Plant Communities Suitable for Green Roofs in Arid Regions
Abstract
:1. Introduction
2. Materials and Methods
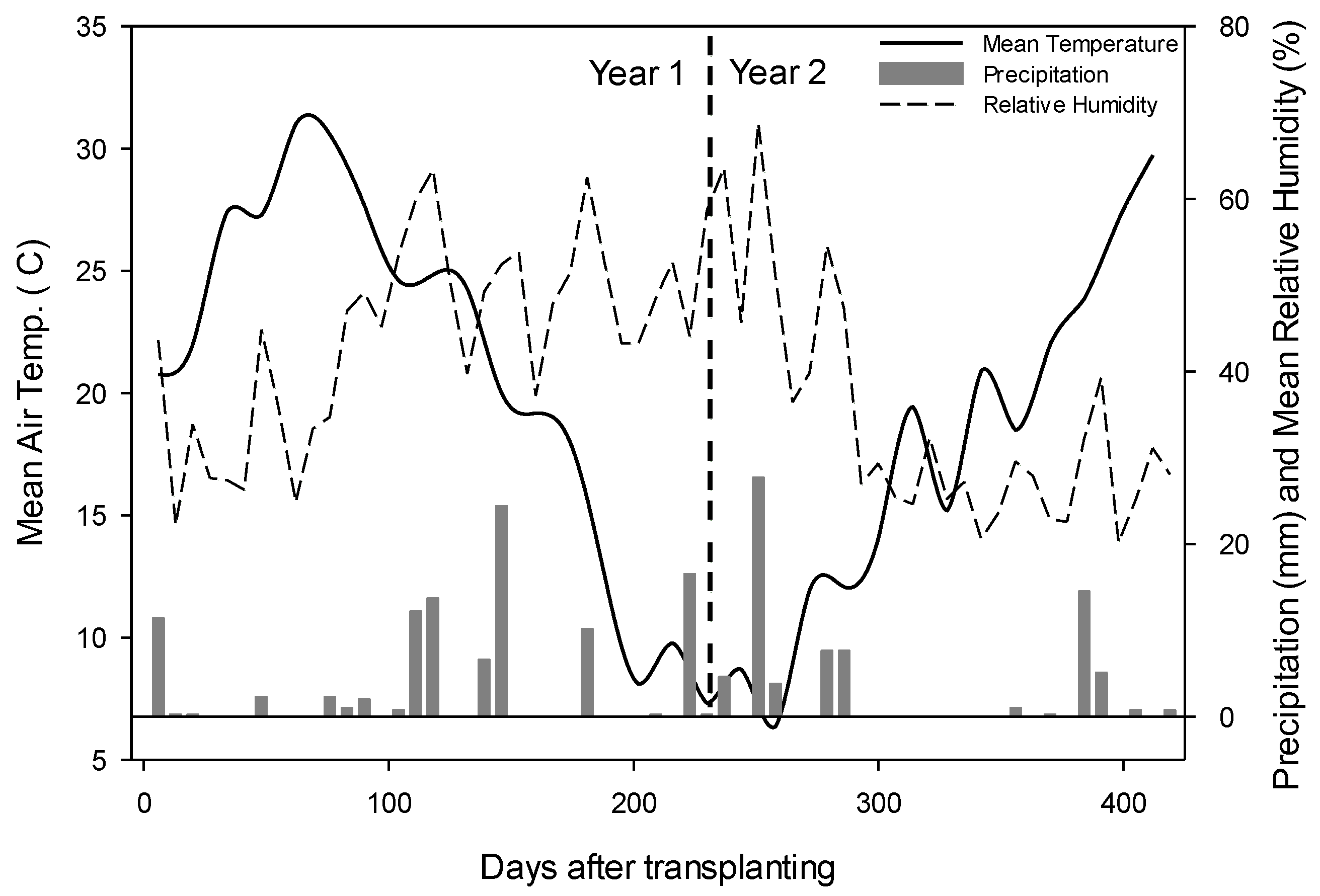
2.1. Site Location and Experiment Setup
2.2. Statistical Analysis
3. Results
3.1. Plant Coverage
3.2. Quality Index
3.3. Relative Water Content
