, cultivation and consumption of yellow-flesh cassava, it is pertinent to study if they are comparable to, or offer any nutritional, functional, or physicochemical advantages over the white-flesh cassava variants in a bid to argue for continued sustenance of efforts to improve its level of acceptance and adoption by the agro-allied industry and the public at large. A positive outcome could encourage public-private investments in breeding and adoption programs in Sub-Saharan Africa. Therefore, the comparison of properties of cassava root, flour, and starch is discussed from a number of literature sources, based on differing flesh colors. For instance, nutritional and chemical properties such as protein, carotenoids, minerals, starch and amylose contents are discussed. Physicochemical and functional properties such as water and oil absorption capacity, swelling power, solubility, and pasting characteristics are also discussed. Factors that influence the differences in properties and effects of processing on the variants are considered. In addition, some peculiar challenges and possible solutions in adoption of the biofortified yellow cassava in Africa are discussed with regard to issues of sustainability. Recommendations based on the review findings are made.
3. Chemical Properties
The literature classifies cassava root as a high calorie food [
4,
31] with a high percentage of carbohydrates (80–90% dry basis) consisting almost entirely of starch [
32]. Nonetheless, cassava root is relatively poor in other nutrients such as proteins, lipids, and vitamins [
17]. Cassava roots generally have a high moisture content, which can differ with variants. A reliable comparison of the moisture contents between variants is possible with dry basis measurements. Most literature reports higher values of moisture contents in yellow-flesh variants. Moisture content of the respective root, flour and starch of yellow-flesh cassava variants is reported to be higher [
16], marginally higher [
21] or significantly higher [
33] than for those of white-fleshed variants (
Table 2). Likewise, Ukenye et al. [
22] reported a higher dry matter content for white-flesh variants compared to yellow-flesh variants, and dry matter of starch extracted from white-flesh variants (89.04–96.41%) was significantly higher than that from yellow-flesh variants (88.47%) [
33]. An association between the concentration of carotenoids and the moisture content of cassava and its products may be possible considering that, in numerous publications, the average moisture content of cassava roots, flour and starch usually ranks in the color sequence: yellow = orange > cream > white [
16,
19,
20,
21,
22,
33]. Despite the relatively higher amount of moisture in yellow-flesh cassava roots, they tend to store better after harvest than their white-flesh counterparts, perhaps due to the additional anti-oxidative effect of carotenoids present [
6]. This beneficial property could be appreciated by farmers, thus encouraging sustainable post-harvest practices and processing. A safe moisture limit for starch storage on the international market is 13% [
34,
35,
36].
The poor nutrients density of cassava [
37,
38,
39] is exemplified in its low protein content. Compared to other roots and tubers, cassava roots have a low protein content of about 1–3% on dry basis [
37], and are particularly poor in sulfur-rich amino acids. Acidic and basic amino acids such as glutamic, aspartic and arginine are, however, relatively plentiful in cassava roots [
32]. Because of this, cassava diet from the roots has to be supplemented by other protein sources. Nonetheless, Montagnac et al. [
4] reported a number of attempts to improve protein in cassava by biofortification and post-harvest processing, with some recorded successes. Protein content (
Table 2) was significantly higher in the flour of white-flesh cassava variants than in the flour of yellow-flesh variants [
21]. In cassava roots reported by Ukenye et al. [
22], the protein content was not significantly different between variants. Protein may be lost during processing of cassava root into flour and starch. Hence, protein content in cassava could be variant-dependent.
The fiber content of cassava roots depends on the variant and age at harvest [
4]. A relatively higher fiber content of 0.62–4.92% has been found in roots of white-flesh cassava variants than in yellow-flesh and cream-flesh variants [
22]. Likewise, crude fiber in cassava flour is generally higher in white-flesh variants than yellow-flesh ones [
16]. The difference in fiber content contributes to the higher dry matter in white-flesh variants than yellow-flesh variants. The isolation procedure undergone during starch extrication rids cassava starch of most fiber. Residual fiber influences texture and in vitro digestibility of cassava starch and flour. Cassava starch is characteristically low in fiber (0.10–0.15%) and lipids (0.11–0.22%) [
38,
39]. About half the lipids in cassava roots are non-polar, or in glycolipid forms (especially galactose diglycerides), but fatty acids such as oleates and palmitates are more commonly found [
32,
40]. Lipids may be involved in the retention of the lipid-soluble carotenoids in yellow-flesh cassava.
