Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions
Abstract
1. Introduction
2. Methods
2.1. Study Regions
2.2. Ladybird Beetle Sampling
2.3. Urban Landscape Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Kaye, J.P.; Groffman, P.M.; Grimm, N.B.; Baker, L.A.; Pouyat, R.V. A distinct urban biogeochemistry? Trends Ecol. Evol. 2006, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Oke, T.R. City size and the urban heat island. Atmos. Environ. 1973, 7, 769–779. [Google Scholar] [CrossRef]
- Lin, B.B.; Egerer, M.H.; Liere, H.; Jha, S.; Bichier, P.; Philpott, S.M. Local- and landscape-scale land cover affects microclimate and water use in urban gardens. Sci. Total Environ. 2018, 610–611, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological light pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Hope, D.; Gries, C.; Zhu, W.; Fagan, W.F.; Redman, C.L.; Grimm, N.B.; Nelson, A.L.; Martin, C.; Kinzig, A. Socioeconomics drive urban plant diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 8788–8792. [Google Scholar] [CrossRef] [PubMed]
- Faeth, S.H.; Warren, P.S.; Shochat, E.; Marussich, W.A. Trophic dynamics in urban communities. Bioscience 2005, 55, 399–407. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M.L. Altered resources, disturbance, and heterogeneity: A framework for comparing urban and non-urban soils. Urban Ecosyst. 2009, 12, 23–44. [Google Scholar] [CrossRef]
- Faeth, S.H.; Saari, S.; Bang, C. Urban Biodiversity: Patterns, Processes and Implications for Conservation, eLS 2012; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Nilon, C.H.; Lepczyk, C.A.; Parker, T.S.; Warren, P.S.; Cilliers, S.S.; Goddard, M.A.; Hahs, A.K.; Herzog, C.; Katti, M.; et al. Hierarchical filters determine community assembly of urban species pools. Ecology 2016, 97, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Mackin-Rogalska, R.; Pinowski, J.; Solon, J.; Wojcik, Z. Changes in vegetation, avifauna, and small mammals in a suburban habitat. Pol. Ecol. Stud. 1988, 14, 293–330. [Google Scholar]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 5347, 450–453. [Google Scholar] [CrossRef]
- Seto, K.C.; Fragkias, M.; Gu, B. A Meta-Analysis of Global Urban Land Expansion. PLoS ONE 2011, 6, e23777. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Guneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.S. Incorporating critical elements of city distinctiveness into urban biodiversity conservation. Biodivers. Conserv. 2015, 24, 683–700. [Google Scholar] [CrossRef]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mougeot, L.J.A. Urban agriculture: Definition, presence, potentials and risks, and policy challenges. In Growing Cities, Growing Food: Urban Agriculture on the Policy Agenda. A Reader on Urban Agriculture; Bakker, N., Dubbeling, M., Gündel, S., Sabel-Koschella, U., De Zeeuw, H., Eds.; DSE/ETC: Feldafing, Germany, 2000; pp. 99–117. [Google Scholar]
- Lovell, S.T. Multifunctional urban agriculture for sustainable land use planning in the United States. Sustainability 2010, 2, 2499–2522. [Google Scholar] [CrossRef]
- Zezza, A.; Tasciotti, L. Urban agriculture, poverty, and food security: Empirical evidence from a sample of developing countries. Food Policy 2010, 35, 265–273. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban biodiversity: Patterns and mechanisms. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.F.; Hafernik, J.; Levy, J.; Moore, V.L.; Rickman, J.K. Insect conservation in an urban biodiversity hotspot: The San Francisco Bay Area. J. Insect Conserv. 2002, 6, 247–259. [Google Scholar] [CrossRef]