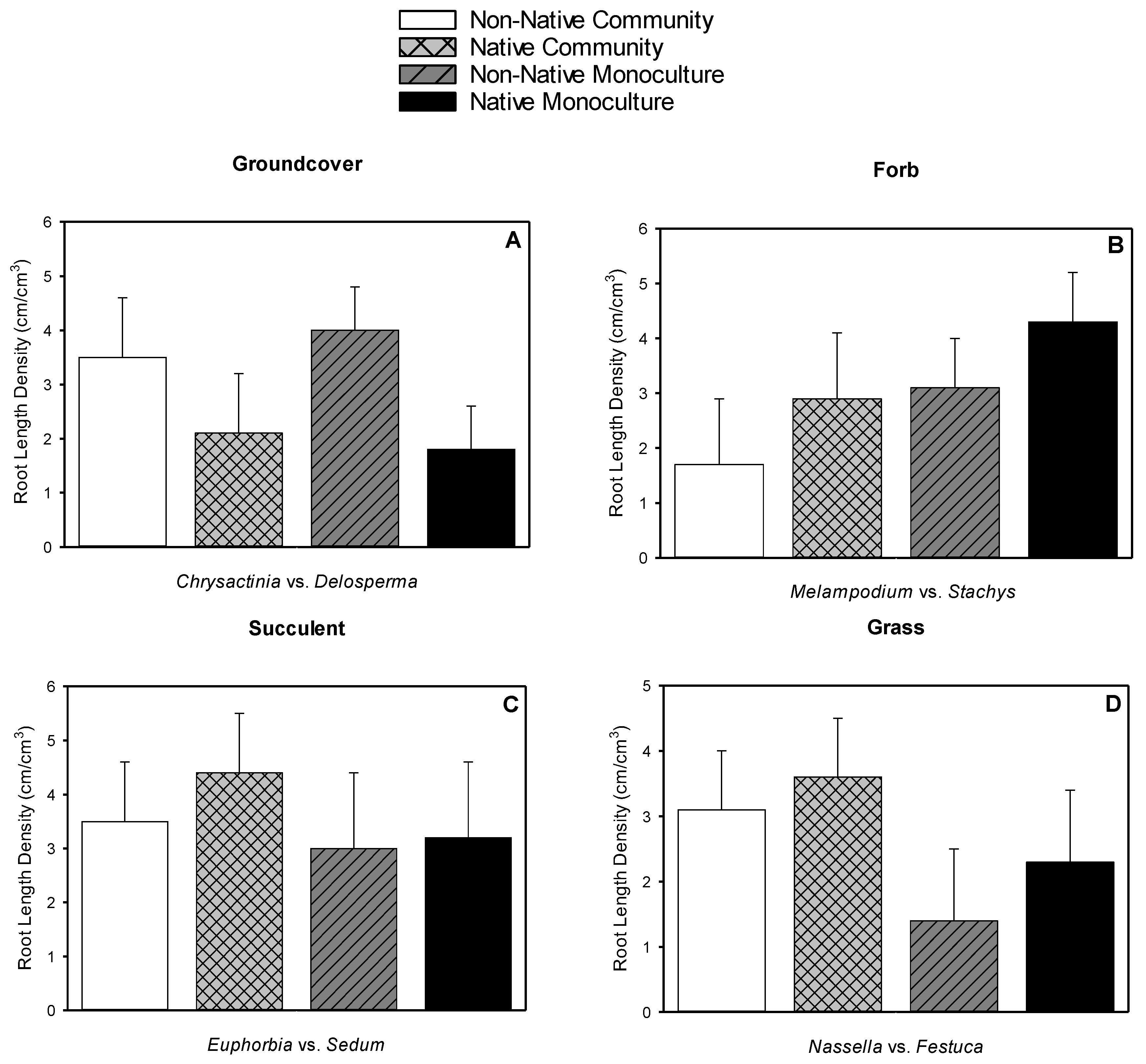
3.4. Root Length
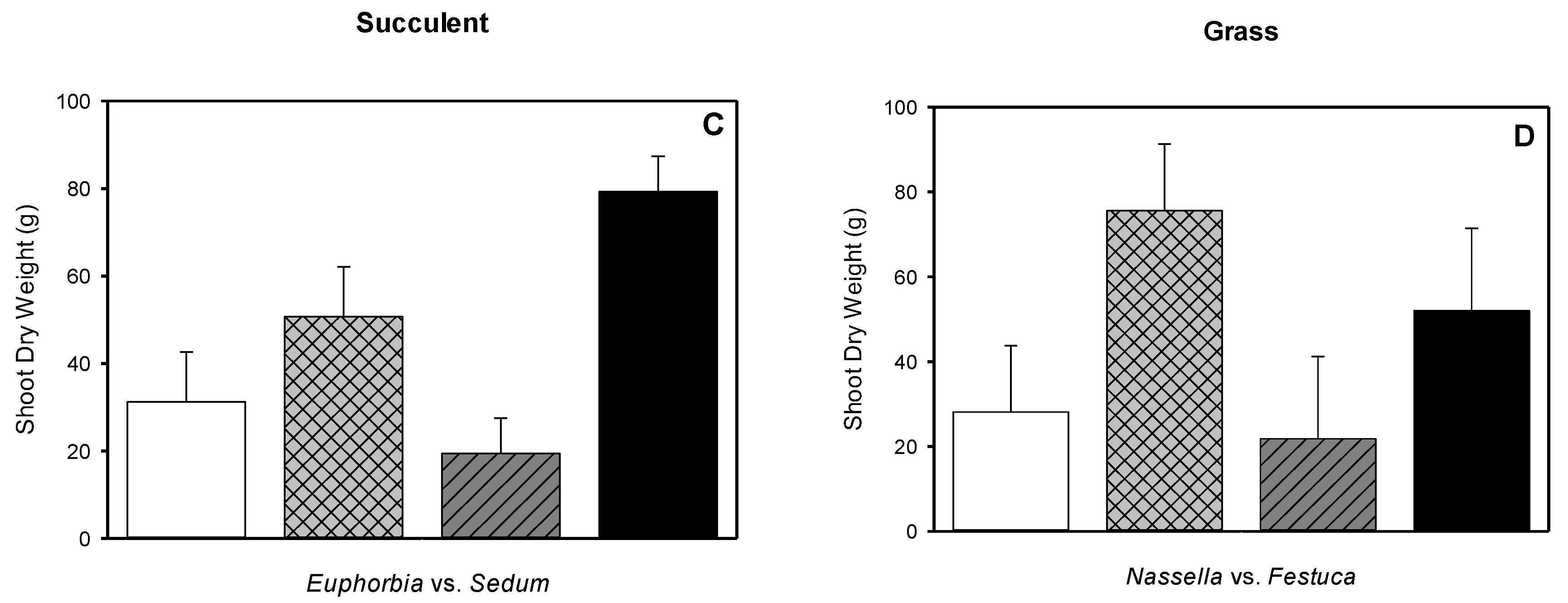
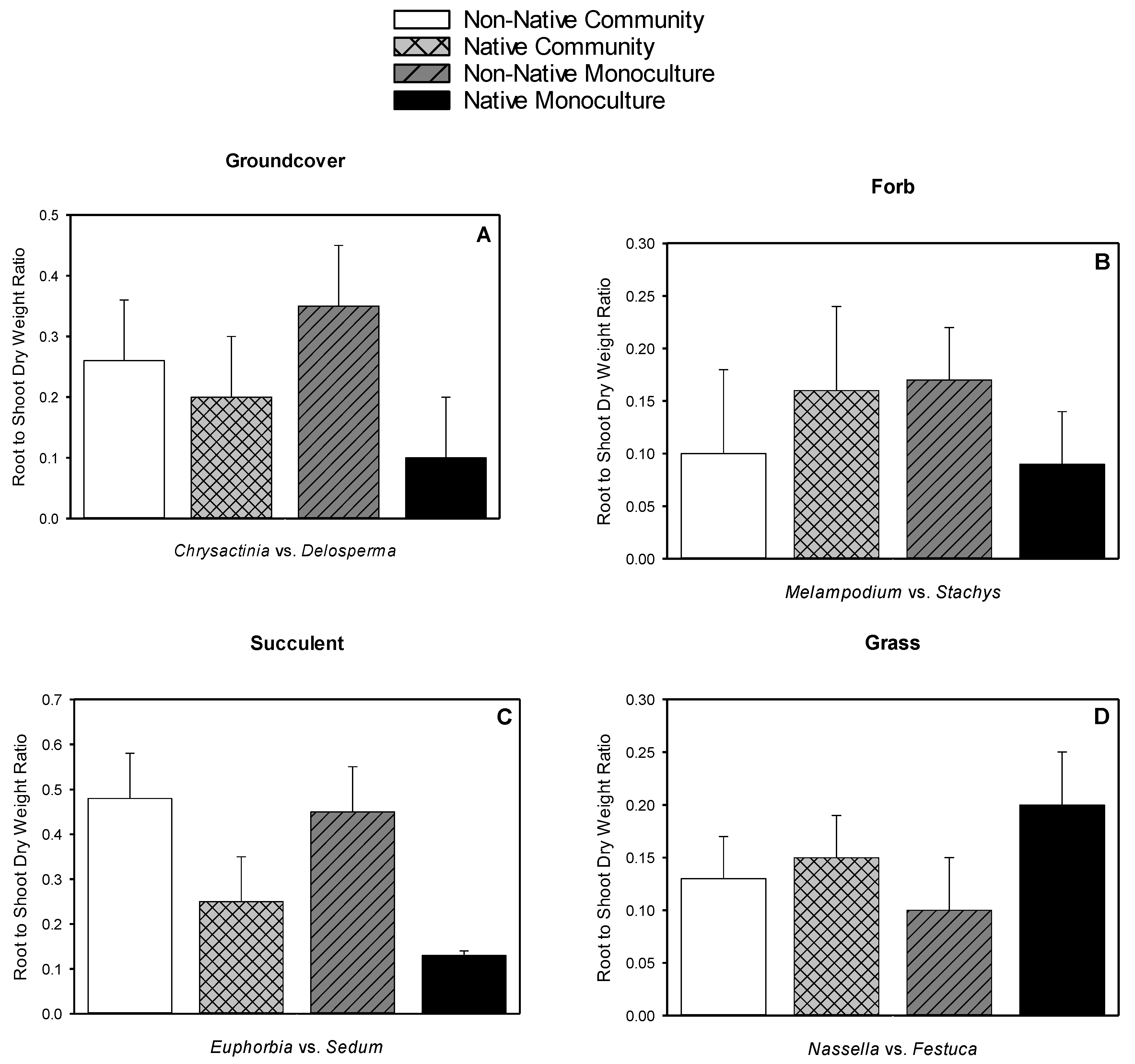
3.5. Root Dry Weight, Shoot Dry Weight, Root-to-shoot Dry Weight Ratio, Root Length Density
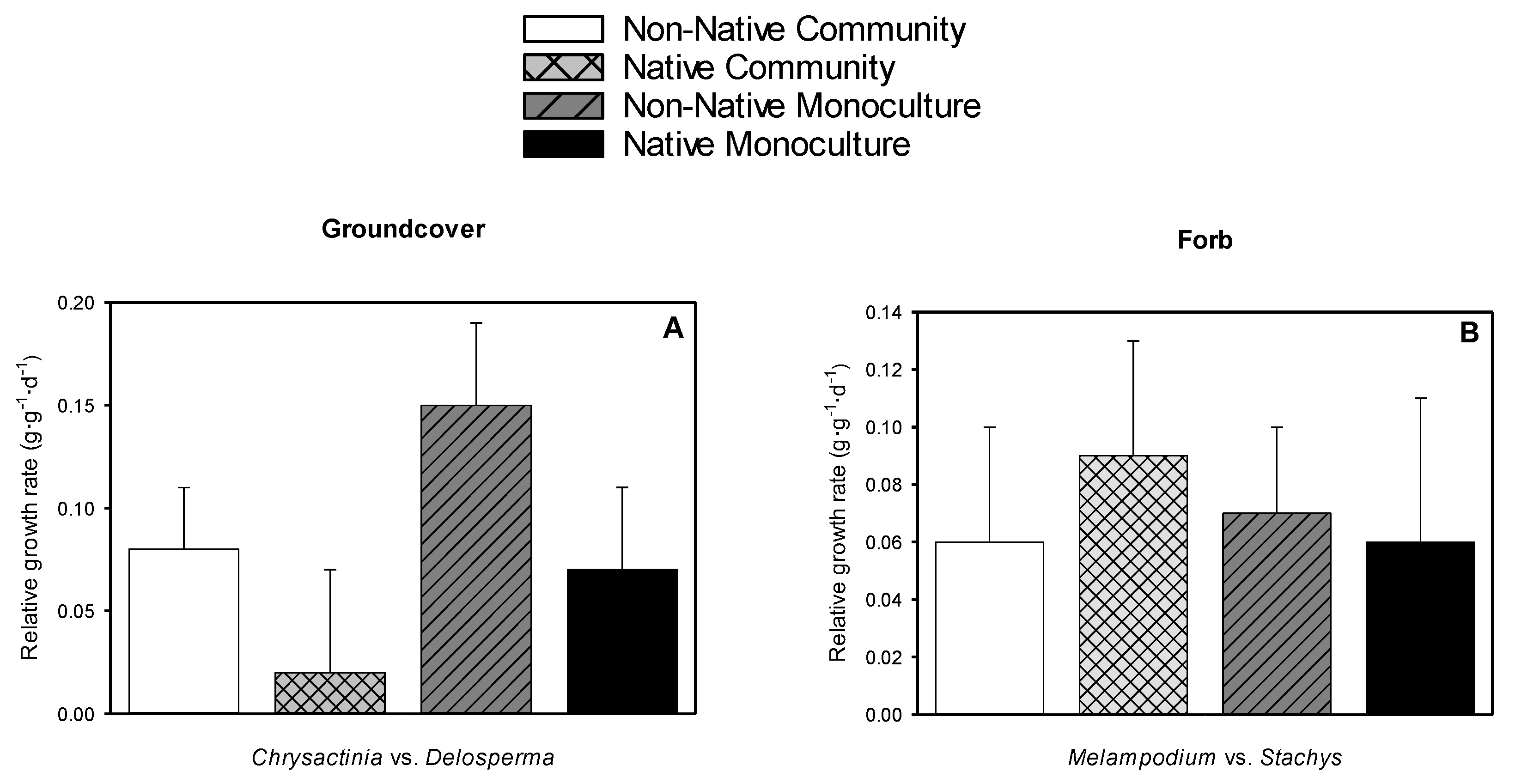
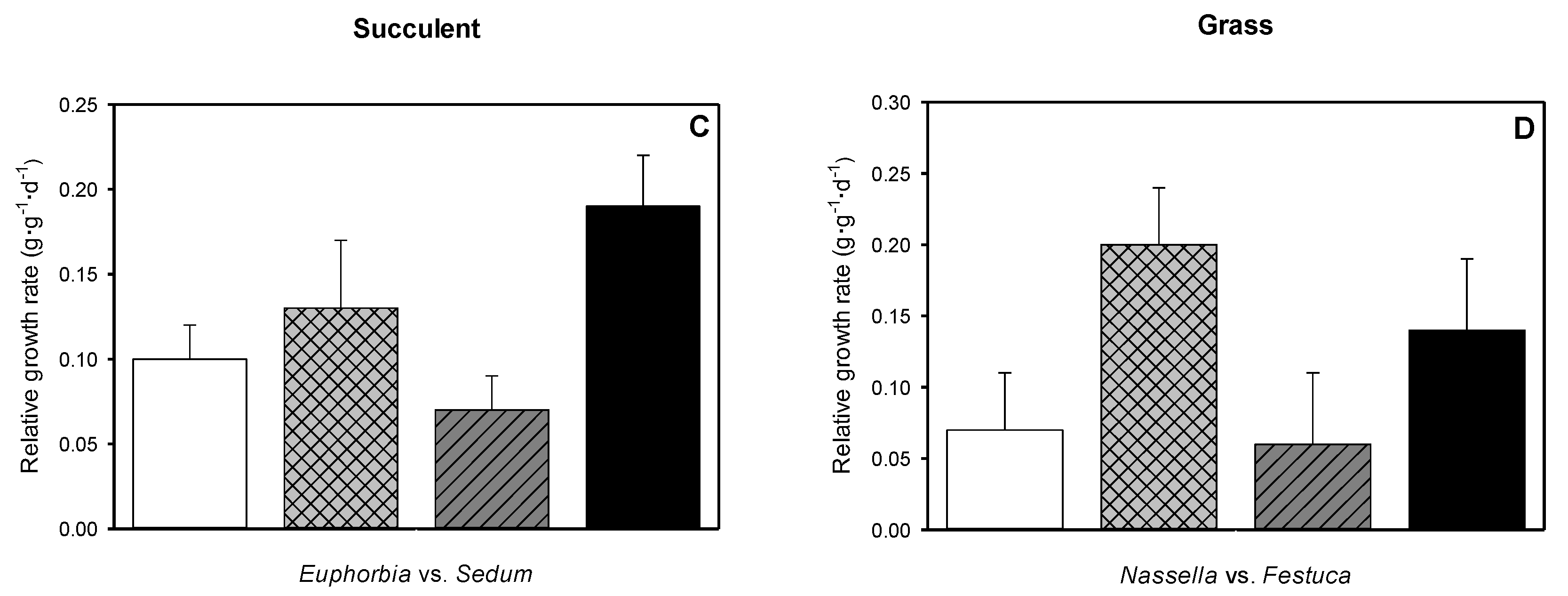
3.6. Relative Growth Rate
3.7. Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Green Roof Industry Survey 2016 Executive Summary. Available online: https://static1.squarespace.com/static/58e3eecf2994ca997dd56381/t/5967869229687ff1cfbb6ada/1499956889472/GreenRoofIndustrySurvey2016ExecutiveSummary.pdf (accessed on 10 May 2018).