Cassava is not particularly rich in all mineral nutrients; hence, diets based on cassava alone may not fulfill adequate mineral nutritional requirement in humans. Chavez et al. [
6] analyzed 20 variants of cassava collected from several core clones and quantified the major minerals present. Average content of zinc, iron, calcium, magnesium, sodium, potassium and sulfur (dry basis) were 6.4 mg/kg, 9.6 mg/kg, 590 mg/kg, 1153 mg/kg, 66.4 mg/kg, 8903 mg/kg, and 273 mg/kg, respectively. Other minerals were only found in negligible quantities. Total phosphorus content in cassava is as low as 70–120 mg/kg of root [
41]. An average phosphorus content of 1284 mg/kg has been reported for cassava roots [
6], and the phosphate content did not vary by flesh color. Phosphate in starches of seven yellow-flesh and white-flesh Indonesian variants were similar, and had negligible amounts (23.5–25.3 nmol/mg) of phosphorus [
8], attached mostly at C-3 and C-6 positions on anhydro-glucose units. In other tuber crops, especially potato, the phosphate content is relatively high, and influences a number of physicochemical and functional properties [
26]. However, the use of cassava in industrial food and non-food use is almost unrivalled. Due to its comparably superior solubility and paste clarity, low gelatinization temperature and low retrogradation tendency [
26], the industrial use of cassava in food applications, textile processing and in confectioneries endears it to the industry. In addition, cassava flour and starch are more popular, cheaper, and more available in commercial quantities than those of potato across regions of Sub-Saharan Africa.
Other micronutrients of more recent significance and interest in cassava are pro-vitamin A carotenoids and vitamin C. Vitamin C, which is important for mineral absorption in the gut, is found in relatively higher amounts than carotenoids in fresh cassava. For instance, among more than 500 cassava root lines evaluated by Chavez et al. [
6], vitamin C averaged 9.5 mg/100 g in fresh cassava although it is much more susceptible to losses during processing than carotenes. Minimal processing of cassava by methods susceptible to oxidation is recommended, if some vitamin C is to be retained. However, much of the processing techniques used for converting cassava to edible, safe food cannot guarantee its retention. Carotenoids are important for healthy body metabolism and disease prevention. Of the carotenoids found in yellow-flesh cassava roots, β-carotenes are present in higher concentrations than other carotenoids involved in the biosynthesis of vitamin A [
42]. They are important micronutrients in global health issues, particularly in developing countries [
43,
44,
45]. Many studies have measured carotenoids in cassava (
Table 3) with significant differences existing between the variants. The Indian yellow-flesh variant Narayanakappa had lower total carotenoids and β-carotene (3.1 µg/g, and 2.3 µg/g, respectively) contents than three other orange-flesh variants [
1]. The higher color intensity of orange-fleshed variants could indicate higher concentrations in carotenoids than the yellow-flesh cassava. The concentrations differed by variant when some processing techniques were employed [
15]. Total carotenoids of roots of three yellow-flesh cassava variants from Nigeria ranged between 2.6–7.3 µg/g (average 4.9 µg/g), and varied significantly depending on the variant [
46]. These values are close to those reported (6.26–7.76 µg/g) for other variants of yellow-flesh cassava by Omodamiro et al. [
23], but considerably higher than that of white-flesh variants (0.35 µg/g). Again, root and flour from six yellow-flesh cassava variants were about three to six folds richer in carotenoids than from white-flesh variants [
16]. Thus, the value addition offered by yellow-flesh cassava over white-flesh cassava as a vector for biofortification cannot be overemphasized. Ceballos et al. [
47] determined total carotenoid content of six variants of yellow-flesh cassava from Colombia as 8.32–16.40 µg/g (wb), which consists of 70% all-trans β-carotene and 5% each of the isomers 9-cis and 13-cis β-carotene. The proximal parts of the roots had a higher concentration of total carotenoids, β-carotenes, and higher dry matter than central and distal parts, signifying inhomogeneous distribution of carotenoids in cassava, and could be informative in preferred choice of parts for consumption. Visual inspection of cassava root color intensity could be used as a casual, non-empirical indicator for carotenoid concentration as conducted by Ukenye et al. [
22], where color intensity and carotenoid content ranked: yellow-flesh > cream-flesh > white-flesh.