- Quistberg, R.D.; Bichier, P.; Philpott, S.M. Landscape and local correlates of bee abundance and species richness in urban gardens. Environ. Entomol. 2016, 45, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.B.; Gratton, C. Measuring natural pest suppression at different spatial scales affects the importance of local variables. Environ. Entomol. 2012, 41, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Egerer, M.H.; Arel, C.; Otoshi, M.D.; Quistberg, R.D.; Bichier, P.; Philpott, S.M. Urban arthropods respond variably to changes in landscape context and spatial scale. J. Urban Ecol. 2017, 3. [Google Scholar] [CrossRef]
- Egerer, M.H.; Bichier, P.; Philpott, S.M. Landscape and local habitat correlates of lady beetle abundance and species richness in urban agriculture. Ann. Entomol. Soc. Am. 2016, 110, 97–103. [Google Scholar] [CrossRef]
- Hall, D.M.; Camilo, G.R.; Tonietto, R.K.; Ollerton, J.; Ahrné, K.; Arduser, M.; Ascher, J.S.; Baldock, K.C.R.; Fowler, R.; Frankie, G.; et al. The city as a refuge for insect pollinators. Conserv. Biol. 2017, 31, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N.; et al. Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 2015, 282, 20142849. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H.; Minckley, R.L.; Kervin, L.J.; Roulston, T.H.; Williams, N.M. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 2006, 16, 632–644. [Google Scholar] [CrossRef]
- Lin, B.B.; Philpott, S.M.; Jha, S. The future of urban agriculture and biodiversity-ecosystem services: Challenges and next steps. Basic Appl. Ecol. 2015, 16, 189–201. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Harvey, C.T.; Gross, K.; Ives, A.R. Biodiversity and biocontrol: Emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 2003, 6, 857–865. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; O’Neil, R.J. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Evans, E.W. Lady beetles as predators of insects other than Hemiptera. Biol. Control 2009, 51, 255–267. [Google Scholar] [CrossRef]
- Oberholtzer, L.; Dimitri, C.; Pressman, A. Organic Agriculture in U.S. Urban Areas: Building Bridges between Organic Farms and Education. In Proceedings of the IFOAM Organic World Congress 2014, Istanbul, Turkey, 13–15 October 2014. [Google Scholar]
- US Census Bureau. American Community Survey 5-Year Estimates, County; US Census Bureau: Suitland, MD, USA, 2014.
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93. Available online: https://www.zin.ru/Animalia/Coleoptera/addpages/Andrey_Ukrainsky_Library/References_files/Gordon85a.htm (accessed on 1 March 2018).
- Discover Life Discover Life. Available online: www.discoverlife.org (accessed on 10 September 2015).
- Iowa State University Department of Entomology BugGuide. Available online: www.bugguide.net (accessed on 10 October 2014).
- Oksanen, J. Community Ecology Package. Available online: https://github.com/vegandevs/vegan (accessed on 15 April 2018).
- Hijmans, R.J. Geographic Data Analysis and Modeling; R Package Raster Version 2.6-7; R Foundation: Vienna, Austria, 2015; Available online: https://cran.r-project.org/web/packages/raster/ (accessed on 1 October 2017).
- R Development Core Team. R development core team. In R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2016; Volume 55, pp. 275–286. [Google Scholar]
- Xian, G.Z.; Homer, C.G.; Dewitz, J.; Fry, J.; Hossain, N.; Wickham, J. Change of impervious surface area between 2001 and 2006 in the conterminous United States. Photogramm. Eng. Remote Sens. 2011, 77, 758–762. [Google Scholar]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.L. A revision of the American Coccinellidae. J. N. Y. Entomol. Soc. 1899, 7, 71–169. [Google Scholar]
- Hodek, I.; Honěk, A. Ecology of Coccinellidae; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 54. [Google Scholar]
- Egerer, M.H.; Liere, H.; Bichier, P.; Philpott, S.M. Cityscape quality and resource manipulation affect natural enemy biodiversity in and fidelity to urban agroecosystems. Landsc. Ecol. 2018, 1–14. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Rango, J.; Fagan, W.F.; Faeth, S.H. Ground arthropod community structure in a heterogeneous urban environment. Landsc. Urban Plan. 2001, 52, 257–274. [Google Scholar] [CrossRef]