- McIntyre, L.; Snodgrass, E.C. The Green Roof Manual: A Professional Guide to Design, Installation, and Maintenance; Timber Press: Portland, OR, USA, 2010; ISBN 1604690496. [Google Scholar]
- Baumann, N. Ground-nesting birds on green roofs in Switzerland: preliminary observations. Urban Habitats 2006, 4, 37–50. [Google Scholar]
- Brenneisen, S. Space for urban wildlife: Designing green roofs as habitats in Switzerland. Urban Habitats 2006, 4, 27–36. [Google Scholar]
- Brenneisen, S. The Benefits of Biodiversity from Green Roofs: Key Design Consequences. In Proceedings of the 1st North American Green Roof Conference: Greening Rooftops for Sustainable Communities, Chicago, IL, USA, 29–30 May 2003; The Cardinal Group: Toronto, ON, CA, USA; pp. 323–329. [Google Scholar]
- Getter, K.L.; Rowe, D.B. The role of extensive green roofs in sustainable development. HortScience 2006, 41, 1276–1285. [Google Scholar]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Köhler, M.; Liu, K.K.; Rowe, B. Green roofs as urban ecosystems: Ecological structures, functions, and services. BioScience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Nabhan, G.P.; St Antoine, S. The loss of floral and faunal story: The extinction of experience. In The Biophilia Hypothesis; Kellert, S.R., Wilson, E.O., Eds.; Island Press: Washington, DC, USA, 1993; pp. 229–250. ISBN 1559631473. [Google Scholar]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1984; p. 157. ISBN 0674074424. [Google Scholar]
- Heerwagen, J.; Kellert, S.R.; Mador, M. Biophilic Design: The Theory, Science and Practice of Bringing Buildings to Life; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 3–15. ISBN 1118174240. [Google Scholar]
- Kellert, S.R.; Wilson, E.O. The Biophilia Hypothesis; Island Press: Washington, DC, USA, 1995; pp. 31–41. ISBN 1559631473. [Google Scholar]
- Kosareo, L.; Ries, R. Comparative environmental life cycle assessment of green roofs. Build. Environ. 2007, 42, 2606–2613. [Google Scholar] [CrossRef]
- Pedersen, K.L. Green Roofs for Sustainable Urban Development: The Oslo Case Study. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2014. [Google Scholar]
- Carter, T.; Jackson, C.R. Vegetated roofs for stormwater management at multiple spatial scales. Landsc. Urban Plan. 2007, 80, 84–94. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B. Effect of substrate depth and planting season on Sedum plug survival on green roofs. J. Environ. Hort. 2007, 25, 95–99. [Google Scholar]
- Hilten, R.N.; Lawrence, T.M.; Tollner, E.W. Modeling stormwater runoff from green roofs with HYDRUS-1D. J. Hydrol. 2008, 358, 288–293. [Google Scholar] [CrossRef]
- Van Renterghem, T.; Botteldooren, D. Numerical evaluation of sound propagating over green roofs. J. Sound Vib. 2008, 317, 781–799. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Q.; Gong, P. Quantifying air pollution removal by green roofs in Chicago. Atmos. Environ. 2008, 42, 7266–7273. [Google Scholar] [CrossRef]
- Takebayashi, H.; Moriyama, M. Surface heat budget on green roof and high reflection roof for mitigation of urban heat island. Build. Environ. 2007, 42, 2971–2979. [Google Scholar] [CrossRef]
- Bass, B. Green Roofs and Green Walls: Potential Energy Savings in the Winter. In Proceedings of the Adaption and Impacts Research Division, Environment Canada, University of Toronto, Toronto, ON, CA, USA, 31 March 2007; Available online: https://www.researchgate.net/profile/Brad_Bass/publication/242663071_Green_Roofs_and_Green_Walls_Potential_Energy_Savings_in_the_Winter/links/0c96052b4deec56cc2000000.pdf (accessed on 3 March 2018).
- Dunnett, N.; Nolan, A. The Effect of Substrate Depth and Supplementary Watering on the Growth of Nine Herbaceous Perennials in a Semi-extensive Green Roof. Acta Hort. 2002, 643, 305–309. [Google Scholar] [CrossRef]
- Durhman, A.K.; Rowe, D.B.; Rugh, C.L. Effect of substrate depth on initial growth, coverage, and survival of 25 succulent green roof plant taxa. HortScience 2007, 42, 588–595. [Google Scholar]
- Durhman, A.K.; Rowe, D.B.; Rugh, C.L. Effect of watering regimen on chlorophyll fluorescence and growth of selected green roof plant taxa. HortScience 2006, 41, 1623–1628. [Google Scholar]
- Hotovec, L.; Gioannini, R.; VanLeeuwen, D.M.; Hilaire, R.S. Shallow substrates support the growth of contrasting plant types installed in irrigated, arid-climate green roofs. J. Environ. Hort. 2017, 35, 146–155. [Google Scholar]
- Ntoulas, N.; Nektarios, P.A.; Nydrioti, E. Performance of Zoysia matrella ‘Zeon’in shallow green roof substrates under moisture deficit conditions. HortScience 2013, 48, 929–937. [Google Scholar]
- Thuring, C.E.; Berghage, R.D.; Beattie, D.J. Green roof plant responses to different substrate types and depths under various drought conditions. HortTechnology 2010, 20, 395–401. [Google Scholar]
- Getter, K.L.; Rowe, D.B. Substrate depth influences Sedum plant community on a green roof. HortScience 2009, 44, 401–407. [Google Scholar]
- Bousselot, J.M.; Klett, J.E.; Koski, R.D. Moisture content of extensive green roof substrate and growth response of 15 temperate plant species during dry down. HortScience 2011, 46, 518–522. [Google Scholar]
- Castleton, H.; Stovin, V.; Beck, S.; Davison, J. Green roofs; building energy savings and the potential for retrofit. Energy Build. 2010, 42, 1582–1591. [Google Scholar] [CrossRef]