On a dry basis, total carotenoids and β carotene contents quantified for three cassava roots [
48] were 9.06–21.95 µg/g and 7.16–13.50 µg/g, respectively, with HPLC chromatograms revealing the presence of isomeric forms of carotene: 13-cis-β- carotene and 9-cis-β-carotene in relatively lower quantities. A similar study was conducted by Oliveira et al. [
49] for 12 bitter yellow-flesh cassava roots, where total carotenoids and β-carotene (isomeric forms: all-E-β carotene, 13-Z-β carotene and 9-Z-β carotene) ranged between 1.97–16.33 µg/g and 1.37–7.66 µg/g, respectively, while the total carotenoid content for flour of five other bitter yellow-flesh variants was 3.65–18.92 µg/g. Thus, carotenoids in cassava can be unstable during processing, and converted to other more stable forms. Two genes have been thought to be implicated in defining concentration of carotenes found in several cassava variants, one coding for transportation of products of precursors, and the other for accumulation [
6,
50]. These genes could be manipulated for breeding purposes in developing cassava of increasingly higher carotenoids content. Carotenoid concentration in cassava can be considered a qualitative trait, determined by a few genes, and not readily repressed or promoted due to effects of the environment [
51]. The study [
51], which describes the individual and interactive effects of genotype (G), year of cultivation (Y), and location (L) on the fresh yield and total carotenoid quality of 24 yellow-flesh and 3 white-flesh variants in Nigeria, showed that G had the strongest impact on total carotenoids, while the location was the decisive factor for high fresh weight yields. Each factor had a significant effect on both qualities, but Y and interaction effect G-L-Y were only partially significant. Interaction effects Y-L and G-L were strongly significant, but G-Y was not significant. Interacting principal component analysis (IPCA) was helpful in selecting the genotypes and locations with best strengths and minimal compromise for the targeted qualities.
Amylose and amylopectin play crucial compositional and functional roles in cassava starch, influencing properties such as crystallinity, gelatinization, retrogradation, gelling, and pasting. Cassava starch with low amylose has higher crystallinity corresponding to a reduced amorphous band [
52], and high-amylose starch retrogrades relatively easily [
53]. Amylopectin molecular structure, as determined by degree of branching, molecular weight, and chain length can be influenced by the activity of starch branching enzymes of different polymorphic forms [
54]. When determined from the roots directly, amylose content of yellow-flesh cultivars was similar to that of white-flesh variants [
22]. A contrasting observation was made for other roots/tubers, such as sweet potatoes [
55] and yam bean (
Pachyrhizus tuberosus) tuber, where amylose content in yellow-flesh variants was significantly higher than in white-flesh variants [
56]. Significant difference (
p < 0.01) was found between amylose of cassava flour of 40 yellow-flesh (15.71–22.25%) variants and three white-flesh (18.18–20.29%) variants from Nigeria [
21], when studied for effect of drying method and variety. The difference in amylose may have partly contributed to the higher paste peak viscosity of flours from most yellow-flesh variants compared to white-fleshed ones in the study. Amylopectin plays a more significant role, however, in the pasting properties of cassava [
57]. Amylose content of cassava starch varies widely between 14–24% [
41], but starches of waxy (amylopectin-rich) variants may contain as low as 0–3.4% amylose [
58]. Amylose of starches of seven variants from Southern China ranged from 13.5–24.65%, varying significantly with location, environment, and variant [
7]. Among these variants, the yellow-flesh ones had similar amylose content to white-flesh ones (
Table 3). A similar trend was found in other works [
33,
59,
60], where similar methods were used for amylose determination. Again, amylose in starches of 17 Indonesian variants was similar regardless of flesh color, ranging 17.1–21.3% [
8]. No difference was found between the amylose content in starches of yellow-flesh variants (17.4–20.2%) and white-flesh variants (16.8–21.3%). Any variations in values of amylose content reported could be due to different methods of determination employed.
