Assessing Soil Metal Levels in an Industrial Environment of Northwestern China and the Phytoremediation Potential of Its Native Plants
Abstract
1. Introduction
2. Materials and Methods
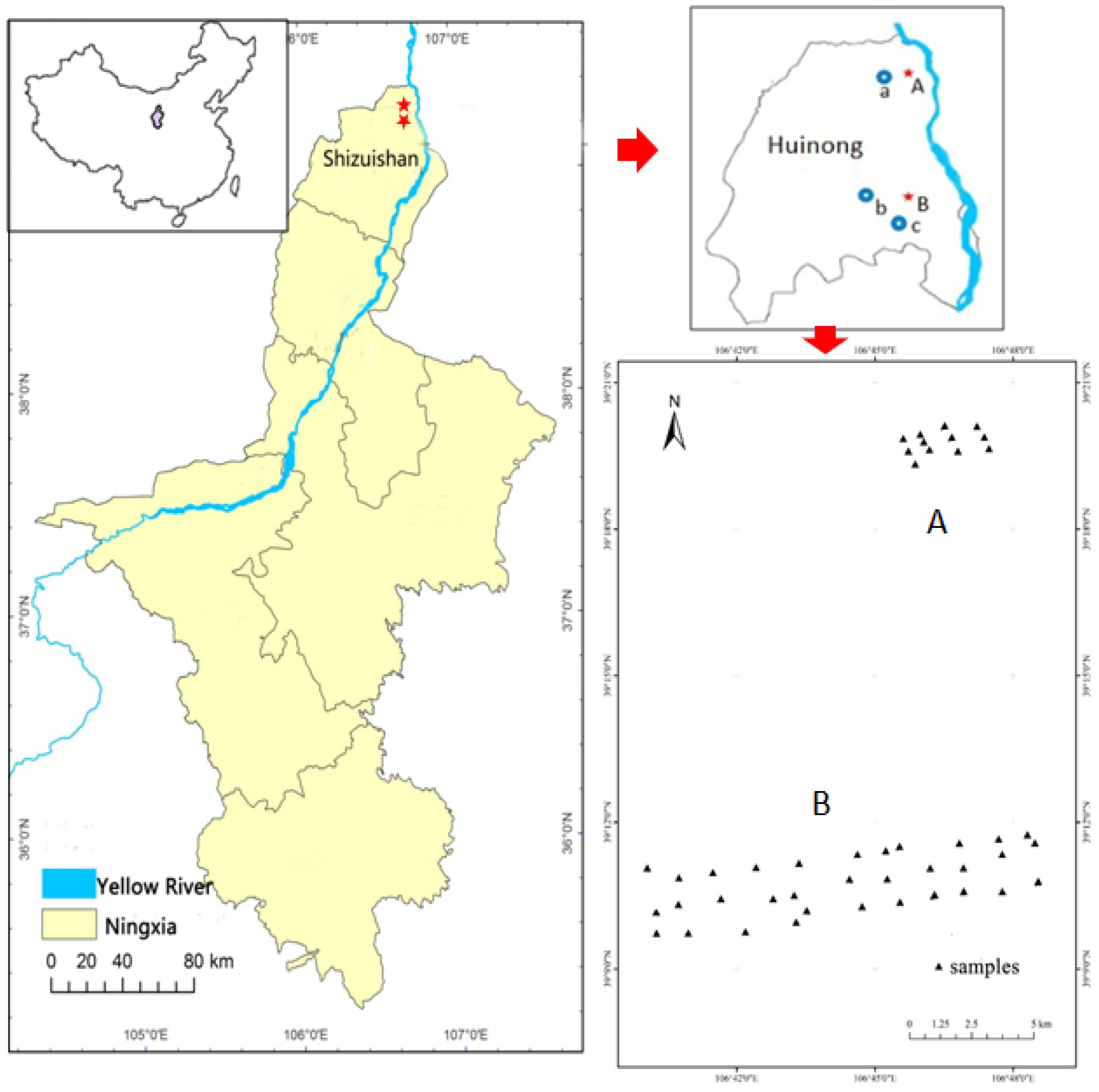
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Assessment of Soil Pollution
- (1)
- The single factor pollution index is expressed as follows:where Pi is the single factor pollution index of heavy metal i, and a larger Pi value indicates that the heavy metal pollution of soils is more serious. Ci is the measured value of the heavy metal i. Si is the second level standard of the Environmental Quality Standard for soils of heavy metal i.