- Livingston, E.; Miller, C.; Lohr, M. Green Roof Design and Implementation in Florida. In Proceedings of the Second Annual Greening Rooftops for Sustainable Communities Conference, Awards and Trade Show, Portland, OR, USA, 2–4 June 2004; The Cardinal Group: Toronto, ON, CA, USA, 2004; pp. 353–364. [Google Scholar]
- Blanusa, T.; Monteiro, M.M.V.; Fantozzi, F.; Vysini, E.; Li, Y.; Cameron, R.W. Alternatives to Sedum on green roofs: can broad leaf perennial plants offer better ‘cooling service’? Build. Environ. 2013, 59, 99–106. [Google Scholar] [CrossRef]
- Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau (FLL). Guidelines for the Planning, Execution and Upkeep of Green-Roof Sites; FLL: Bonn, Germany, 2002. [Google Scholar]
- Altieri, M.A.; Letourneau, D.K. Vegetation management and biological control in agroecosystems. Crop Prot. 1982, 1, 405–430. [Google Scholar] [CrossRef]
- Dewey, D.W.; Johnson, P.G.; Kjelgren, R.K. Species composition changes in a rooftop grass and wildflower meadow: implications for designing successful mixtures. Native Plants J. 2004, 5, 56–65. [Google Scholar] [CrossRef]
- Dunnett, N.; Nagase, A.; Hallam, A. The dynamics of planted and colonising species on a green roof over six growing seasons 2001–2006: Influence of substrate depth. Urban Ecosyst. 2008, 11, 373–384. [Google Scholar] [CrossRef]
- Dunnett, N.; Kingsbury, N. Planting Green Roofs and Living Walls; Timber Press: Portland, OR, USA, 2008; ISBN 9780881929119. [Google Scholar]
- Duffield, M.; Jones, W.D. Plants for Dry Climates: How to Select, Grow and Enjoy; De Capo Press: Boston, MA, USA, 1981; p. 172. ISBN 0895860422. [Google Scholar]
- Jones, W.D.; Sacamano, C.M. Landscape Plants for Dry Regions: More than 600 Species from Around the World; De Capo Press: Boston, MA, USA, 2000; pp. 107, 153, 215. ISBN 1555611907. [Google Scholar]
- Wasowski, S.; Wasowski, A. Native Texas Plants: Landscaping Region by Region; Taylor Trade Publishing: Boulder, CO, USA, 2003; p. 198. ISBN 1589796586. [Google Scholar]
- Mielke, J. Native Plants for Southwestern Landscapes; University of Austin Press: Austin, TX, USA, 1993; p. 144. ISBN 0292751478. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Nagase, A.; Dunnett, N. Drought tolerance in different vegetation types for extensive green roofs: Effects of watering and diversity. Landsc. Urban Plan. 2010, 97, 318–327. [Google Scholar] [CrossRef]
- Hilaire, R.S. Landscape Plants for the Lower Rio Grande Basin; Sentia Publishing: Austin, TX, USA, 2012; p. 18. ISBN 9781732060173. [Google Scholar]
- Horvath, B. The Plant Lover’s Guide to Sedums; Timber Press: Portland, OR, USA, 2014; p. 120. ISBN 1604693924. [Google Scholar]
- Plant Hardiness Zone Map for the United States. Available online: http://planthardiness.ars.usda.gov/PHZMWeb/ (accessed on 7 March 2015).
- U.S. Climate Data. Available online: http://www.usclimatedata.com/climate/las-cruces/new-mexico/united-states/usnm0492/2017/1 (accessed on 7 March 2015).
- Clouston, B. Landscape Design with Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1989; p. 416. ISBN 0434902349. [Google Scholar]
- Barnard, C.M.; Potter, L.D. New Mexico Grasses: A Vegetative Key; University of New Mexico Press: Albuquerque, NM, USA, 1984; p. 92. ISBN 0826307442. [Google Scholar]
- Bennett, J. Dryland Gardening; Plants that Survive and Thrive in Tough Conditions; Firefly Books: Richmond Hill, ON, USA, 2005; p. 76. ISBN 9781554070312. [Google Scholar]
- Handreck, K.A.; Black, N.D. Growing Media for Ornamental Plants and Turf; University of New South Wales Press: Kensington, UK, 2002; p. 14. ISBN 0868407968. [Google Scholar]
- Bousselot, J.M.; Klett, J.E.; Koski, R.D. Evaluating a natural zeolite as an amendment for extensive green roof substrate. J. Environ. Hort. 2012, 30, 201–206. [Google Scholar]
- Liu, R.; Coffman, R. Lightweight aggregate made from dredged material in green roof construction for stormwater management. Materials 2016, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Gutschick, V.; Kay, L. Nutrient-limited growth rates: quantitative benefits of stress responses and some aspects of regulation. J. Exp. Bot. 1995, 46, 995–1009. [Google Scholar] [CrossRef]
- Bsoul, E.; Hilaire, R.S.; VanLeeuwen, D.M. Bigtooth maples exposed to asynchronous cyclic irrigation show provenance differences in drought adaptation mechanisms. J. Am. Soc. Hort. Sci. 2006, 131, 459–468. [Google Scholar]
- Ramsey, F.L.; Schafer, D.W. The Statistical Sleuth: A Course in Methods of Data Analysis, 2nd ed.; Duxbury/Thomson Learning Inc.: Pacific Grove, CA, USA, 2002; ISBN 0534386709. [Google Scholar]
- Piepho, H.-P. A SAS macro for generating letter displays of pairwise mean comparisons. Commun. Biometry Crop Sci. 2012, 7, 4–13. [Google Scholar]
- Saxton, A.M. A Macro for Converting Mean Separation Output to Letter Groupings in Proc Mixed. In Proceedings of the 23rd SAS Users Group International, Nashville, TN, USA, 22–25 March 1998; pp. 1243–1246. [Google Scholar]
- Lumsden, R.D.; Walter, J.F. Development of Gliocladium virens for damping-off disease control. Can. J. Plant Path. 1996, 18, 463–468. [Google Scholar] [CrossRef]
- Burns, J.; Benson, D. Biocontrol of damping-off of Catharanthus roseus caused by Pythium ultimum with Trichoderma virens and binucleate Rhizoctonia fungi. Plant Dis. 2000, 84, 644–648. [Google Scholar] [CrossRef]
- French, J.; New Mexico State University, Las Cruces, NM, USA. Personal communication, 2016.