Starch content of cassava can be determined chemically or enzymatically, but starch yield is the amount of starch physically recoverable from cassava root. Total starch content (
Table 3) of flour from 40 yellow-flesh cassava variants (67.08–81.18%) was similar to that of flour of three white-flesh variants (70.48–82.42%) from Nigeria, when studied for effect of drying method and variety [
21]. Starch yield from four yellow-flesh cassava variants was lower than starch yield from white and cream-flesh variants, with total carbohydrates following the same trend [
22]. Again, starch extracted from six roots and flours of recently released yellow-flesh variants was lower on the average in comparison to that from white-flesh variants [
16]. These reports indicate that the starch content of white-flesh cassava variants is significantly higher than that of yellow-flesh variants, although genotypic differences [
21] and age can also cause differences in starch contents between these variants. This might reveal that there is possibly a reduced activity of granule bound starch synthase (GBBS), soluble starch synthase (SSS) and starch branching enzymes (SBE) for starch synthesis [
61] in yellow-flesh variants.
Sugars such as sucrose, glucose, fructose and maltose have been quantified in cassava roots [
62]. Overall, about 17% sucrose has been found in sweet variants of cassava roots [
31]. Anggraini et al. [
8] found all yellow-flesh variants studied were sweet, while white-flesh variants were either bitter or sweet depending on the variant. The amount of compounds responsible for the bitterness, such as tannins and cyanogens, was significantly lower in yellow-flesh roots than in white-flesh roots [
7] and may influence taste. In contrast, similar or even higher amounts of sugars have been found in some white-flesh variants compared to yellow-flesh variants [
33]. In fact, Oliveira et al. [
49] has reported 17 known bitter yellow-flesh cassava variants cultivated in Brazil. Total sugars were found to be significantly higher among flours of yellow-flesh cassava variants (
Table 3) than flours of white-flesh cassava variants in the study by Maziya-Dixon et al. [
21]. Hence, this might be the plausible reason for mild to sweet taste among most yellow-flesh variants. These findings imply that the differences in variants and cultivation conditions could be strong factors influencing sugar composition in cassava.
The ash content is an important component of cassava, and is an indication of the mineral richness and non-volatiles content of cassava [
4]. Total non-incinerable matter (ash) of cassava was reported to be similar between both white-flesh and yellow-flesh variants [
22]. Ash content reported for cassava flour and starch made from white cassava were within similar range as for yellow-flesh variants [
16,
21,
33]. Among these cassava materials, starch had the lowest ash content. Cassava leaves, however, have a significantly higher ash content than the roots [
4]. Processing of cassava has been reported to significantly reduce ash content of the roots, with a similar trend for minerals [
4]. Hence, severe processing techniques such as those involving application of high temperature and chemicals and excessive fermentation, washing and milling treatments could significantly reduce ash in cassava-dominated diets.
5. Relationships (Correlation or Regression) between Physicochemical and Functional Properties of Cassava
A few studies demonstrate the relationships between physicochemical and functional properties of cassava. Flesh color has been positively correlated with total carotenoids in some works [
48,
99]. Low dry matter has also been associated with carotenoid contents of cassava variants [
65,
100]. A common theory that postharvest physiological deterioration (PPD) and shelf storability may be influenced by pro-vitamin A content of yellow cassava roots due to the anti-oxidative nature of the compounds may be true. Postharvest physiological deterioration of cassava includes vascular streaking, tissue softening, rotting, and discoloration resulting from biochemical changes. A report by Chavez et al. [
6] correlated PPD of 30 cassava variants with vitamins after six days in storage and revealed there was significant evidence to support this hypothesis, but conclusions could not be drawn since there was a low correlation. It was further established that >0.5 mg/kg carotene ensured that PPD did not result in reduction of carotene nutrients exceeding 30%. Toxicity, as measured by cyanogenic content, varied significantly in different parts of the cassava plant. Concentration of cyanogenic compounds in the root, stem, and leaf of cassava are independent of one another because total cyanogens in those parts are influenced by age at maturity or harvest, precipitation/rainfall, genetics, fertilization, soil type, location, etc. [
101,
102,
103,
104].
