Abstract
In this study, the effect of Al2O3-water and ZnO-water nanofluids, with and without ethylene glycol (EG), on the efficiency of a flat plate solar collector was investigated. Two systems were set up and the nanofluids with and without EG were examined at the same time. The volume fraction of the nanoparticles and EG were 0.25% and 25%, respectively. The study was conducted on three mass flow rates: 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s. ASHRAE Standard 93-2010 was used to calculate the efficiency. The efficiency of the system was compared to distilled water (base fluid). The results also showed that an increase in the mass flow rate and use of the EG increased efficiency. Furthermore, in comparison with the base fluid, the maximum increase in efficiency (15.13%) was observed at 0.09 kg/s when using a Al2O3-water/EG nanofluid.
1. Introduction
To date, fossil fuels have been used to supply the majority of our energy demand, as these are much cheaper and more feasible than alternative energy sources. On the other hand, their negative impact on the environment has been a major concern, which has led scientists to search for alternative energy sources, such as solar energy. According to recent findings, it has been found that solar energy is superior to fossil fuels because solar energy is cleaner and does not cause any environmental pollution. Therefore, solar energy has become a widely used energy source for heating water, especially in countries with hot climates. Although other systems are available, stationary (non-concentrating) and concentrating systems are the main types of water-heating systems in use.
The stationary collector uses the same area to intercept and absorb solar radiation, whereas the reflecting surfaces of the concentrating solar collector generally have a concave shape for intercepting and focusing the solar radiation into a smaller area and, therefore, increases the radiation flux [1].
Recently, many researchers have examined the efficiency of solar collectors. The studies have usually been classified into six topics: the new heat transfer fluids (nanofluid) in solar thermal collectors, novel materials, integrated solar thermal collectors, heat pipe solar collectors, novel geometries, and hybrid thermal collectors [2].
The efficiency of the flat plate solar collector is dependent on many factors, including the position of the sun, weather conditions, the orientation and the tilt angle of the panel, the material composition and mounting structure of the panel, the mass flow rate, and the type of working fluid.
Innovative heat transfer fluids have been suggested as a method to increase the efficiency of energy systems due to their thermal conductivity, which is dependent upon the mixing of solid nanoparticles with conventional heat transfer fluids, such as water, ethylene glycol, and oil. These mixtures are called nanofluids, a term developed by Choi [3].
Yousefi et al. studied Al2O3 nanofluid (with and without surfactant) as a working fluid in a flat plate solar collector. Triton X-100 was used as a surfactant, and Al2O3 nanofluids were tested in terms of their nanoparticle concentration at two different weight fractions: 0.2% and 0.4%. During the test periods, the mass flow rate was stabilized at three different rates: 1 L/min, 2 L/min, and 3 L/min, in order. The ASHRAE 93-2010 [4] standard was used to calculate the efficiency. The results demonstrated that the efficiency of the solar collector compared to water as a working fluid was enhanced by 28.3% when using a 0.2 wt % Al2O3 nanofluid. The maximum increase in efficiency was 15.63% when using the surfactant [5].
Babu et al. studied the efficiency of a flat plate solar collector with and without ZnO/water nanofluid. The nanofluid was prepared with a 0.1% weight fraction and mass flow rates were chosen as 0.0084 kg/s, 0.0167 kg/s, and 0.025 kg/s. Results indicated that the efficiency of the flat plate solar collector increased by 7.03% at 0.0084 kg/s, 6.59% at 0.0167 kg/s, and 4.13% at 0.025 kg/s when using the ZnO-water nanofluid compared to water [6].
Bhatti et al. have numerically investigated entropy generation for non-Newtonian Eyring-Powell nanofluids. The results indicated that the nanofluid concentration profile affected the Brownian motion parameter and Lewis number inversely; a decrease in temperature profile caused an increase in the Prandtl number [7].
Hayat et al. investigated the effect of different thicknesses of Riga plates on the boundary layer flow. Heat transfer properties are studied with convective boundary conditions and heat generation/absorption. The impacts of velocity, temperature and nanoparticles volume fraction distributions are shown graphically. The results showed that the increase of the Hartman number occurred with the decrease of velocity distribution [8].