- (2)
- The Nemerow synthetic pollution index is expressed as follows:where PN is the Nemerow synthetic pollution index in every sampling site, (Ci / Si)max is the corresponding maximum value in the single factor pollution index, is the corresponding weighted average value in the single factor pollution index, and is the weight coefficient of different heavy metals. The grade standard of the single factor pollution index and the Nemerow synthetic pollution index are showed in Table 2 [35,36].
2.4. Statistical Analysis
3. Results
3.1. Heavy Metal Concentration in Soils
3.2. Pollution Assessment of Heavy Metals
3.3. Relationship between Metal Levels in Soil and Plants
3.4. Heavy Metal Concentration in Plants
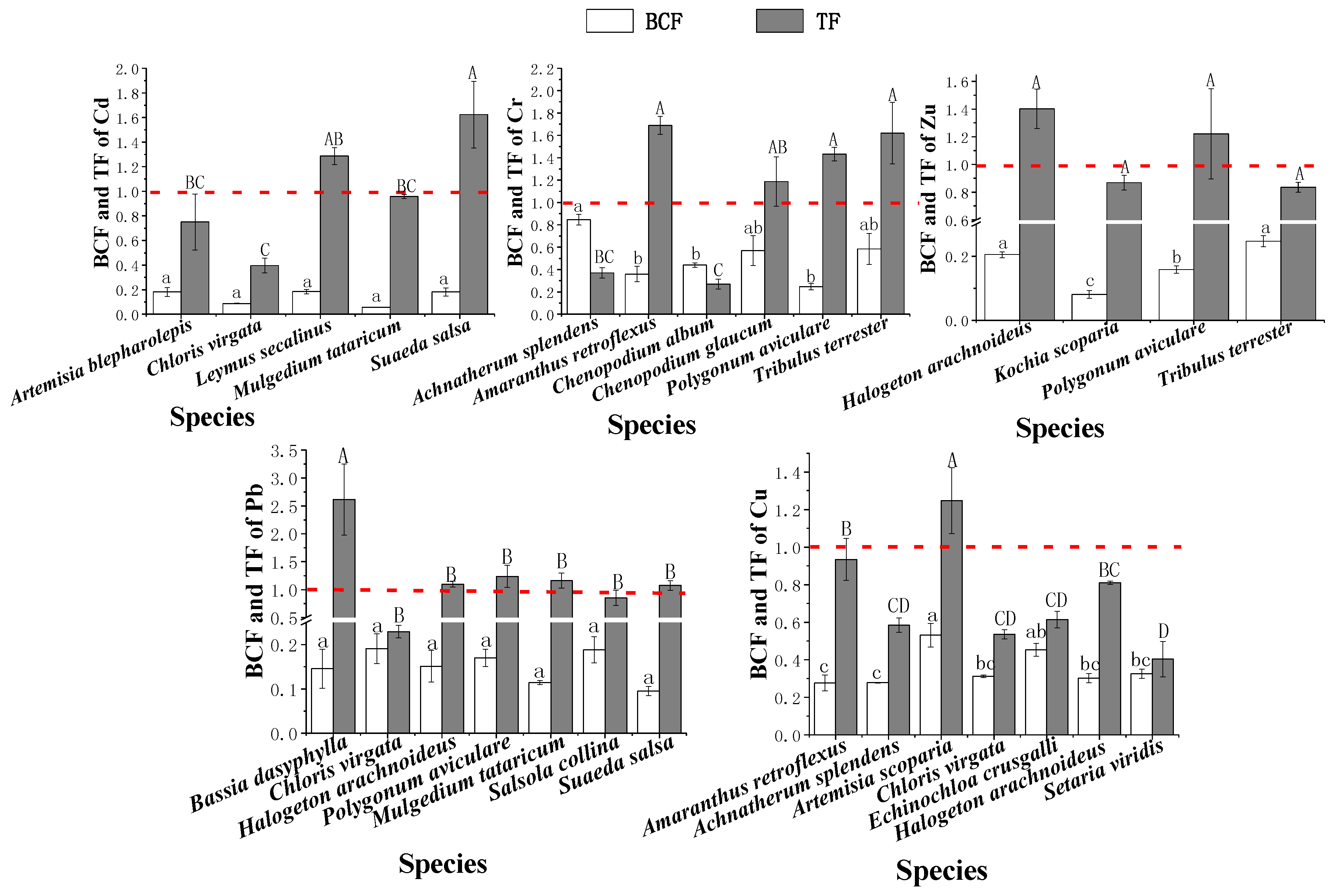
3.5. Bioconcentration and Translocation Factors in Native Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carr, R.; Zhang, C.; Moles, N.; Harder, M. Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyser and GIS. Environ. Geochem. Health 2008, 30, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chai, L.; Li, H.; Yang, Z.; Yang, W. A 10-year statistical analysis of heavy metals in river and sediment in Hengyang segment, Xiangjiang River basin, China. Sustainability 2018, 10, 1057. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Bai, Y.R.; Wang, J.Y. Distribution of urban soil heavy metal and pollution evaluation in different functional zones of Yinchuan city. Environ. Sci. 2016, 37, 710–716. [Google Scholar]
- Malayeri, B.E.; Chehregani, A.; Mohsenzadeh, F.; Kazemeini, F.; Asgari, M. Plants growing in a mining area: Screening for metal accumulator plants possibly useful for bioremediation. Toxicol. Environ. Chem. 2013, 95, 434–444. [Google Scholar] [CrossRef]
- Li, J.; He, M.; Han, W.; Gu, Y.F. Analysis and assessment on heavy metal sources in the coastal soils developed from alluvial deposits using multivariate statistical methods. J. Hazard. Mater. 2009, 164, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, P.; Yang, F.; Yang, F.; Sun, D.L.; Zhang, D.X.; Zhou, Y.K. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Qu, L.; Wang, T.; Luo, L.L.; Chen, H.; Dahlgren, R.A.; Zhang, M.H. Distribution and source analysis of heavy metal pollutants in sediments of a rapid developing urban river system. Chemosphere 2018, 207, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.H.; Wang, J.J.; Zhang, Z.Q. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, Q.W.; Sun, L.N.; Zeng, Q.; Liu, S.J.; Pan, J.; Liu, X.L. Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb-Zn mine site, China. Environ. Earth Sci. 2011, 64, 1317–1321. [Google Scholar] [CrossRef]