- Noggle, G.R.; Fritz, G.J. Introductory Plant Physiology; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1976; p. 446. ISBN 0135020964. [Google Scholar]
- MacIvor, J.S.; Lundholm, J. Performance evaluation of native plants suited to extensive green roof conditions in a maritime climate. Ecol. Eng. 2011, 37, 407–417. [Google Scholar] [CrossRef]
- Monterusso, M.A.; Rowe, D.B.; Rugh, C.L. Establishment and persistence of Sedum spp. and native taxa for green roof applications. HortScience 2005, 40, 391–396. [Google Scholar]
- Ondra, N.J. Grasses: Versatile Partners for Uncommon Garden Design; Storey Publishing: North Adams, MA, USA, 2002; p. 112. ISBN 9781580174237. [Google Scholar]
- Getter, K.L.; Rowe, D.B.; Cregg, B.M. Solar radiation intensity influences extensive green roof plant communities. Urban For. Urban Green. 2009, 8, 269–281. [Google Scholar] [CrossRef]
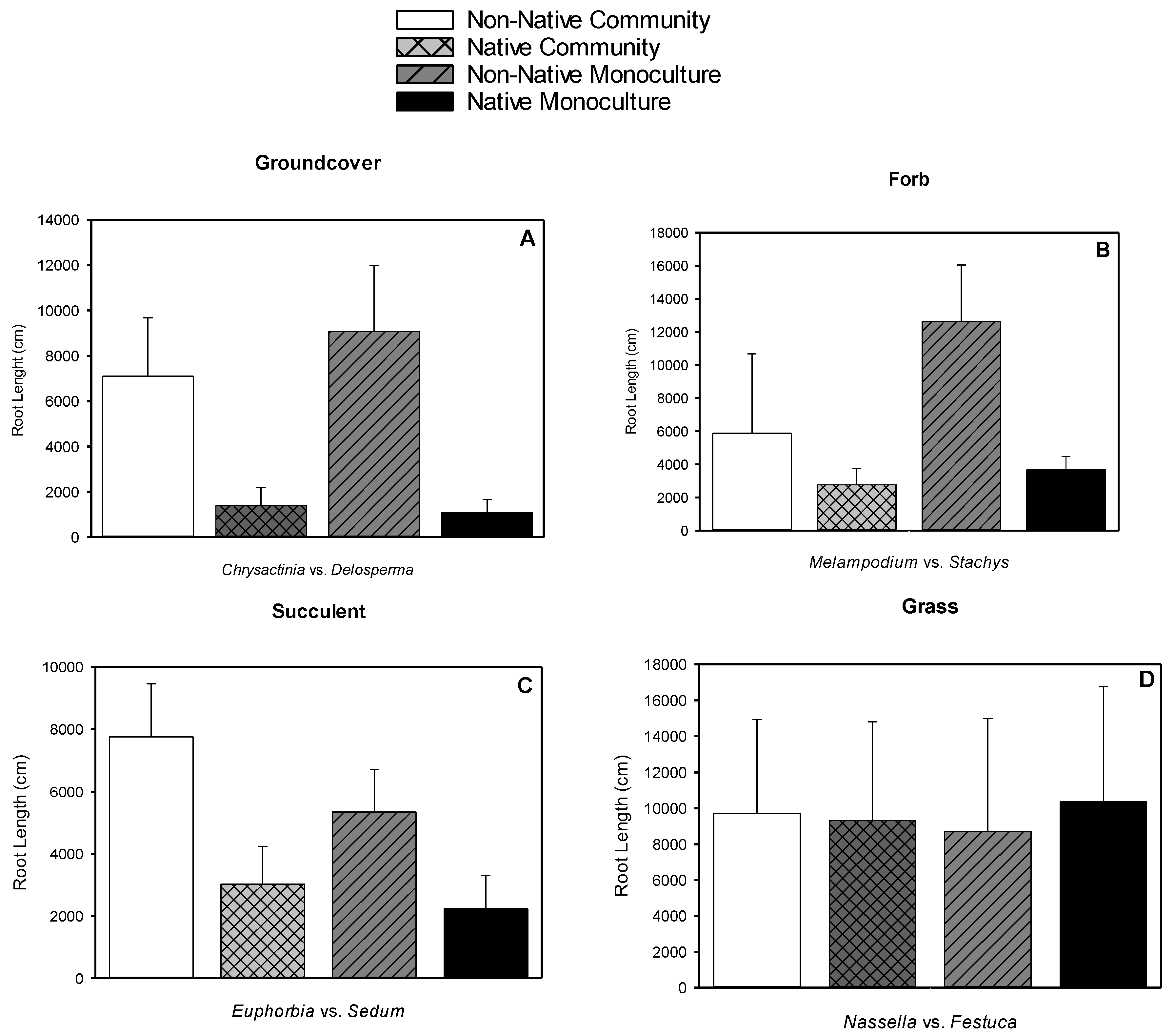
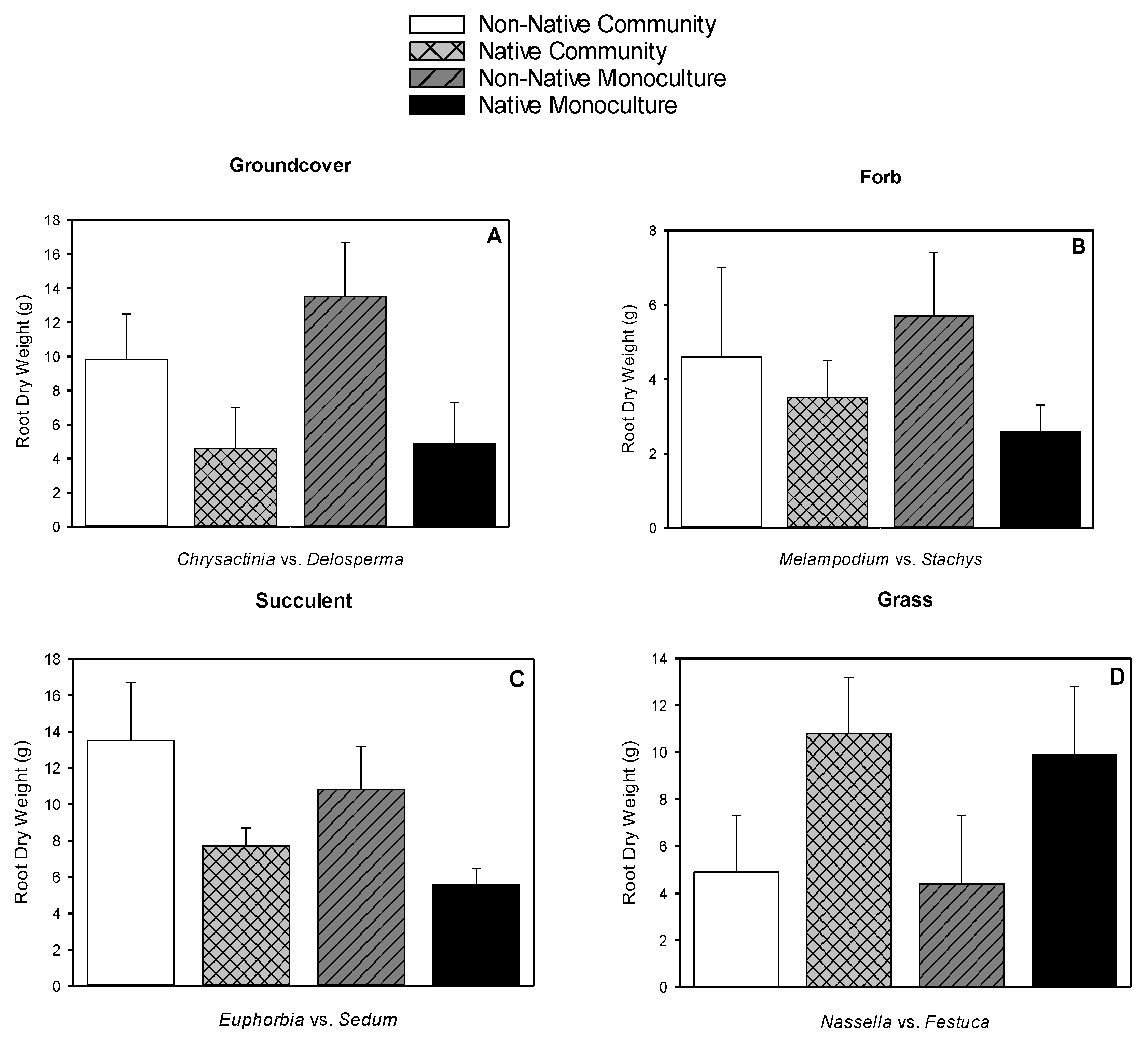
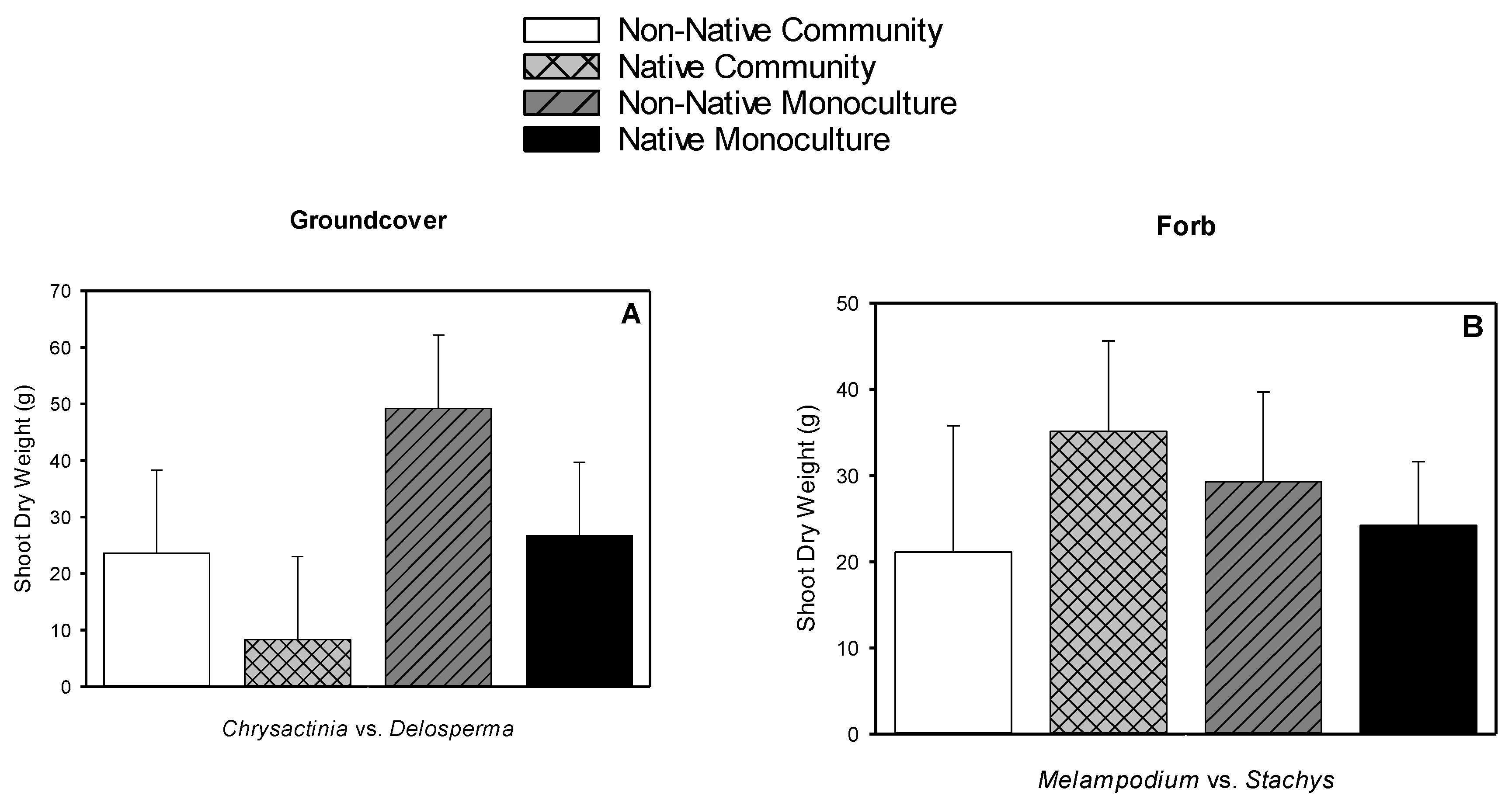
| Species | Type | Typical Spread at Maturity (cm2) | Leaf Area (cm2) | Shoot Dry Weight (g) | Root Dry Weight (g) | Root-to-shoot Dry Weight Ratio |
|---|---|---|---|---|---|---|
| Chrysactinia mexicana | native groundcover | 930–3721 1 | 49 | 6.0 | 2.0 | 0.33 |
| Delosperma nubigenum | non-native groundcover | 8281 2 | 348 | 9.0 | 3.2 | 0.35 |
| Melampodium leucanthum | native forb | 400–3721 1 | 190 | 6.3 | 2.1 | 0.35 |
| Stachys byzantina | non-native forb | 900–3600 3 | 253 | 2.7 | 5.4 | 1.98 |
| Euphorbia antisyphilitica | native succulent | 3721 1 | 244 | 19.1 | 4.8 | 0.26 |
| Sedum kamtschiaticum | non-native succulent | 900 4 | 612 | 4.6 | 5.3 | 1.10 |
| Nassella tenuissima | native grass | 1600–6400 5 | 283 | 14.6 | 8.4 | 0.58 |
| Festuca glauca | non-native grass | 225–625 6 | 29 | 4.2 | 3.8 | 0.91 |
| Irrigation | |||
|---|---|---|---|
| Date | Duration (Minutes per Day) | Frequency (Days per Week) | Weekly Amount (Liters per Week) |
| 2016 | |||
| 13 May | 13 | 7 | 34.3 |
| 18 May | Off | ||
| 3 June | 10 | 7 | 26.6 |
| 17 August | 6 | 7 | 16.1 |
| 28 September | 6 | 4 | 9.2 |
| 14 November | 5 | 3 | 5.7 |
| 2017 | |||
| 15 March | 6 | 4 | 9.2 |
| 17 April | 4 | 7 | 10.5 |
| 3 May | 6 | 7 | 16.1 |
| Taxa | Dead (no.) | Overall Loss (%) | Within Species (%) | Setting | |
|---|---|---|---|---|---|
| Monoculture (%) | Community (%) | ||||
| Chrysactinia | 7 | 35 | 58 | 71 | 29 |
| Delosperma | 1 | 5 | 8 | 0 | 100 |
| Euphorbia | 0 | 0 | 0 | 0 | 0 |
| Festuca | 5 | 25 | 42 | 80 | 20 |
| Melampodium | 2 | 10 | 17 | 50 | 50 |
| Nassella | 0 | 0 | 0 | 0 | 0 |