The experimental results of the previous studies were represented as graphs and equations which showed the collector efficiency versus a reduced temperature parameter, (Ti − Ta)/GT [9,10,11,12,13].
To date, no study has examined the performance of a flat plate solar collector using nanofluids with EG and without EG simultaneously. The aim of this study is to investigate the efficiency of commonly used solar collectors under hot climate conditions with nanofluids. Furthermore, the efficiency was investigated by using ethylene glycol (EG) with nanofluids under hot climate conditions.
2. Methodology
Two systems were designed and constructed. Initially, only base fluid (distilled water) measurements were made to calibrate the data loggers, rotameter, and to detect the fluid leaks. The fluid was loaded into the system by a water pump and air was removed by vacuum breaker.
Inlet and outlet temperature of the working fluid, temperature of the plate, and ambient temperature were measured by a data logger in Nicosia (PASCO, model number: PS-2002). During the experiments, all temperature values fell in the wide range (−35 °C to +135 °C) of the sensors, which could be measured in Kelvin [14]. Solar radiation was measured by a pyranometer (Kipp Zonen, model number: SMP II).
The results of the tests with the base fluid were found to be very similar with previous studies [5,12,15]. The tests were performed between 9 a.m. and 3 p.m. during November. All represented data were recorded every 30 min. Each test was carried out within two days and the best experimental data was picked. The experimental results contained the performance of the solar collector using water, Al2O3-water, ZnO-water with EG and without EG. The concentration of nanoparticles and EG were chosen as 0.25% and 25%, respectively. The solar collector was examined for different mass flow rates of 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s for each type of working fluid. The tests were performed with EG and without EG at the same time for each nanofluid, and the tests were also carried out for base fluid and water/EG mixture. One of the systems used was for the nanofluid with EG and the other one was used for the nanofluid without EG.
2.1. Experimental Setup
In this study, two systems with same technical specifications were designed and constructed. The schematic and real pictures of the experimental setup are shown in Figure 1. The specifications of the experimental setups are demonstrated in Table 1.
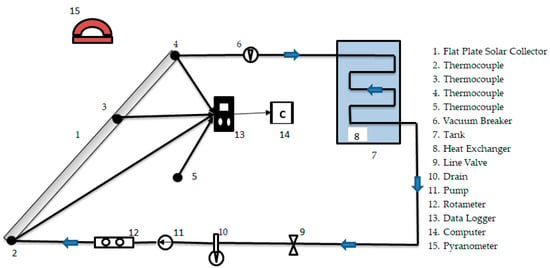
Figure 1.
The schematic of experimental setup.

Table 1.
Specifications of the solar collector.
The systems were installed at Cyprus International University (Nicosia, Cyprus) located on 35.17° latitude and 33.36° longitude in the northern hemisphere.
The absorber plate and tubes of the collectors (1 in Figure 1) were manufactured from galvanized sheet and the tilt angle (β) of each collector was 45°. The specifications of the collectors are shown in Table 1.
As shown in 7 in Figure 1, the system had a tank to soak up the heat energy from the collector cycle. The capacity of the tanks were 60 L and the tanks were insulated by foam with a thickness of 2 cm. Additionally, the tanks had copper heat exchangers (8 in Figure 1). Experimental setups had a circulating pump (Wita U65) and each pump (11 in Figure 1) had a maximum flow rate with 1.33 kg/s [16]. In addition to this, the systems had a rotameter (12 in Figure 1) in front of the pump to fix the flow rate value.
2.2. Nanofluid Preparation
In this study, two different nanoparticles, with and without ethylene glycol (EG) were studied. Aluminum oxide (Al2O3) and zinc oxide (ZnO) were chosen as nanoparticles with a 0.25% volume fraction. Al2O3 (CAS number: 1344-28-1), ZnO (CAS number: 1314-13-2), and EG (CAS number: 107-21-1) were provided from Merck Millipore. The nanofluids prepared with EG were determined at a 25:75 ratio of EG:water (distilled water). The densities of ZnO and Al2O3 were 3.94 g/cm3 [17] and 5.61 g/cm3 [18], respectively. The size of nanoparticles were 0.196 μm and 68.12 μm for ZnO and Al2O3, respectively. According to Wang and Mujumdar [19], assessment with several data showed that the improvement in the thermal conductivity of nanofluids improved with a reduction in particle sizes. This study can be improved by using different nanoparticle size.