Water absorption capacity of three yellow-flesh variants had a significant negative correlation with breakdown viscosity of the starches. A significant negative linear regression coefficient has been observed between starch granule damage and gelatinization properties of cassava starch, such as enthalpy and onset of gelatinization, especially when grown under less water stress [
71].
A study on 79 cassava variants in China revealed no relationship between amylose, peak viscosity and clarity of the starches [
105], but a report by Charles et al. [
106] found correlations between these properties. The amount of rainfall cassava received during cultivation was negatively correlated to starch yield and dry matter [
7], while starch content significantly correlated positively with dry matter (0.54,
p < 0.01). In the same work, freeze–thaw stability correlated positively to retrogradation (0.355,
p < 0.05), indicating that cassava starches that are stable to freeze–thaw cycles may not readily retrograde. Starch granule volume, and surface area correlated positively with viscosity (0.350, 0.336,
p < 0.05).
Pasting properties of cassava as well as other tuber crops have been found to negatively correlate with protein content [
107]. Since amylose content is a major composition of cassava starch, there was a positive correlation with pasting temperature and peak time (
r = 0.45). Higher proportions of amylose in the starch require longer periods to leach out of the granule matrix before complete solubilization and swelling occurs [
68,
107]. Starch–fiber interaction may have a negative effect on peak viscosity (
r = −0.544) and swelling power (
r = −0.805), but a positive correlation with pasting temperature (
r = 0.422) [
68,
107]. Swelling of cassava starch is negatively correlated with pasting temperature (
r = −0.629), but positively correlated with peak viscosity,
r = 0.588 [
68].
6. Effects of Processing on Nutrients in White-Flesh and Biofortified Yellow-Flesh Cassava Roots and Flour
Cassava is safe for consumption only after undergoing appropriate processing, which may include one or a combination of some treatments such as boiling, frying, fermenting, drying, baking, or size-reduction, all of which contribute to reducing cyanogenic glucosides [
108]. However, the downside of these processing operations is often a resultant reduction of nutrients, or conversion to other forms than the original nutrients. For instance, production of flour and other foods from cassava often leads to a loss of vital micronutrients, some of which are discussed below.
In a study of 28 clonal variants selected by Chavez et al. [
6] from a large genetic pool of cassava, boiling of the roots led to an average reduction of carotene by 34%. In addition, flour produced after oven drying and sun drying lost an average of 44% and 73% carotene, respectively. In India, Narayanakappa, a yellow-fleshed cassava, and three other colored-flesh variants, all of high-carotene content, were processed by boiling, frying, sun drying and oven drying [
1,
15], and the effect on the concentration of total carotenoids and β-carotene were studied. Destruction of total carotenoids and beta carotene was found to be in the order: sun drying (44–67% and 43–79%) > frying (20–51% and 16–56%) > boiling (16–52% and 19–49%) > oven drying (16–45% and 5–36%) [
15]. Boiling intensified the color of the cassava chips, possibly due to starch gelatinization, however, the profound release of carotenoids from cassava matrices could also be a reason [
20]. Frying depleted carotenoids, which are known to be fat-soluble. Diminished retention was highest for variant
Acc-3, which had the highest amounts of carotenoids and β-carotene. Rapid deterioration of carotenoids during exposure to light and heat can be attributed to their sensitivity towards oxidation and isomerization. Similar results have been reported in other studies [
6,
23,
109,
110]. Retention of carotenoids after processing is essential in adopting yellow-flesh cassava as a means of combating vitamin A deficiency in affected populations.
Other forms of processing affect retention of carotenoids in cassava. Grated cassava mash, fermented cassava mash, and fermented-cooked cassava dough (fufu) retained 97.68–98.48%, 94.68–96.66%, and 86.42–90.24% of original total carotenoids in yellow-flesh cassava (6.26–7.76 µg/g), respectively [
23]. Drying the product from the “odorless” non-fermented method resulted in a significant loss of carotenoids, the severity being higher with sun drying than oven drying. Generally, the acceptability of fufu by sensory panelists, in terms of color, was best with the traditional method [
23], because the method enhanced carotenoids retention better than sun drying and oven drying methods.