- Morse, C.C.; Huryn, A.D.; Cronan, C. Impervious surface area as a predictor of the effects of urbanization on stream insect communities in Maine, USA. Environ. Monit. Assess. 2003, 89, 95–127. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Insect extinction by urbanization: A long term study in Rome. Biol. Conserv. 2011, 144, 370–375. [Google Scholar] [CrossRef]
- Gaston, K.J.; Quinn, R.M.; Blackburn, T.M.; Eversham, B.C. Species-range size distributions in Britain. Ecography 1998, 21, 361–370. [Google Scholar] [CrossRef]
- Gibb, H.; Hochuli, D.F. Habitat fragmentation in an urban environment: Large and small fragments support different arthropod assemblages. Biol. Conserv. 2002, 106, 91–100. [Google Scholar] [CrossRef]
- Parris, K.M. Urban amphibian assemblages as metacommunities. J. Anim. Ecol. 2006, 75, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.E.; Ruttenberg, B.I.; Gaines, S.D.; Kinlan, B.P. The relationship between dispersal ability and geographic range size. Ecol. Lett. 2007, 10, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Lizée, M.H.; Manel, S.; Mauffrey, J.F.; Tatoni, T.; Deschamps-Cottin, M. Matrix configuration and patch isolation influences override the species-area relationship for urban butterfly communities. Landsc. Ecol. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Penone, C.; Kerbiriou, C.; Julien, J.F.; Julliard, R.; Machon, N.; Le Viol, I. Urbanisation effect on Orthoptera: Which scale matters? Insect Conserv. Divers. 2013, 6, 319–327. [Google Scholar] [CrossRef]
- Dupouey, J.L.; Dambrine, E.; Laffite, J.D.; Moares, C. Irreversible impact of past land use on forest soils and biodiversity. Ecology 2002, 83, 2978–2984. [Google Scholar] [CrossRef]
- Pautasso, M. Scale dependence of the correlation between human population presence and vertebrate and plant species richness. Ecol. Lett. 2007, 10, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Meineke, E.K.; Dunn, R.R.; Sexton, J.O.; Frank, S.D. Urban warming drives insect pest abundance on street trees. PLoS ONE 2013, 8, e59687. [Google Scholar] [CrossRef] [PubMed]
- Ernsting, G.; Isaaks, A. Ectotherms, temperature, and trade-offs: Size and number of eggs in a carabid beetle. Am. Nat. 2000, 155, 804–813. [Google Scholar] [PubMed]
- Helden, A.J.; Leather, S.R. Biodiversity on urban roundabouts-Hemiptera, management and the species-area relationship. Basic Appl. Ecol. 2004, 5, 367–377. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; Mccarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Richard, P.; Norton, B.A.; Thompson, K.; et al. A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 2008, 97. [Google Scholar] [CrossRef]
- Roy, H.E.; Brown, P.M.J.; Adriaens, T.; Berkvens, N.; Borges, I.; Clusella-Trullas, S.; Comont, R.F.; De Clercq, P.; Eschen, R.; Estoup, A.; et al. The harlequin ladybird, Harmonia axyridis: Global perspectives on invasion history and ecology. Biol. Invasions 2016, 18, 997–1044. [Google Scholar] [CrossRef]
- Camacho-Cervantes, M.; Ortega-Iturriaga, A.; Del-Val, E. From effective biocontrol agent to successful invader: The harlequin ladybird (Harmonia axyridis) as an example of good ideas that could go wrong. PeerJ 2017, 5, e3296. [Google Scholar] [CrossRef] [PubMed]
- Ingebritsen, S.E.; Jones, D.R. Santa Clara Valley, California; U.S. Geological Survey: Menlo Park, CA, USA, 1999; Volume 1182, pp. 15–22.
- UCCE. Master Gardener Program Home, Garden, Turf, and Landscape Pests. Available online: http://ipm.ucanr.edu/ (accessed on 1 March 2018).
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Jurjavcic, N.L.; O’Brien, J.M. Conservation’s disenfranchised urban poor. Bioscience 2002, 52, 601–606. [Google Scholar] [CrossRef]
- Martin, N.A. Dusky Lady Beetle—Nephus binaevatus. Available online: http://nzacfactsheets.landcareresearch.co.nz/Index.html (accessed on 1 March 2018).