The nanofluids were prepared by using a homogenizer (DAIHAN Scientific Co. Ltd., Korea, model number: HG-15D) and were weighed using an electronic balance (Sartorius, Göttingen, Germany, model number: KD-KC).
One liter (1 L) of each type of nanofluid was prepared. Nanoparticles with water or with water-EG mixture were stirred for 30 min with the homogenizer.
The volume concentration at the dispersed fluid is represented by the following equation [20]:
where Φv, Φm, ρn, and ρf, are the volume concentration, mass concentration, density of the nanoparticles, and the density of the fluid, respectively.
The heat capacity of the nanofluid is calculated as follows [21]:
where cp,nf, cp,n, and cp,f are heat capacity of the nanofluid, the nanoparticles, and the fluid, respectively.
2.3. Energy Analysis
ASHRAE Standard 93-2010 was used to evaluate the thermal performance of the flat plate solar collectors. Firstly, the effect of solar rays hitting the collector surface was investigated by using solar angles and the area of the collector. In this calculation, refracted solar radiation was excluded and total absorbed radiation was calculated; collector losses were also considered.
Additionally, heat removal factor (FR) was determined by dividing the actual output with fluid inlet temperature. Then, FR value with total absorbed energy were used to find the system efficiency.
When the inlet and outlet fluid temperatures as well as the flow rate of the working fluid were measured, the useful energy could be calculated using the formula below [4]:
The instant collector efficiency depends on the relationship among useful energy, total incident radiation, and the area of the collector surface [4]:
where , cp,nf, To, Ti, A, and GT are the mass flow rate, heat capacity of the nanofluid, outlet fluid temperature, inlet fluid temperature, collector area, and solar radiation, respectively.
The equation of useful energy can also be shown in terms of the energy absorbed and the energy lost, as given by Equation (5):
where FR, (τα), and UL are the heat removal factor, absorptance-transmittance product, and overall loss coefficient of the solar collector, respectively. To calculate the thermal efficiency, as shown in Equations (5) and (6), we divided by the energy input [4]:
2.4. Climate Conditions
This study was carried out in Cyprus, which has the highest solar irradiation in Europe, comprising more than 300 days of sunny weather. In Cyprus, the annual irradiation is 2.000 kWh/m2 on a tilted surface of 27.58°, which is much higher than the sunniest area of the world’s largest market, Germany [22]. The minimum mean temperature is 4 °C during the winter, whereas the maximum mean temperature is 30 °C during the summer.
Figure 2 represents an average recorded data of solar radiation (W/m2) and ambient temperature (°C) between 9 a.m. and 3 p.m. in November.
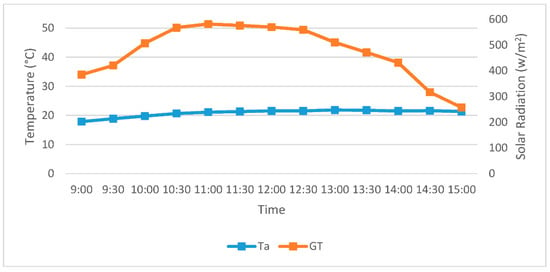
Figure 2.
Average solar radiation and ambient temperature between 9 a.m. and 3 p.m. in November.
Table 2 shows the meteorology values of Cyprus per month [23]. As shown in Table 2, the highest global irradiance is 8.12 kWh/m2/day in June. On the other hand, the highest ambient temperature is 29.4 °C in August.

Table 2.
Meteorology values of Cyprus.