- Yang, Q.W.; Xu, Y.; Liu, S.J.; He, J.F.; Long, F.Y. Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol. Environ. Saf. 2011, 74, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, A.X.; Wang, J.H.; Ma, Z.H.; Pan, L.G.; Feng, X.Y. Effect of land use type on metals accumulation and risk assessment in soil in the peri-urban area of Beijing, China. Hum. Ecol. Risk Assess. 2015, 22, 265–278. [Google Scholar] [CrossRef]
- Sarkar, B. Heavy Metals in the Environment; CRC Express Inc.: Boca Raton, FL, USA, 1991; pp. 195–196. [Google Scholar]
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative. Environ. Health Perspect. 2014, 122, 594–600. [Google Scholar]
- Liang, L.C.; Liu, W.T.; Sun, Y.B.; Huo, X.H.; Li, S.; Zhou, Q.X. Phytoremediation of heavy metal-contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2016, 25, 269–281. [Google Scholar] [CrossRef]
- Padmapriya, S.; Murugan, N.; Ragavendran, C.; Thangabalu, R.; Natarajan, D. Phytoremediation potential of some agricultural plants on heavy metal contaminated mine waste soils, Salem District, Tamilnadu. Int. J. Phyth. 2016, 18, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Basharat, Z.; Novo, L.; Yasmin, A. Genome editing weds CRISPR: What is in it for phytoremediation? Plants 2018, 7, 51. [Google Scholar] [CrossRef]
- Cui, S.; Zhou, Q.; Chao, L. Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environ. Geol. 2007, 51, 1043–1048. [Google Scholar] [CrossRef]
- Chehregani, A.; Mohsenzade, F.; Vaezi, F. Introducing a new metal accumulator plant and the evaluation of its ability in removing heavy metals. Toxicol. Environ. Chem. 2009, 91, 1105–1114. [Google Scholar] [CrossRef]
- Lorestani, B.; Cheraghi, M.; Yousefi, N. Accumulation of Pb, Fe, Mn, Cu and Zn in plants and choice of hyperaccumulator plant in the industrial town of Vian, Iran. Arch. Biol. Sci. 2011, 63, 739–745. [Google Scholar] [CrossRef]
- Antosiewicz, D.M.; Escudĕ-Duran, C.; Wierzbowska, E.; Skłodowska, A. Indigenous plant species with the potential for the phytoremediation of arsenic and metals contaminated soil. Water Air Soil Pollut. 2008, 193, 197–210. [Google Scholar] [CrossRef]