- Cadotte, M.; Lovett-Doust, J. Ecological and taxonomic differences between rare and common plants of southwestern Ontario. Ecoscience 2002, 9, 397–406. [Google Scholar] [CrossRef]
- Deguines, N.; Julliard, R.; Flores, M.; Fontaine, C. Functional homogenization of flower visitor communities with urbanization. Ecol. Evol. 2016, 6, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Lovett-doust, J. Ecological patterns and biological invasions: Using regional species inventories in macroecology. Biol. Invasions 2006, 8, 809–821. [Google Scholar] [CrossRef]
- Ossola, A.; Nash, M.A.; Christie, F.J.; Hahs, A.K.; Livesley, S.J. Urban habitat complexity affects species richness but not environmental filtering of morphologically-diverse ants. PeerJ 2015, 3, e1356. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, A.C.J.; Landry, K.M.; Alyokhin, A. V Abundance of native and non-native lady beetles (Coleoptera: Coccinellidae) in different habitats in Maine. Ann. Entomol. Soc. Am. 2008, 101, 1078–1087. [Google Scholar] [CrossRef]
- Roy, H.E.; Brown, P.M.J.; Comont, R.F.; Poland, R.L.; Sloggett, J.J. Ladybirds; Pelagic Publishing Ltd.: Exeter, UK, 2013; Volume 10, ISBN 190780739X. [Google Scholar]
- Hagen, K.S. Biology and ecology of predaceous coccinellidae. Annu. Rev. Entomol. 1962, 7, 289–326. [Google Scholar] [CrossRef]
- Rees, M.; Roe, J.H.; Georges, A. Life in the suburbs: Behavior and survival of a freshwater turtle in response to drought and urbanization. Biol. Conserv. 2009, 142, 3172–3181. [Google Scholar] [CrossRef]