3. Results and Discussion
3.1. Efficiency vs. (Ti − Ta)/GT for Nanofluid without Ethylene Glycol
Figure 3 shows the efficiency (η) vs. (Ti − Ta)/GT (reduced temperature parameter) for water, Al2O3, and ZnO at 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s, respectively. This study was carried out to compare the efficiency of Al2O3-water and ZnO-water nanofluids with the efficiency of water. For three working fluids, the efficiency of the solar collector is demonstrated in Figure 3: base fluid, Al2O3-water, and ZnO-water nanofluids. The efficiency was calculated to be similar for all working fluids. The Al2O3-water nanofluid was more efficient than the ZnO-water nanofluid, which was more efficient than the base fluid. This was predominantly caused by the thermal conductivity of the nanoparticles, which were 2.3 W/mK [24] and 3.89 W/mK [18] for ZnO and Al2O3, respectively. According to the findings, higher thermal conductivity leads to higher efficiency.
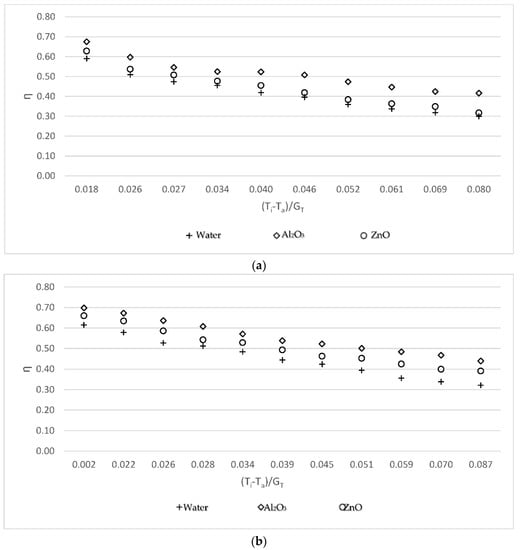
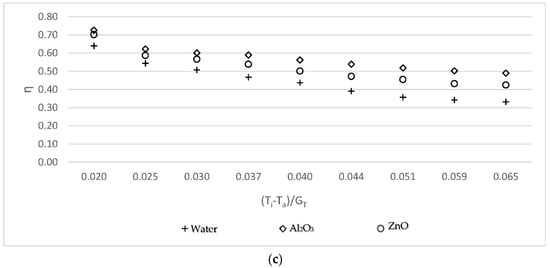
Figure 3.
Variations of the collector efficiency versus the reduced temperature parameter (a) for the 0.05 kg/s mass flow rate; (b) for the 0.07 kg/s mass flow rate; and (c) for the 0.09 kg/s mass flow rate.
Using water as the working fluid, the maximum efficiencies of the flat plate solar collector obtained at 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s mass flow rates were 59.21%, 62.17%, and 64.36%, respectively. The minimum efficiencies of the flat plate solar collector were 28.12%, 31.55%, and 32.05%, respectively. As shown in Figure 3, the efficiency increased by 8.02%, 9.55%, and 11.15% for three different mass flow rates when using Al2O3; in comparison, the efficiency increased by 4.17%, 5.29%, and 5.81% when using ZnO.
3.2. Efficiency vs. (Ti − Ta)/GT for Nanofluid with Ethylene Glycol
Figure 4 shows the efficiency of the solar collector for three working fluids: base fluid/EG, Al2O3-water/EG, and ZnO-water/EG nanofluids. The efficiency was calculated to be similar for all working fluids. The Al2O3-water/EG nanofluid was more efficient than ZnO-water/EG nanofluid, which was more efficient than the base fluid/EG. According the results, the ethylene glycol had a greater effect on the efficiency than the nanofluid without ethylene glycol in terms of obtaining higher values.
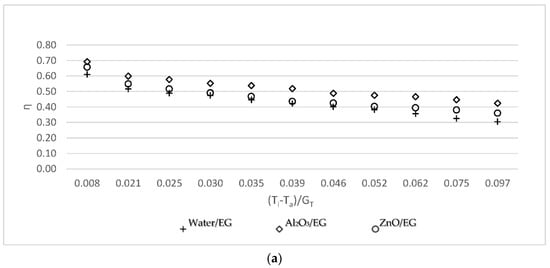
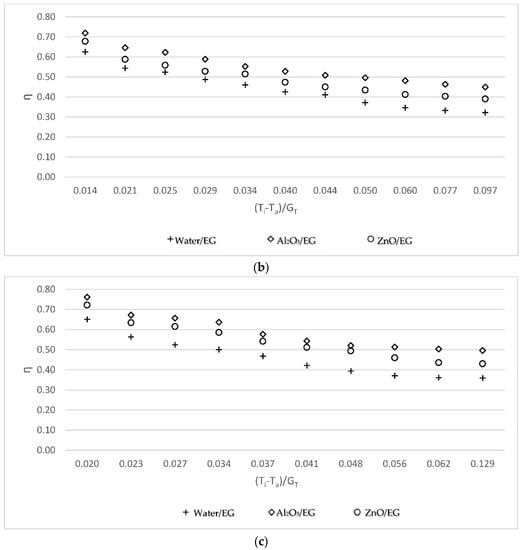
Figure 4.