- Bini, C. From Soil Contamination to Land Restoration; Novaence Pub: New York, NY, USA, 2010. [Google Scholar]
- Chen, B.D.; Zhu, Y.G.; Duan, J.; Xiao, X.Y.; Smith, S.E. Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ. Pollut. 2007, 147, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xie, X.M. Effect of heavy metals on substrate utilization pattern, biomass, and activity of microbial communities in a reclaimed mining wasteland of red soil area. Ecotoxicol. Environ. Saf. 2007, 66, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, W.; Müller, S.; Kanchanakool, N.; Zech, W. Urban soil contamination in Bangkok: Heavy metal and aluminium partitioning in topsoils. Geoderma 1998, 86, 211–228. [Google Scholar] [CrossRef]
- Wang, J.Z.; Peng, S.C.; Chen, T.H.; Zhang, L. Occurrence, source identification and ecological risk evaluation of metal elements in surface sediment: Toward a comprehensive understanding of heavy metal pollution in Chaohu Lake, Eastern China. Environ. Sci. Pollut. Res. 2016, 23, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, A.; Li, J.; Yan, L.D.; Li, J. Groundwater quality evaluation and health risk assessment in the Yinchuan Region, northwest China. Expo Health 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Rodríguez-Bocanegra, J.; Roca, N.; Febrero, A.; Bort, J. Assessment of heavy metal tolerance in two plant species growing in experimental disturbed polluted urban soil. J. Soils Sedim. 2018, 18, 2305–2317. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements a review of their distribution. Ecol. Phytochem. 1989, 1, 81–126. [Google Scholar]
- Gergel, S.E.; Turner, M.G.; Miller, J.R.; Melack, J.M.; Stanley, E.H. Landscape indicators of human impacts to riverine systems. Aquat. Sci. 2002, 64, 118–128. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Gemmell, R.P. Colonization of industrial wasteland. J. Appl. Ecol. 1978, 15, 1201–1213. [Google Scholar]
- Wang, T.; Wei, H.; Zhou, C.; Gu, Y.W.; Li, R.; Chen, H.C.; Ma, W.C. Estimating cadmium concentration in the edible part of Capsicum annuum, using hyperspectral models. Environ. Monit. Assess. 2017, 189, 548–561. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration. State Bureau of Technology Supervision. Environmental Quality Standard for Soils (GBl5618-1995). Available online: http://english.mep.gov.cn/standards_reports/standards/Soil/Quality_Standard3/200710/W020070313485587994018.pdf (accessed on 31 July 2018).