- Parris, K.M.; Hazell, D.L. Biotic effects of climate change in urban environments: The case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biol. Conserv. 2005, 124, 267–276. [Google Scholar] [CrossRef]
- US Climate Data California Climate Data. Available online: http://www.usclimatedata.com/climate.php (accessed on 1 November 2017).
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity is a vital element of landscape structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef]
- Rudd, H.; Vala, J.; Schaefer, V. Importance of backyard habitat in a comprehensive biodiversity conservation strategy: A connectivity analysis of urban green spaces. Restor. Ecol. 2002, 10, 368–375. [Google Scholar] [CrossRef]
- Levy, J.M.; Connor, E.F. Are gardens effective in butterfly conservation? A case study with the pipevine swallowtail, Battus philenor. J. Insect Conserv. 2004, 8, 323–330. [Google Scholar] [CrossRef]
- Burks, J.M.; Philpott, S.M. Local and landscape drivers of parasitoid abundance, richness, and composition in urban gardens. Environ. Entomol. 2017, 46, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Plascencia, M.; Philpott, S.M. Floral abundance, richness, and spatial distribution drive urban garden bee communities. Bull. Entomol. Res. 2017, 107, 658–667. [Google Scholar] [CrossRef] [PubMed]
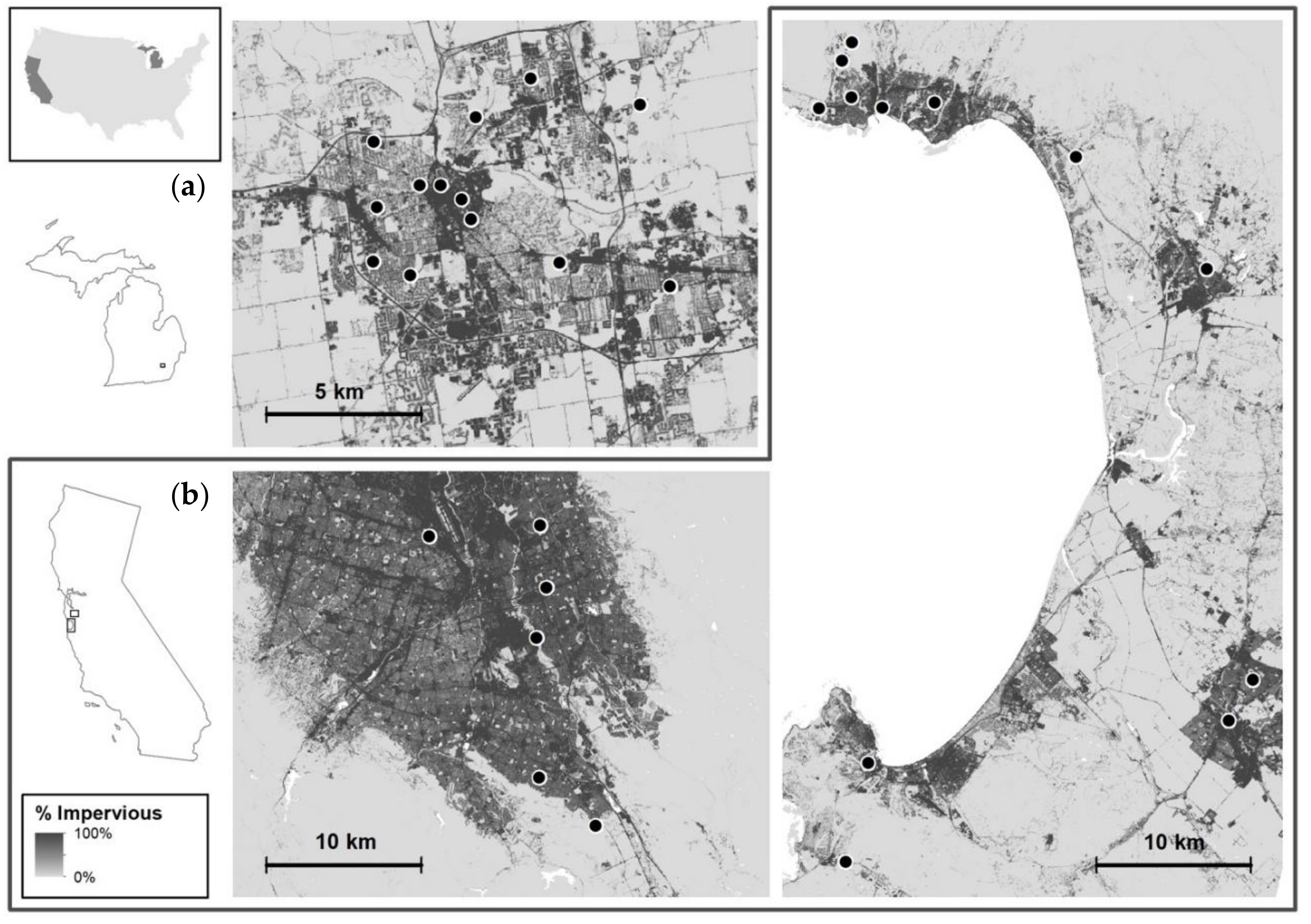
| Species | Region Observed | Feeds on | Ecological Function in Agroecosystems | Origin | Distribution in US |
|---|---|---|---|---|---|
| Adalia bipunctata | CA | aphids and mites | predator/pest control | native | West coast, Northeast, few Midwest records (historically most of US and Canada) |
| Coccinella californica | CA | mostly aphids | predator/pest control | native | West coast CA |