Variations of collector efficiency using working fluid/EG versus the reduced temperature parameter (a) for the 0.05 kg/s mass flow rate; (b) for the 0.07 kg/s mass flow rate; and (c) for the 0.09 kg/s mass flow rate.
When making a comparison between Figure 3 and Figure 4, the effect of EG can be seen. To observe this effect, two experimental setups were constructed and the tests were performed simultaneously with EG and without EG.
In comparison with water/EG, the efficiency increase at these mass flow rates was 8.56%, 10.28%, and 13.34%, respectively when using Al2O3-water/EG, and the efficiency enhanced by 4.41%, 5.68%, and 6.86%, respectively, when using ZnO-water/EG, as can be seen in Figure 4.
For the nanofluid with EG, the removed energy parameter, FRUL, was lower while the absorbed energy parameter, FR(τα), was greater than the base fluid without EG. Therefore, the efficiency of the collector using the nanofluid with EG was greater than the base fluid with or without EG [9].
When water with EG was compared the water without EG at the mass flow rates of 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s, the efficiency increased by 1.31%, 1.63%, and 1.79%, respectively. The efficiency increase in Al2O3-water/EG at these mass flow rates was 1.85%, 2.36%, and 3.98%, respectively, when compared to Al2O3-water nanofluid. Additionally, the efficiency of ZnO with EG at these mass flow rates increased by 1.55%, 2.02%, and 2.84%, respectively, compared to ZnO tested without EG.
3.3. Effect of the Mass Flow Rate
The typical example of the recorded data is represented in Figure 5 for all nanofluids, showing the impact of the mass flow rates. The similar results were observed in three different mass flow rates. The efficiency of the solar collector increased by enhancing the mass flow rates. Thus, these results demonstrate that increasing the Reynolds number enhances the efficiency [5].
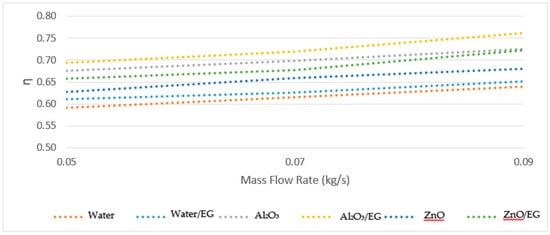
Figure 5.
Experimental curve of the nanofluids.
When all the working fluids were compared in terms of the three different mass flow rates, the maximum efficiency increase was 10.41% when using Al2O3-water/EG. On the other hand, the minimum efficiency increase was 5.15% when using water only.
3.4. Comparison with Previous Studies
Yousefi et al. studied the flat plate solar collector by using 0.2 wt % Al2O3 as working fluid; they found that the efficiency increase was 28.30% at 3 L/min mass flow rate. According to their results, this increase was a result of the nanofluid having a higher absorbed energy parameter, FR(τα), than the water [5].
Babu et al. studied nanofluid with ZnO and they observed a 4.13% efficiency increase at 0.025 kg/s mass flow rate. They concluded that this increase was dependent on the amount of heat energy absorbed by flat plate solar collector, high outlet temperature of the working fluid, and the weight concentration of the nanoparticles [6].
The results found in this study on the influence of ZnO was nearly similar with the results found in this study. However, there was a difference between the results of Al2O3 and the results of this study. This difference was based on using different amounts of Al2O3 and the application of different mass flow rates.