Evaluation of retained micronutrients, such as zinc, iron and total carotenoids, was made after three yellow-flesh cassava variants TMS 01/1371, 01/1235, and 94/0006 were processed traditionally by four methods: boiling, fermenting (raw fufu), fermenting and cooking (cooked fufu) and fermenting and roasting (gari) [
46]. Boiling led to losses of about 4–52% of carotenoids, 3.6–20.6% of iron, and 2.7–21.7% of zinc. Fermentation significantly increased the average carotenoid content of the cassava roots from 4.9 µg/g to 8.64 µg/g according to wet basis measurements. A possible reason could be that, as major compositions of cassava (carbohydrates, moisture, and fiber) reduce by hydrolysis during fermentation, the proportion of other minor compositions such as carotenoids will apparently increase. Dry basis measurements could have given a more accurate trend of what transpired during processing [
111]. Fermentation also significantly reduced the average iron and zinc content from 7.47 mg/kg to 7.13 mg/kg, and 8.95 mg/kg to 5.58 mg/kg, respectively. Fermentation leaches minerals due to the acidic nature of fermentate (fufu), and oxidative activities of microbes that use these micronutrients for development and growth. Boiling fermented cassava paste to achieve a doughy consistency further led to a reduction in the average carotenoid (3.64 µg/g) and zinc content (6.23 mg/kg), thus retaining less carotenoids (21.5%) and zinc (34.1%) than the uncooked paste. Only 32.5% iron was retained. The high temperature employed may have resulted in the rapid oxidation, isomerization, and destruction of vitamin A precursors [
112,
113,
114], and leaching of the minerals. Again, fermenting and subsequent roasting (gari) was reported to increase average carotenoids and iron of three yellow-flesh variants from 4.9 µg/g to 10.6 µg/g, and from 7.5 mg/kg to 8.2 mg/kg. respectively. This is due to the dry nature of gari, and the fresh weight basis by which the determinations were made. Hence, the increase in the carotenoids and iron content was not an increase in actual amounts. There was an average retention of about 45% and 22% of carotenoids and iron. respectively, while 90% of zinc was lost. Heating, increase in surface area, and agitation [
115,
116,
117,
118,
119,
120,
121] during gari production may have increased carotenoids available for quantification. Chopping (to increase surface area) and brief heating of colored-flesh cassava roots was adjudged to have contributed to the bioavailability of carotenoids and vitamin A by disrupting cell wall and protein–carotenoids complexes [
20].
High performance liquid chromatograms [
47] revealed that boiling can reduce total carotenoids and all-trans-β-carotenes of yellow-flesh cassava from 32.6 µg/g to 27.2 µg/g and 22.7 µg/g to 15.3 µg/g, respectively, on dry weight basis. The reduction of all-trans β-carotenes has been characterized as isomerization to 13-cis and 15-cis-β-carotene in boiled cassava [
47,
122]. Dry matter also reduced from 34.4% to 29.7% after boiling for 30 min. However, high retention (86.6%) of total carotenoids was observed in the work [
47], and it was postulated that high dry matter in yellow-flesh variants might enhance retention of carotenoids in general.
Individual and interaction effects [
48] of variants and processing methods showed significant differences in the retention of β-carotene among yellow-flesh cassava variants. This is an important criterion in the selection of variants to be adopted for vitamin A bio-fortification. A ranking of the processing methods vis-à-vis β-carotene retention was: oven drying (71.9%) > shadow drying (59.2%) > boiling (55.7%) > sun drying (37.9%) > gari production (34.1%). Storage of cassava up to four weeks in any form (flour, chip or root) after sun drying and oven drying resulted in reducing the retained β-carotene by slightly above 50%, depending on the severity of size-reduction (flour > chip > root). Vacuum storage reduced residual β-carotene, possibly due to the permeability of packaging used, and not as a result of the vacuum created. Generally, a reduction of oxygen species in an environment could deter oxidation of carotenoids.
Processing of five bitter yellow-flesh variants of cassava from Brazil [
49] to flour reduced total carotenoids by an average of 50%, with further reduction of 33–99% during storage for up to 19 days. By the fourth week in storage, total carotenoids of the variants had completely diminished.