| Cycloneda polita | CA | mostly aphids | predator/pest control | native | West coast US and British Columbia |
| Hippodamia convergens | CA | mostly aphids | predator/pest control | native | Throughout US and western Canada |
| Hyperaspis quadrioculata | CA | aphids and scale insects | predator/pest control | native | Central to south CA |
| Nephus binaevatus | CA | aphids and scale insects | predator/pest control | non-native | Central to south CA |
| Psyllobora vigintimaculata | CA | fungus | fungus and mildew control | native | Throughout US and Canada |
| Scymnus cervicalis | CA | mites and scale insects | predator/pest and mite control | native | East US to south CA |
| Scymnus coniferarum | CA | mites and scale insects | predator/pest and mite control | native | CA and scattered west NA records |
| Scymnus marginicollis | CA | mites and scale insects | predator/pest and mite control | native | CA to British Columbia; scattered NA records |
| Scymnus nebulosus | CA | mites and scale insects | predator/pest and mite control | native | South CA to Canada |
| Stethorus punctum | CA | mites and scale insects | predator/pest and mite control | native | West coast US; Northeast, west to north Great Plains |
| Coleomegilla maculata | MI | mostly aphids | predator/pest control | native | East NA to southwest US |
| Cryptolaemus montrouzieri | MI | mites and scale insects | predator/pest and mite control | non-native | Throughout US |
| Hippodamia variegata | MI | mostly aphids | predator/pest control | native | Northeastern to middle US and Canada |