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Yang, L.Q.; Huang, B.; Hu, W.Y.; Chen, Y.; Mao, M.C.; Yao, L.P. The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China. Chemosphere 2013, 103, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65, 21–33. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 411, 579. [Google Scholar] [CrossRef] [PubMed]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ikea, M.; Nakazatob, H.; Saekib, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Rai, U.N.; Upadhyay, A.K.; Singh, N.K.; Dwivedia, S.; Tripathia, R.D. Seasonal applicability of horizontal sub-surface flow constructed wetland for trace elements and nutrient removal from urban wastes to conserve Ganga River water quality at Haridwar, India. Ecol. Eng. 2015, 81, 115–122. [Google Scholar] [CrossRef]
- China National Environment Monitoring Station. China Soil Background Valued; China Sciences Press: Beijing, China, 1990; pp. 339–386. (In Chinese) [Google Scholar]
- Alam, S.; Ahmad, I.; Din, Z.U.; Bangash, F.K. Variations of contaminants in the road side agricultural soil of Thana Malakand Agency. J. Chem. Soc. Pak. 2008, 30, 800–804. [Google Scholar]
- Gallardo, A.; Parama, R. Spatial variability of soil elements in two plant communities of NW Spain. Geoderma 2007, 139, 199–208. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.H.; Zeng, G.M.; Yuan, X.Z.; Li, X.D.; Liang, J.; Wang, X.Y.; Tang, X.J.; Bai, B. Spatial risk assessment and sources identification of heavy metals in surface sediments from Dongting Lake, Middle China. J. Geochem. Explor. 2013, 132, 75–83. [Google Scholar] [CrossRef]
- Yao, S.C.; Li, S.J. Sedimentary records of eutrophication for the last 100 years in Caohu Lake. Acta Sedimentol. Sin. 2004, 22, 343–347. [Google Scholar]
- Cui, X.; Sun, X.L.; Hu, P.J.; Yuan, C.; Luo, Y.M.; Wu, L.H.; Christie, P. Concentrations of heavy metals in suburban horticultural soils and their uptake by Artemisia selengensis. Pedosphere 2015, 25, 878–887. [Google Scholar] [CrossRef]
- Ent, A.V.D.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar]
- Dahmani-Muller, H.; Oort, F.V.; Gélie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, C.X.; Ni, X.L.; Li, J. Physiological ecological responses of Ulmus pumila “Jinye” and Ligustrum obtusifolium to lead stress. J. Chongqing Norm. Univ. 2018, 35, 127–134. (In Chinese) [Google Scholar]
- Yan, J.W.; Li, C.X.; Cui, Z.; Liu, Y. Effects of cadmium on growth, cadmium accumulation, and photosynthetic physiology of Buddleja alternifolia Maxim. seedlings under drought stress. Acta Ecol. Sin. 2017, 37, 7242–7250. (In Chinese) [Google Scholar]
| Family | Species | Important Value | Classification |