Processing could, in addition, lead to a reduction of cassava toxicity. Breeding to reduce toxicity is an ongoing sustained effort by scientists and breeders in research institutes in Africa and Asia. La Frano et al. [
20] determined toxic cyanogenic contents of white-flesh (5.27 ppm) and yellow-flesh (5.51–280 ppm) cassava variants for safety reasons, and found a complete absence after they were processed by boiling into porridge, while 99% and 96% of β-carotene was retained, respectively. Generally, white-flesh cassava variants have a negligible β-carotene content, losing less than 1% during boiling [
20], but variants with a high initial carotene content lose it much more readily during processing [
6,
48]. Furthermore, in the work of Diallo et al. [
123], three cassava variants in Senegal were processed into four products—chips, flour, gari, and attieke—and their cyanide concentration (wb) was evaluated for the extent of detoxification. The chips retained 15.1–51.6% of cyanogenic toxicity, but gari, flour and attieke retained as low as 0–1.8%, 0–2.8%, and 1.1–5.4% cyanogens, respectively. These levels were below the allowed toxicity recommendation (<10 ppm) of the Codex Alimentarius Commission [
124] for cassava flour, and are thus regarded safe for consumption.
In Nigeria, value-addition has been achieved in using yellow-flesh variants in producing gari instead of the traditional practice ofadding red palm oil to the white cassava granules to improve the vitamin A content of the food. Not only are production costs reduced, but a healthy form of fortification is also acquired by the yellow-flesh variants with yields close to the white-flesh ones. From a culinary and health/nutrition perspective, adding palm oil may not be acceptable in gari due to phase separation in water-based processes as well as for cholesterol-related issues.
Vitamin C retention is very low in processed cassava roots and flour. Only 36.6%, 6.2%, and 0.005% of vitamin C was retained after boiling, oven-drying and sun drying, respectively [
6]. Pregnant women, lactating mothers and children [
125] need the vital minerals (iron and zinc) and pro-vitamin A to prevent anemia, diarrhea, stunted growth, and defective eyesight. Evidence from trials with American women [
20] and Kenyan children [
17] has shown that feeding biofortified cassava is more efficient in the bioavailability of pro-vitamin A compounds than white-flesh cassava. Some new yellow-flesh cassava roots in Nigeria could potentially supply about 25% of the daily vitamin A requirement if consumed in sufficient amounts [
16]. Hence, processes retaining much of these micronutrients are beneficial, especially when losses are unavoidable [
47]. An indirect, but efficient way to reduce nutrient losses in cassava during processing could be to significantly improve the nutrient content of the raw, unprocessed crop. Efforts to achieve this by biofortification in increasing the protein, minerals, starch and β-carotene concentrations in cassava, has been reported [
4]. This has been the focus of majority of cassava breeders in research institutes in Africa and Asia. Post-harvest technique, such as solid-state fermentation has also been used to increase protein in cassava [
126]. In addition, minimal processing sufficient enough to make cassava edible and safe should be encouraged. Excessive fermentation, drying, boiling, cooking, retting, and frying cause major losses in nutrients [
4], and should be avoided.
7. Practical Considerations for Sustainability in Adoption of Biofortified Yellow-Flesh Cassava
To argue for the sustained cultivation of biofortified yellow cassava and the production, utilization and consumption of its products, it is important to have detailed information on properties of the biofortified variant in comparison to the conventional white-flesh cassava. The arguments focus on the advantages offered by the biofortified variants, such as substitutability, nutrition, safety for consumption, storage life and relative ease of harvest and post-harvest handling. Many of the data discussed in this work show that biofortified yellow-flesh cassava can be a suitable, and arguably better, substitute compared to the conventional white-flesh cassava. The argument can be made based on the considerations discussed below, which has evidently, and can potentially, make biofortified yellow-flesh cassava more sustainable in many aspects than white-flesh cassava.