4. Conclusions
This study was conducted to determine the effect of nanofluids on the performance of flat plate solar collectors. The tests were performed at three mass flow rates of 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s for Al2O3-water and ZnO-water nanofluids with a 0.25% volume fraction. Moreover, the tests were performed using nanofluids with a 25:75 ratio of EG:water mixture and without EG. The ASHRAE 93-2010 standard was used for calculating the efficiencies. The highlights of the study are summarized as follows:
- ✔
- Efficiency of the solar collector was enhanced by increasing the mass flow rate.
- ✔
- Nanofluid enhanced the acquired energy parameter of the solar collector.
- ✔
- The nanofluid with EG increased the efficiency compared to the nanofluid without EG.
- ✔
- When EG was added to the base fluid, the efficiency increases of the system were noted as 2.21%, 2.62%, and 2.78%, at three mass flow rates of 0.05 kg/s, 0.07 kg/s, and 0.09 kg/s, respectively.
- ✔
- The efficiency increase in Al2O3-water at these three mass flow rates was recorded as 2.75%, 3.29%, and 5.27% when using EG.
- ✔
- The efficiency of the system when using ZnO-water increased by 2.45%, 2.99%, and 4.05% for the three different mass flow rates when EG was added.
Author Contributions
E.A. and S.A. designed and constructed the system; M.G. prepared the nanofluids; and all of authors analyzed the data.
Acknowledgments
This study was supported by the Paralik Solar Collector Manufacturing Ltd., Nicosia, Cyprus and Sağsan San. Tic. Ltd., Famagusta, Cyprus.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Nomenclature | |
| EG | Ethylene glycol |
| Al2O3 | Aluminum oxide |
| ZnO | Zinc oxide |
| ASHRAE | The American Society of Heating, Refrigerating and Air-Conditioning Engineers |
| CAS | Chemical Abstracts Service |
| cp,nf | Heat capacity of the nanofluid (j/gK) |
| cp,n | Heat capacity of the nanoparticles (j/gK) |
| cp,f | Heat capacity of the fluid (j/gK) |
| Q | Useful energy (j) |
| m | Mass flow rate (kg/s) |
| To | Outlet fluid temperature (K) |
| Ti | Inlet fluid temperature (K) |
| Ta | Ambient temperature (K) |
| A | Solar collector area (m2) |
| GT | Solar radiation (W/m2) |
| FR | Heat removal factor |
| (τα) | Absorptance-transmittance product |
| UL | Overall heat loss coefficient (W/m2K) |
| η | Efficiency |
| L | Length (cm) |
| W | Width (cm) |
| T | Thickness (cm) |
| Greek Symbols | |
| Фv | Volume concentration |
| Фm | Mass concentration |
| ρn | Density of nanoparticle (g/cm3) |
| ρf | Density of fluid (g/cm3) |
| β | Collector tilt angle (°) |
| ∆T | Outlet fluid temperature and inlet fluid temperature differences (°C) |
References
- Kalogirou, S.A. Solar Energy Engineering Processes and Systems, 1st ed.; Elsevier: London, UK, 2009; pp. 121–214. ISBN 978-0-12-374501-9. [Google Scholar]
- Colangelo, G.; Favale, E.; Miglietta, P.; Risi, A. Innovation in Flat Solar Thermal Collectors: A Review of the Last Ten Years Experimental Results. Renew. Sustain. Energy Rev. 2016, 57, 1141–1159. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat Transfer Enhancement of Nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc. ASHRAE 93-2010 (RA 2014), Methods of Testing to Determine the Thermal Performance of Solar Collectors; ASHRAE: Atlanta, GA, USA, 2014. [Google Scholar]
- Yousefi, T.; Veysi, F.; Shojaeizadeh, E.; Zinadini, S. An Experimental Investigation on the Effect of Al2O3-H2O Nanofluid on the Efficiency of Flat-Plate Solar Collectors. Renew. Energy 2012, 39, 293–298. [Google Scholar] [CrossRef]