| Propylea quatuordecimpunctata | MI | mostly aphids | predator/pest control | non-native | Throughout NA (native to the Palaearctic) |
| Coccinella septempunctata | MI, CA | mostly aphids | predator/pest control | non-native | Throughout NA (native to the Old World) |
| Cycloneda sanguinea | MI, CA | mostly aphids | predator/pest control | native | West to south CA; NC and FL |
| Harmonia axyridis | MI, CA | mostly aphids | predator/pest control | non-native | Throughout US and southern Canada, except northern Rockies |
| Olla v-nigrum | MI, CA | mostly aphids | predator/pest control | native | Throughout US, except ME and Pacific Northwest |
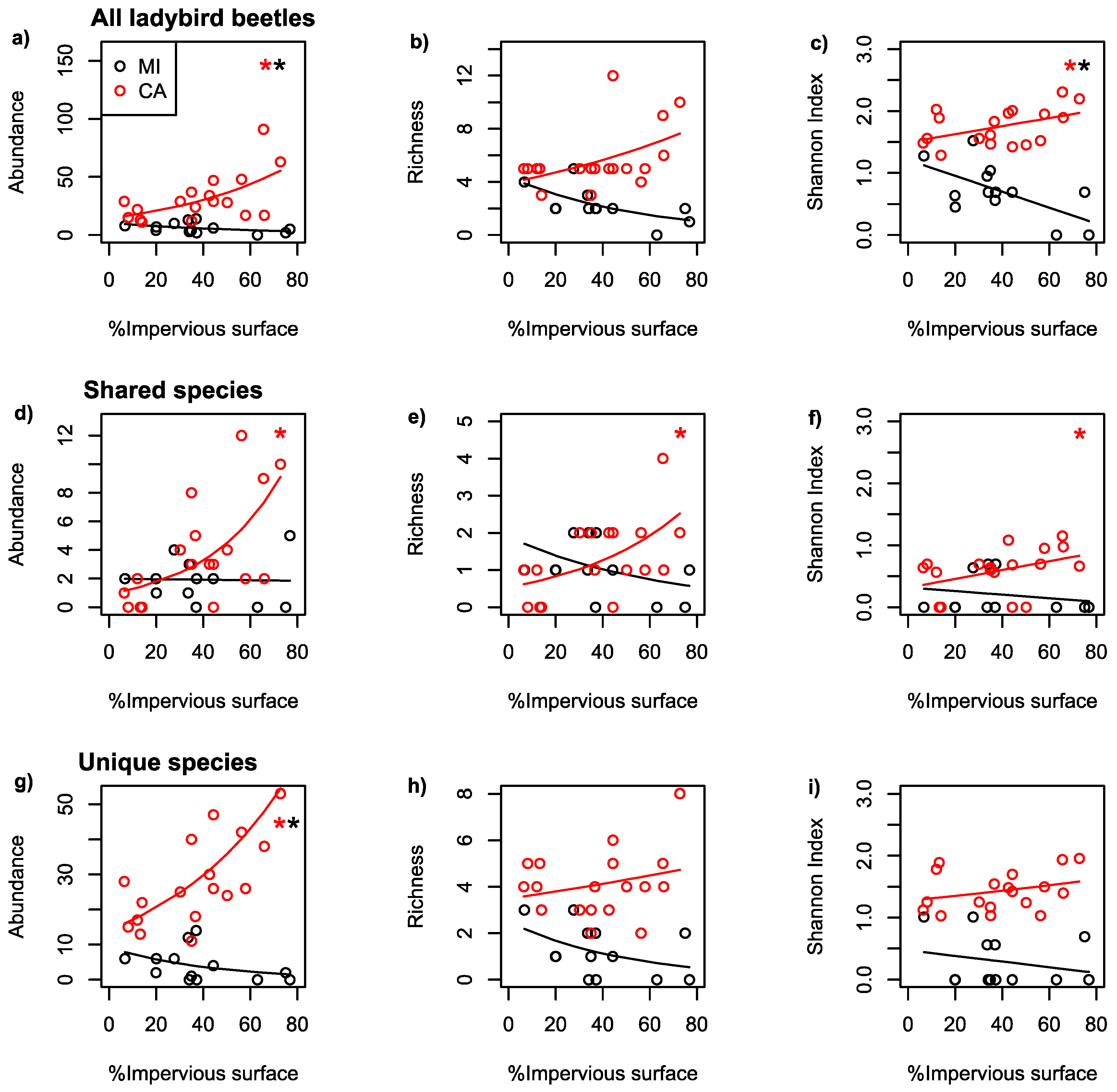
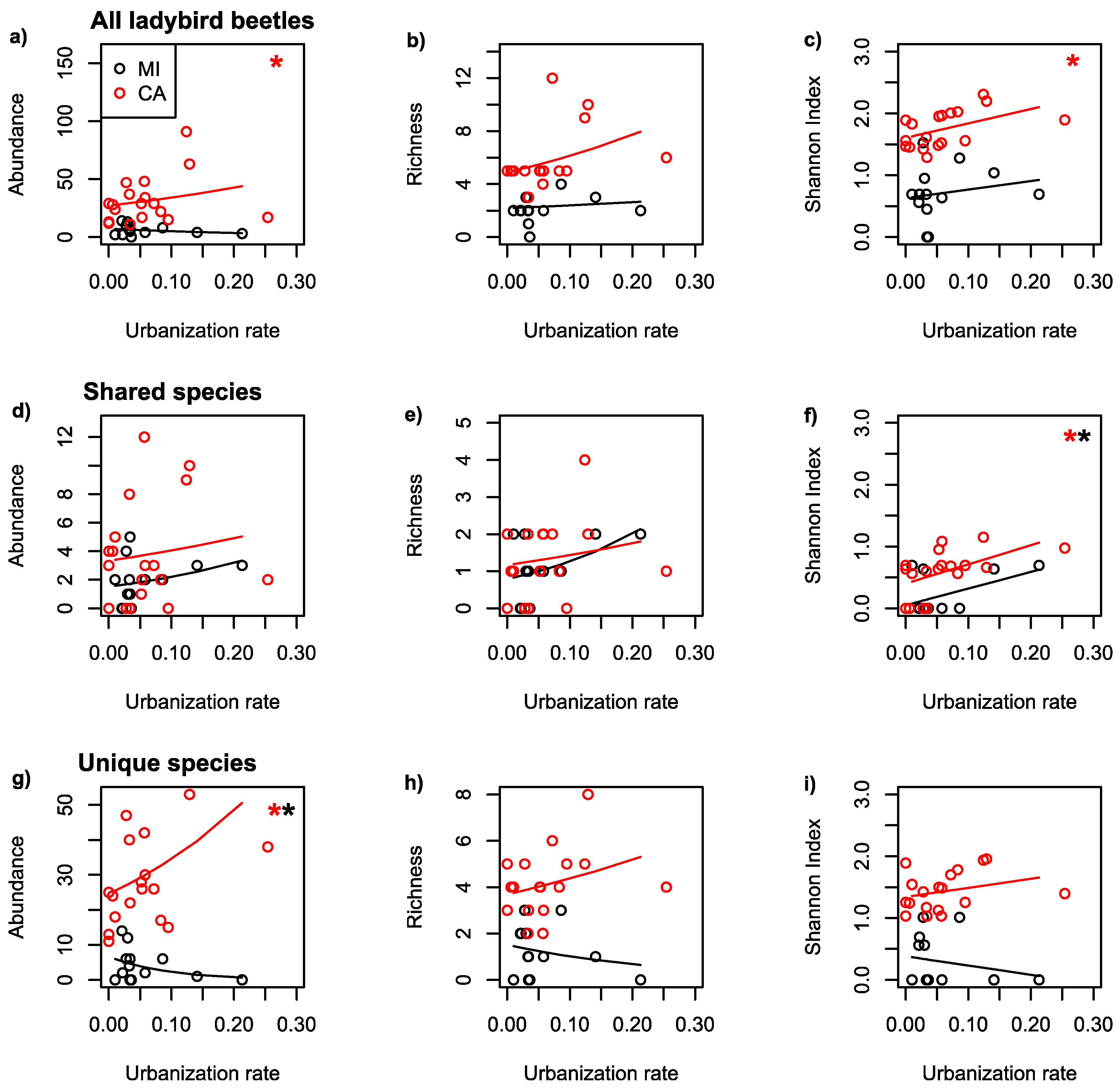
| Dataset | Region | Scale | Predicted | Predictor | Coefficient | p-Value |
|---|---|---|---|---|---|---|
| All | MI | 500 | AB | IS | −0.015 | 0.01 |
| All | CA | 100 | AB | IS | 0.019 | <0.001 |
| All | MI | 500 | RI | IS | −0.018 | 0.08 |
| All | CA | 100 | RI | IS | 0.009 | 0.06 |
| All | MI | 500 | SH | IS | −0.013 | 0.02 |
| All | CA | 100 | SH | IS | 0.006 | 0.05 |
| Shared | MI | 500 | AB | IS | −0.001 | 0.92 |
| Shared | CA | 100 | AB | IS | 0.031 | <0.001 |
| Shared | MI | 500 | RI | IS | −0.016 | 0.29 |
| Shared | CA | 100 | RI | IS | 0.021 | 0.05 |
| Shared | MI | 500 | SH | IS | −0.003 | 0.54 |
| Shared | CA | 100 | SH | IS | 0.007 | 0.09 |
| Unique | MI | 500 | AB | IS | −0.023 | 0.004 |
| Unique | CA | 100 | AB | IS | 0.018 | <0.001 |
| Unique | MI | 500 | RI | IS | −0.020 | 0.16 |
| Unique | CA | 100 | RI | IS | 0.004 | 0.46 |
| Unique | MI | 500 | SH | IS | −0.005 | 0.44 |
| Unique | CA | 100 | SH | IS | 0.003 | 0.76 |