|---|---|---|---|
| Gramineae | Agropyron cristatum | 0.040 | Perennial |
| Achnatherum splendens | 0.034 | Perennial | |
| Chloris virgata | 0.060 | Annual herb | |
| Echinochloa crusgalli | 0.110 | Annual herb | |
| Leymus secalinus | 0.043 | Perennial | |
| Phragmites japonica | 0.101 | Perennial | |
| Setaria viridis | 0.071 | Annual herb | |
| Tragus racemosus | 0.026 | Annual herb | |
| Zygophyllaceae | Peganum harmala | 0.090 | Perennial |
| Tribulus terrester | 0.064 | Annual herb | |
| Asteraceae | Artemisia blepharolepis | 0.084 | Perennial |
| Artemisia scoparia | 0.087 | Perennial | |
| Artemisia verbenacea | 0.097 | Perennial | |
| Cirsium setosum | 0.036 | Perennial | |
| Mulgedium tataricum | 0.021 | Perennial | |
| Scorzonera divaricata | 0.036 | Perennial | |
| Sonchus oleraceus | 0.038 | Annual herb | |
| Xanthium sibiricum | 0.053 | Annual herb | |
| Chenopodiaceae | Bassia dasyphylla | 0.053 | Annual herb |
| Chenopodium album | 0.048 | Annual herb | |
| Chenopodium glaucum | 0.067 | Annual herb | |
| Chenopodium serotinum | 0.052 | Annual herb | |
| Halogeton arachnoideus | 0.080 | Annual herb | |
| Kochia scoparia | 0.050 | Annual herb | |
| Salsola collina | 0.093 | Annual herb | |
| Salicornia europaea | 0.028 | Annual herb | |
| Suaeda glauca | 0.060 | Annual herb | |
| Suaeda salsa | 0.067 | Annual herb | |
| Polygonaceae | Polygonum aviculare | 0.045 | Annual herb |
| Amaranthaceae | Amaranthus retroflexus | 0.037 | Annual herb |
| Portulacaceae | Portulaca oleracea | 0.020 | Annual herb |
| Convolvulaceae | Convolvulus arvensis | 0.054 | Perennial |
| Typhaceae | Typha orientalis | 0.038 | Perennial |
| Asclepiadaceae | Cynanchum chinense | 0.057 | Perennial |
| Leguminosae | Caragana stenophylla | 0.028 | Shrub |
| Medicago sativa. | 0.034 | Perennial | |
| Lespedeza bicolor | 0.041 | Shrub | |
| Glycyrrhiza uralensis | 0.036 | Perennial |
| Grade | Single Factor Index (Pi) | Pollution Grade | Nemerow Pollution Index (PN) | Pollution Grade |
|---|---|---|---|---|
| 1 | Pi ≤ 1 | No pollution | PN ≤ 0.7 | Clean |
| 2 | 1 < Pi ≤ 2 | Low pollution | 0.7 < PN ≤ 1 | Warn limit |
| 3 | 2 < Pi ≤ 3 | Moderate pollution | 1 < PN ≤ 2 | Slight pollution |
| 4 | Pi > 3 | High pollution | 2 < PN ≤ 3 | Moderate pollution |
| 5 | PN > 3 | Heavy pollution |
| Elements | Range | Mean ± SE | Coefficient of Variation (%) | Distribution Type | Soil Background Content in Ningxia | Over Standard Rate (1) (%) |
|---|---|---|---|---|---|---|
| Cd | 2.1~8.5 | 4.06 ± 0.20 | 33.61 | normal | 0.11 | 100.00 |
| Cr | 59.8~132.3 | 100.27 ± 1.92 | 12.86 | normal | 60.00 | 97.78 |