| Sedum | 1 | 5 | 8 | 100 | 0 |
| Stachys | 4 | 20 | 33 | 50 | 50 |
| Total Loss | 20 | ||||
| Measurement Date | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | |||||||||
| Setting | Origin | July (53 days z) | Aug. (81 days) | Sept. (116 days) | Oct. (144 days) | Nov. (172 days) | Apr. (326 days) | May (354 days) | June (389 days) | July (417 days) |
| Comm | NN | 579 z | 676 | 683 | 693 | 721 | 691 | 688 | 684 | 402 |
| Comm | N | 250 | 275 | 454 | 432 | 424 | 156 | 181 | 174 | 191 |
| Mono | NN | 509 | 586 | 664 | 655 | 645 | 553 | 566 | 566 | 635 |
| Mono | N | 190 | 210 | 383 | 398 | 400 | 359 | 384 | 482 | 339 |
| Origin Differences ± S.E. | NN, N | 324 ± 48 | 388 ± 58 | 254 ± 81 | 259 ± 95 | 271 ± 105 | 364 ± 124 | 344 ± 133 | --- | --- |
| Significance | p value | |||||||||
| Setting | 0.2136 | 0.2127 | 0.5984 | 0.7125 | 0.6476 | 0.8026 | 0.7681 | 0.5502 | 0.3361 | |
| Origin | 0.0002 | 0.0001 | 0.0136 | 0.0264 | 0.0329 | 0.0190 | 0.0325 | 0.0862 | 0.2100 | |
| Setting × Origin | 0.9213 | 0.8305 | 0.7550 | 0.9792 | 0.8064 | 0.2072 | 0.2577 | 0.1982 | 0.8243 | |
| Measurement Date | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | |||||||||
| Setting | Origin | July (53 days y) | Aug. (81 days) | Sept. (116 days) | Oct. (144 days) | Nov. (172 days) | Apr. (326 days) | May (354 days) | June (389 days) | July (417 days) |
| Comm | NN | 254 | 353 c z | 490 c | 723 c | 713 c | 317 | 420 | 593 | 524 |
| Comm | N | 1040 | 1929 a | 3754 a | 3986 a | 3541 a | 776 | 1027 | 1168 | 1144 |
| Mono | NN | 337 | 507 c | 620 c | 682 c | 708 c | 288 | 384 | 432 | 649 |
| Mono | N | 814 | 1243 b | 1797 b | 1946 b | 1566 b | 674 | 948 | 1015 | 1185 |
| Setting Differences ± S.E. | Mono, Comm | --- | --- | 914 ± 254 | 1041 ± 277 | 990 ± 240 | --- | --- | --- | --- |
| Origin Differences ± S.E. | NN, N | 632 ± 116 | 1157 ± 176 | 2220 ± 254 | 2265 ± 277 | 1843 ± 240 | 422 ± 130 | 586 ± 166 | 579 ± 216 | --- |
| Significance | p value | |||||||||
| Setting | 0.5528 | 0.1679 | 0.0071 | 0.0056 | 0.0033 | 0.6258 | 0.7393 | 0.4873 | 0.7877 | |
| Origin | 0.0006 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | 0.0118 | 0.0078 | 0.0279 | 0.0873 | |
| Setting × Origin | 0.2185 | 0.0436 | 0.0034 | 0.0070 | 0.0034 | 0.7783 | 0.8991 | 0.9839 | 0.8911 | |
| Measurement Date | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | |||||||||
| Setting | Origin | July (53 days z) | Aug. (81 days) | Sept. (116 days) | Oct. (144 days) | Nov. (172 days) | Apr. (326 das) | May (354 days) | June (389 days) | July (417 days) |
| Comm | NN | 126 | 177 | 275 | 276 | 259 | 239 | 307 | 477 | 704 |