- Babu, S.A.; Raja, M. An Experimental Investigation on the Effect of ZnO/Water Nanofluid on the Efficiency of Flat-Plate Solar Collector. Adv. Nat. Appl. Sci. 2016, 7, 40–48. [Google Scholar]
- Bhatti, M.M.; Abbas, T.; Rashidi, M.M. Entropy Generation as a Practical Tool of Optimisation for Non-Newtonian Nanofluid Flow through a Permeable Stretching using SLM. J. Comput. Des. Eng. 2017, 4. [Google Scholar] [CrossRef]
- Hayat, T.; Abbas, T.; Ayub, M.; Farooq, M.; Alsaedi, A. Flow of Nanofluid due to Convectively Heated Riga Plate with Variable Thickness. J. Mol. Liquid 2016, 222. [Google Scholar] [CrossRef]
- Zamzamian, A.; Rad, M.K.; Neyestani, M.K.; Jamal-Abad, M.T. An Experimental Study on the Effect of Cu-Synthesized/EG Nanofluid on the Efficiency of Flat-Plate Solar Collectors. Renew. Energy 2014, 71, 658–664. [Google Scholar] [CrossRef]
- Montoya-Marquez, O.; Flores-Prieto, J.J. The Effect of Inclination on the Efficiency in a Medium-Temperature Flat Plat Solar Collector. Energies 2017, 10, 71. [Google Scholar] [CrossRef]
- Noghrehabadi, A.; Hajidavalloo, E.; Moravej, M. Experimental Investigation of Efficiency of Square Flat-Plate Solar Collector Using SiO2/Water Nanofluid. Case Stud. Therm. Eng. 2016, 8, 378–386. [Google Scholar] [CrossRef]
- Moghadam, A.J.; Farzane-Gord, M.; Sajadi, M.; Hoseyn-Zadeh, M. Effects of Cu/Water Nanofluid on the Efficiency of a Flat-Plate Solar Collector. Exp. Therm. Fluid Sci. 2014, 58, 9–14. [Google Scholar] [CrossRef]
- He, Q.; Zeng, S.; Wang, S. Experimental Investigation on the Efficiency of Flat-Plate Solar Collectors with Nanofluids. Appl. Therm. Eng. 2015, 88, 165–171. [Google Scholar] [CrossRef]
- PASCO. PASPORT Temperature Sensor—PS-2125: PASCO. Available online: www.pasco.com/prodCatalog/PS/PS-2125_pasport-temperature-sensor/index.cfm (accessed on 11 March 2018).
- Michael, J.J.; Iniyan, S. Performance of Copper Oxide/Water Nanofluid in a Flat Plate Solar Water Heater under Natural and Forced Circulations. Energy Convers. Manag. 2015, 95, 160–169. [Google Scholar] [CrossRef]
- U 65—WITA®. Available online: www.wita.de/en/products/pump-technology/wita-u-55-kopie/2 (accessed on 11 March 2018).
- Merck Millipore: ZnO—SDS Report. Available online: www.merckmillipore.com/search/1314-13-2 (accessed on 22 May 2018).
- Merck Millipore: Al2O3—SDS Report. Available online: www.merckmillipore.com/search/1344-28-21 (accessed on 22 May 2018).
- Wang, X.Q.; Mujumdar, A.S. Heat Transfer Characteristics of Nanofluis: A Review. Int. J. Therm. Sci. 2007, 46. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y. Hydrodynamic and Heat Transfer Study of Dispersed Fluids with Submicron Metallic Oxide Particle. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Ni, R. Measurement of the Specific Heat Capacity of Water-Based Al2O3 Nanofluid. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Zinsser, B.; Makrides, G.; Schmitt, W.; Werner, J.H. Annual Energy Yield of 13 Photovoltaic Technologies in Germany and in Cyprus. In Proceedings of the 22nd European Photovoltaic Solar Energy Conference, Milan, Italy, 3–7 September 2007. [Google Scholar]
- Abbasoglu, S.; Adedeji, M.; Şenol, M. Determination of Optimum Tilt and Azimuth Angles for Photovoltaic Systems in Northern Cyprus. In Proceedings of the Solar TR2014, İzmir, Turkey, 19–21 November 2014. [Google Scholar]
- Xu, Y.; Goto, M.; Koto, R.; Tanaka, Y.; Kagawa, Y. Thermal Conductivity of ZnO Thin Film Produced by Reactive Sputtering. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).