| As | 60.3~145.1 | 91.40 ± 3.08 | 22.62 | normal | 12.70 | 100.00 |
| Pb | 18.2~81.6 | 28.50 ± 1.62 | 38.03 | normal | 20.60 | 88.89 |
| Cu | 18.9~42.4 | 28.16 ± 0.695 | 16.56 | normal | 22.10 | 91.11 |
| Zn | 222.6~664.2 | 391.37 ± 16.08 | 27.56 | normal | 58.80 | 100.00 |
| Pi | PN | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cr | As | Pb | Cu | Zn | ||
| Max | 14.1 | 0.53 | 5.8 | 0.23 | 0.42 | 2.21 | 10.47 |
| Min | 3.5 | 0.24 | 2.4 | 0.05 | 0.19 | 0.74 | 2.67 |
| Mean | 6.77 | 0.40 | 3.66 | 0.08 | 0.28 | 1.30 | 5.07 |
| Pollution level | Heavy pollution | Unpolluted | Heavy pollution | Unpolluted | Unpolluted | Light pollution | Serious pollution |
| Species | r | ||||
|---|---|---|---|---|---|
| Cd | Cr | Pb | Cu | Zn | |
| Artemisia blepharolepis | 0.821 * | −0.562 | −0.998 * | −0.996 * | 0.421 |
| Amaranthus retroflexus | 0.027 | 0.727 * | −0.832 * | 0.759 * | −0.669 |
| Artemisia scoparia | 0.295 | 0.181 | −0.491 | 0.523 | 0.454 |
| Achnatherum splendens | −0.675 | 0.573 * | −0.825 * | 0.987 * | 0.029 |
| Bassia dasyphylla | 0.324 | −0.064 | 0.570 | 0.318 | 0.063 |
| Chenopodium album | −0.843 * | 0.960 * | 0.288 | −0.739 | −0.506 |
| Chenopodium glaucum | −0.370 | 0.776 * | 0.129 | −0.180 | −0.216 |
| Chloris virgata | 0.915 * | −0.730 | 0.911 * | 0.991 * | −0.736 * |
| Echinochloa crusgalli | −0.642 * | −0.282 | 0.383 | 0.823 * | 0.350 |
| Halogeton arachnoideus | 0.010 | −0.418 | 0.938 * | 0.883 * | 0.966 * |
| Kochia scoparia | −0.052 | −0.857 * | −0.632 | −0.234 | 0.680 * |
| Leymus secalinus | 0.901 * | −0.811 | −0.762 | −0.766 * | −0.663 |
| Mulgedium tataricum | 0.984 * | −0.840 * | 0.979 * | −0.723 | −0.251 |
| Polygonum aviculare | −0.804 * | 0.901 * | 0.998 * | 0.329 | 0.658 * |
| Peganum harmala | 0.173 | −0.668 | −0.246 | 0.072 | 0.027 |
| Phragmites japonica | 0.141 | −0.118 | −0.333 | 0.087 | −0.393 |
| Salsola collina | 0.297 | 0.411 | 0.884 * | 0.466 | 0.108 |
| Suaeda glauca | 0.050 | −0.691 | −0.342 | −0.101 | −0.578 |
| Suaeda salsa | 0.547 | 0.225 | 0.559 * | −0.250 | −0.698 * |
| Setaria viridis | −0.839 * | −0.818 * | −0.945 * | 0.886 * | −0.608 |
| Tribulus terrester | 0.007 | 0.857 * | 0.424 | −0.329 | 0.716 * |
| Elements | Species | Biomass(g∙plant−1) | Heavy Metals Concentration(mg∙kg−1) | |
|---|---|---|---|---|
| Shoot | Root | |||
| Cd | Artemisia blepharolepis | 46.10 | 0.45 ± 0.09 a | 0.60 ± 0.20 a |
| Chloris virgata | 2.49 | 0.19 ± 0.01 a | 0.50 ± 0.06 a | |
| Leymus secalinus | 23.75 | 0.91 ± 0.12 a | 0.70 ± 0.06 a | |
| Mulgedium tataricum | 5.81 | 0.29 ± 0.02 a | 0.30 ± 0.03 a | |
| Suaeda salsa | 16.48 | 0.75 ± 0.29 a | 0.45 ± 0.07 a | |
| Cr | Achnatherum splendens | 38.10 | 55.21 ± 3.85 a | 152.35 ± 8.60 b |