| Comm | N | 471 | 551 | 732 | 737 | 778 | 582 | 642 | 660 | 574 |
| Mono | NN | 170 | 191 | 228 | 215 | 211 | 271 | 275 | 422 | 431 |
| Mono | N | 350 | 408 | 658 | 715 | 752 | 657 | 653 | 792 | 753 |
| Origin Differences ± S.E. | NN, N | 262 ± 53 | 295 ± 64 | 443 ± 79 | 480 ± 89 | 531 ± 97 | 365 ± 83 | 356 ± 81 | 303 ± 121 | --- |
| Significance | p value | |||||||||
| Setting | 0.4901 | 0.3421 | 0.4680 | 0.6521 | 0.7129 | 0.5362 | 0.9040 | 0.9199 | 0.6613 | |
| Origin | 0.0012 | 0.0017 | 0.0005 | 0.0006 | 0.0006 | 0.0023 | 0.0023 | 0.0366 | 0.3815 | |
| Settingx Origin | 0.1620 | 0.2513 | 0.8648 | 0.8368 | 0.9097 | 0.8019 | 0.7991 | 0.3522 | 0.0596 | |
| Measurement Date | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | |||||||||
| Setting | Origin | July (53 days z) | Aug. (81 days) | Sept. (116 days) | Oct. (144 days) | Nov. (172 days) | Apr. (326 days) | May (354 days) | June (389 days) | July (417 days) |
| Comm | NN | 541 | 457 | 403 | 374 | 345 | 422 | 583 | 598 | 466 |
| Comm | N | 1616 | 2014 | 2317 | 2521 | 2528 | 1412 | 1888 | 1969 | 2993 |
| Mono | NN | 452 | 335 | 335 | 369 | 395 | 472 | 532 | 612 | 304 |
| Mono | N | 1328 | 1873 | 3047 | 3453 | 2724 | 1375 | 1206 | 963 | 1111 |
| Origin Differences ± S.E. | NN, N | 975 ± 132 z | 1547 ± 251 | 2313 ± 302 | 2615 ± 388 | 2256 ± 259 | 947 ± 186 | 989 ± 207 | 861 ± 246 | 1348 ± 353 |
| Significance | p value | |||||||||
| Setting | 0.1904 | 0.6166 | 0.3042 | 0.2660 | 0.6472 | 0.9727 | 0.1139 | 0.0785 | 0.0820 | |
| Origin | <0.0001 | 0.0003 | <0.0001 | 0.0001 | <0.0001 | 0.0009 | 0.0014 | 0.0081 | 0.0051 | |
| Settingx Origin | 0.4716 | 0.9698 | 0.2224 | 0.2613 | 0.7856 | 0.8189 | 0.1656 | 0.0718 | 0.1645 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioannini, R.; Al-Ajlouni, M.; Kile, R.; VanLeeuwen, D.; St. Hilaire, R. Plant Communities Suitable for Green Roofs in Arid Regions. Sustainability 2018, 10, 1755. https://doi.org/10.3390/su10061755
Gioannini R, Al-Ajlouni M, Kile R, VanLeeuwen D, St. Hilaire R. Plant Communities Suitable for Green Roofs in Arid Regions. Sustainability. 2018; 10(6):1755. https://doi.org/10.3390/su10061755
Chicago/Turabian StyleGioannini, Rachel, Malik Al-Ajlouni, Richard Kile, Dawn VanLeeuwen, and Rolston St. Hilaire. 2018. "Plant Communities Suitable for Green Roofs in Arid Regions" Sustainability 10, no. 6: 1755. https://doi.org/10.3390/su10061755
APA StyleGioannini, R., Al-Ajlouni, M., Kile, R., VanLeeuwen, D., & St. Hilaire, R. (2018). Plant Communities Suitable for Green Roofs in Arid Regions. Sustainability, 10(6), 1755. https://doi.org/10.3390/su10061755