First, biofortified yellow-flesh cassava root, flour and starch has many similar physicochemical and functional properties as found for those of white-flesh cassava, and can, therefore, serve as a substitute in any products derived from white-flesh cassava root, flour and starch. This flexibility in utilization is not possible for white-flesh cassava when pro-vitamin A nutrition is of concern, making this additional advantage of nutrition a case for sustained utilization and consumption of biofortified yellow cassava as food. This was already demonstrated by Talsma [
17] in a school children feeding program in Kenya. In addition, the development of biofortified yellow-flesh cassava has evidently explored possibilities of creating value-added cassava variants without genetic modification which is still contended as ethically unacceptable. Biofortification is considered sustainable because it requires a one-time investment only, which further allows farmers flexibility in cultivation. This curbs complete dependence of farmers on manufacturers for cultivation materials supply, as is usually associated with genetically modified crops. Such flexibility is enhanced by the value addition offered by yellow-flesh cassava regarding amount of vitamin nutrient per hectare cultivated. The biofortified cassava can reach subsistent poor farmers and people in remote areas where supplementation programs, which are usually more expensive and unaffordable, scarcely reach [
127].
Second, most biofortified yellow-flesh cassava variants are sweet tasting, containing mild-to-moderate toxic cyanogenic glucosides compared to the majority of white-flesh variants [
7]. Lower concentrations of the toxic compound can guarantee sustainable production plans by reducing time and labor for detoxification during processing, ultimately leading to production of safer foods. In addition, lower toxicity levels could reduce toxic residues in soil and environment of rural communities and urban centers of Africa where large quantities are regularly processed. This issue is of considerable concern to proponents of environmental sustainability since the run-off from these processing centers often result in pollution and contamination of nearby water bodies and the disruption of natural ecosystem of plants and animals.
Third, post-harvest storage of biofortified yellow-flesh cassava is more sustainable, due to its robust and longer shelf-stability [
6,
17] compared to white-flesh cassava. This implies it can secure longer availability periods, while the farmer awaits or is engaged in a new planting cycle. Ongoing unpublished research by the authors has observed that properly waxed yellow-flesh cassava roots have much longer storage life and acceptable quality at 3–5 months in refrigeration at 3 °C, than waxed white-flesh cassava roots which developed unacceptable quality after 3–5 weeks under similar conditions.
Fourth, harvesting and post-harvest handling of some variants of biofortified yellow-flesh cassava has been reported to be less tedious than for white-flesh cassava. Farmers’ response to surveys reveal they were easier to harvest and peel [
127] than conventional white-flesh cassava. This advantage could reduce labor and energy costs, making manual or commercial harvesting and peeling more sustainable.
This work therefore explored comparison of the properties of both variants of cassava for the purpose of assisting farmers, processors and other interest groups in making informed choices on variants with sustainable properties. It may be surmised that, in the near future, the popularity of yellow-flesh cassava may outpace that of the conventional white-flesh cassava [
128] if some measures are taken strategically. Such measures should include a more committed and robust coordination of national governments programs, research groups, and other cassava stakeholders in ensuring rapid and widespread re-orientation, adoption and dissemination of the biofortified yellow cassava and its products to the public. In addition, encouraging market-based approach in cassava value chain to attract private investors [
9,
128] can be helpful.
While the adoption of biofortified orange-flesh sweet potato in Sub-Saharan Africa has achieved remarkable success in countries including South Africa, Mozambique, and Uganda, such cannot be said yet of the biofortified yellow cassava. Originally, pro-vitamins-rich cassava existed in the Amazon regions of South America. They were subsequently bred [
14,
99] and introduced to African countries including Nigeria and Kenya through research cooperation of the International Center for Tropical Agriculture (CIAT), Colombia and the International Institute of Tropical Agriculture, Nigeria. More successes seem to have been achieved in South America regarding the yield, nutrients density [
6,
128] and public acceptance of biofortified yellow cassava variants than in Sub-Saharan Africa. For instance, in northeast Brazil, a survey of 760 farmers reveal 28% of farmers already preferred yellow cassava variants, with about 70% of them been familiar with the yellow varieties [
127]. About 15% of the farmers cultivated the variants as early as the first year of release of the variants. Nevertheless, few successes in acceptance of biofortified yellow cassava in production of gari and fufu, for instance, has been reported in Oyo and Akwa Ibom regions of Nigeria [
128]. One major gap that continues to hamper the adoption of the biofortified variants is the weakened state of the extension service in the agricultural sector of some African countries, for instance, Nigeria [
9].