| All | MI | 500 | AB | UR | −3.524 | 0.15 |
| All | CA | 500 | AB | UR | 2.231 | <0.001 |
| All | MI | 500 | RI | UR | 0.932 | 0.77 |
| All | CA | 500 | RI | UR | 2.292 | 0.11 |
| All | MI | 500 | SH | UR | 1.372 | 0.55 |
| All | CA | 500 | SH | UR | 2.331 | 0.04 |
| Shared | MI | 500 | AB | UR | 3.710 | 0.22 |
| Shared | CA | 500 | AB | UR | 1.925 | 0.29 |
| Shared | MI | 500 | RI | UR | 4.665 | 0.23 |
| Shared | CA | 500 | RI | UR | 2.024 | 0.50 |
| Shared | MI | 500 | SH | UR | 2.698 | 0.09 |
| Shared | CA | 500 | SH | UR | 3.110 | 0.02 |
| Unique | MI | 500 | AB | UR | −10.88 | 0.01 |
| Unique | CA | 500 | AB | UR | 3.376 | <0.001 |
| Unique | MI | 500 | RI | UR | −4.020 | 0.47 |
| Unique | CA | 500 | RI | UR | 1.705 | 0.33 |
| Unique | MI | 500 | SH | UR | −1.506 | 0.48 |
| Unique | CA | 500 | SH | UR | 1.473 | 0.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egerer, M.; Li, K.; Ong, T.W.Y. Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions. Sustainability 2018, 10, 1829. https://doi.org/10.3390/su10061829
Egerer M, Li K, Ong TWY. Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions. Sustainability. 2018; 10(6):1829. https://doi.org/10.3390/su10061829
Chicago/Turabian StyleEgerer, Monika, Kevin Li, and Theresa Wei Ying Ong. 2018. "Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions" Sustainability 10, no. 6: 1829. https://doi.org/10.3390/su10061829
APA StyleEgerer, M., Li, K., & Ong, T. W. Y. (2018). Context Matters: Contrasting Ladybird Beetle Responses to Urban Environments across Two US Regions. Sustainability, 10(6), 1829. https://doi.org/10.3390/su10061829