| Amaranthus retroflexus | 10.80 | 34.70 ± 7.14 a | 21.24 ± 4.33 a | |
| Chenopodium album | 14.30 | 22.41 ± 2.20 a | 69.28 ± 10.20 a | |
| Chenopodium glaucum | 11.28 | 60.74 ± 22.09 a | 59.46 ± 15.65 a | |
| Polygonum aviculare | 13.73 | 63.37 ± 46.86 a | 44.92 ± 23.47 a | |
| Tribulus terrester | 6.85 | 62.27 ± 18.26 a | 66.26 ± 31.13 a | |
| Pb | Bassia dasyphylla | 7.40 | 5.93 ± 1.47 a | 2.30 ± 0.49 a |
| Chloris virgata | 2.49 | 2.46 ± 0.14 a | 10.78 ± 0.08 c | |
| Halogeton arachnoideus | 16.80 | 5.86 ± 0.59 a | 5.32 ± 0.27 b | |
| Mulgedium tataricum | 5.81 | 3.43 ± 0.32 a | 2.98 ± 0.07 ab | |
| Polygonum aviculare | 13.73 | 4.68 ± 0.34 a | 3.93 ± 0.57 ab | |
| Salsola collina | 16.20 | 4.86 ± 0.78 a | 5.98 ± 0.91 b | |
| Suaeda salsa | 16.48 | 2.56 ± 0.34 a | 2.37 ± 0.26 ab | |
| Cu | Achnatherum splendens | 38.10 | 5.72 ± 0.25 a | 9.82 ± 0.22 a |
| Amaranthus retroflexus | 10.80 | 8.31 ± 1.18 ab | 10.01 ± 3.14 a | |
| Artemisia scoparia | 16.80 | 14.00 ± 3.27 c | 11.27 ± 0.70 a | |
| Chloris virgata | 2.49 | 5.78 ± 0.59 a | 10.70 ± 0.61 a | |
| Echinochloa crusgalli | 16.07 | 12.44 ± 0.80 bc | 20.55 ± 3.55 a | |
| Halogeton arachnoideus | 16.80 | 6.87 ± 0.76 ab | 8.47 ± 0.88 a | |
| Setaria viridis | 25.89 | 6.38 ± 1.73 ab | 15.67 ± 1.17 a | |
| Zn | Halogeton arachnoideus | 16.80 | 91.53 ± 26.59 b | 64.03 ± 17.51 a |
| Kochia scoparia | 13.60 | 20.46 ± 0.93 a | 23.23 ± 3.31 a | |
| Polygonum aviculare | 13.73 | 72.16 ± 3.20 ab | 60.02 ± 14.25 a | |
| Tribulus terrester | 6.85 | 93.02 ± 8.90 b | 110.71 ± 6.91 b | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, Y.; Li, C.; Ni, X.; Ma, W.; Wei, H. Assessing Soil Metal Levels in an Industrial Environment of Northwestern China and the Phytoremediation Potential of Its Native Plants. Sustainability 2018, 10, 2686. https://doi.org/10.3390/su10082686
Liu Y, Yang Y, Li C, Ni X, Ma W, Wei H. Assessing Soil Metal Levels in an Industrial Environment of Northwestern China and the Phytoremediation Potential of Its Native Plants. Sustainability. 2018; 10(8):2686. https://doi.org/10.3390/su10082686
Chicago/Turabian StyleLiu, Yuan, Yujing Yang, Changxiao Li, Xilu Ni, Wenchao Ma, and Hong Wei. 2018. "Assessing Soil Metal Levels in an Industrial Environment of Northwestern China and the Phytoremediation Potential of Its Native Plants" Sustainability 10, no. 8: 2686. https://doi.org/10.3390/su10082686
APA StyleLiu, Y., Yang, Y., Li, C., Ni, X., Ma, W., & Wei, H. (2018). Assessing Soil Metal Levels in an Industrial Environment of Northwestern China and the Phytoremediation Potential of Its Native Plants. Sustainability, 10(8), 2686. https://doi.org/10.3390/su10082686






