Seasonal Variations of Dissolved Organic Matter in the East China Sea Using EEM-PARAFAC and Implications for Carbon and Nutrient Cycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pretreatment
2.2. DOC and Nutrient Measurements
2.3. UV-vis Measurements and Fluorescence Analysis
3. Results
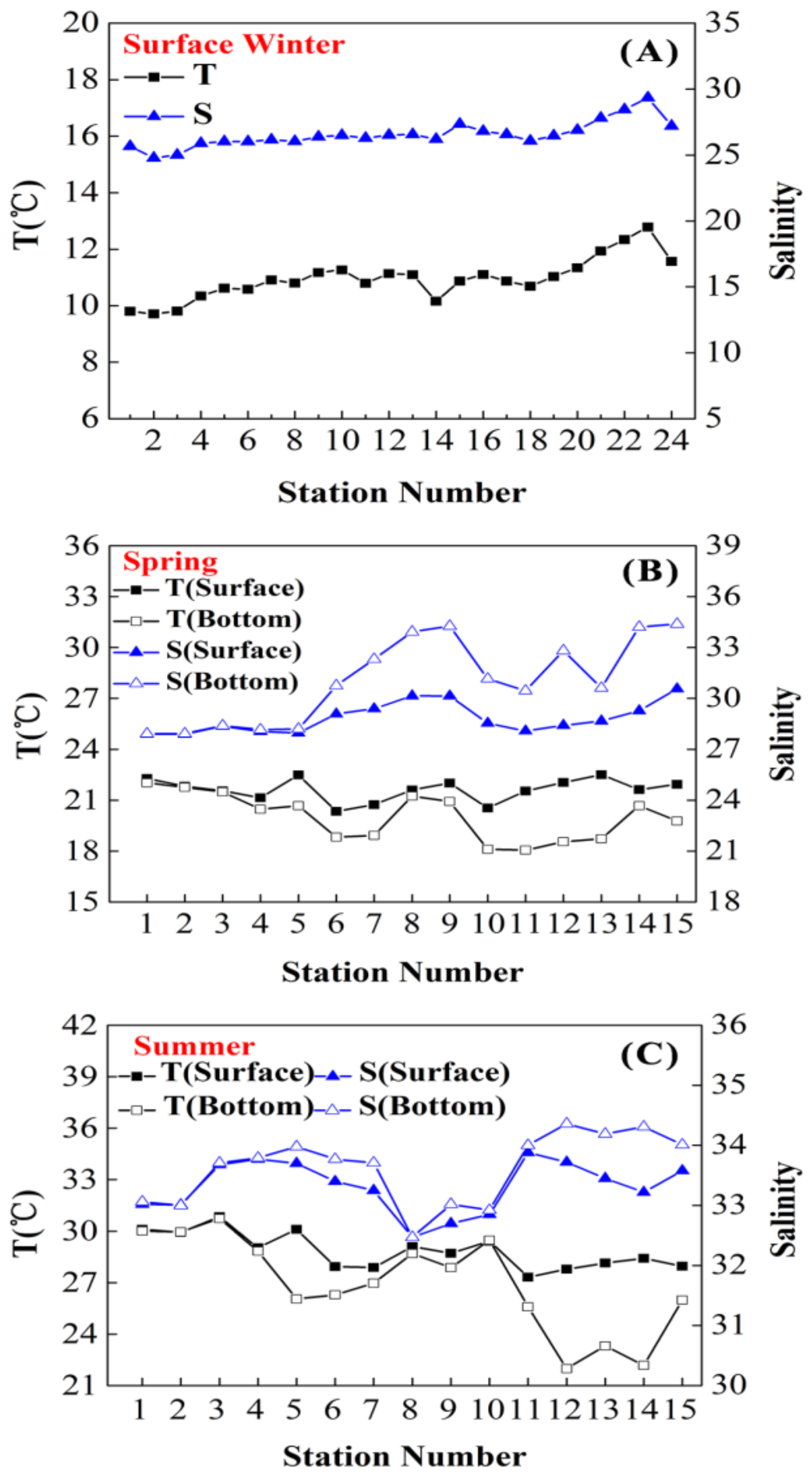
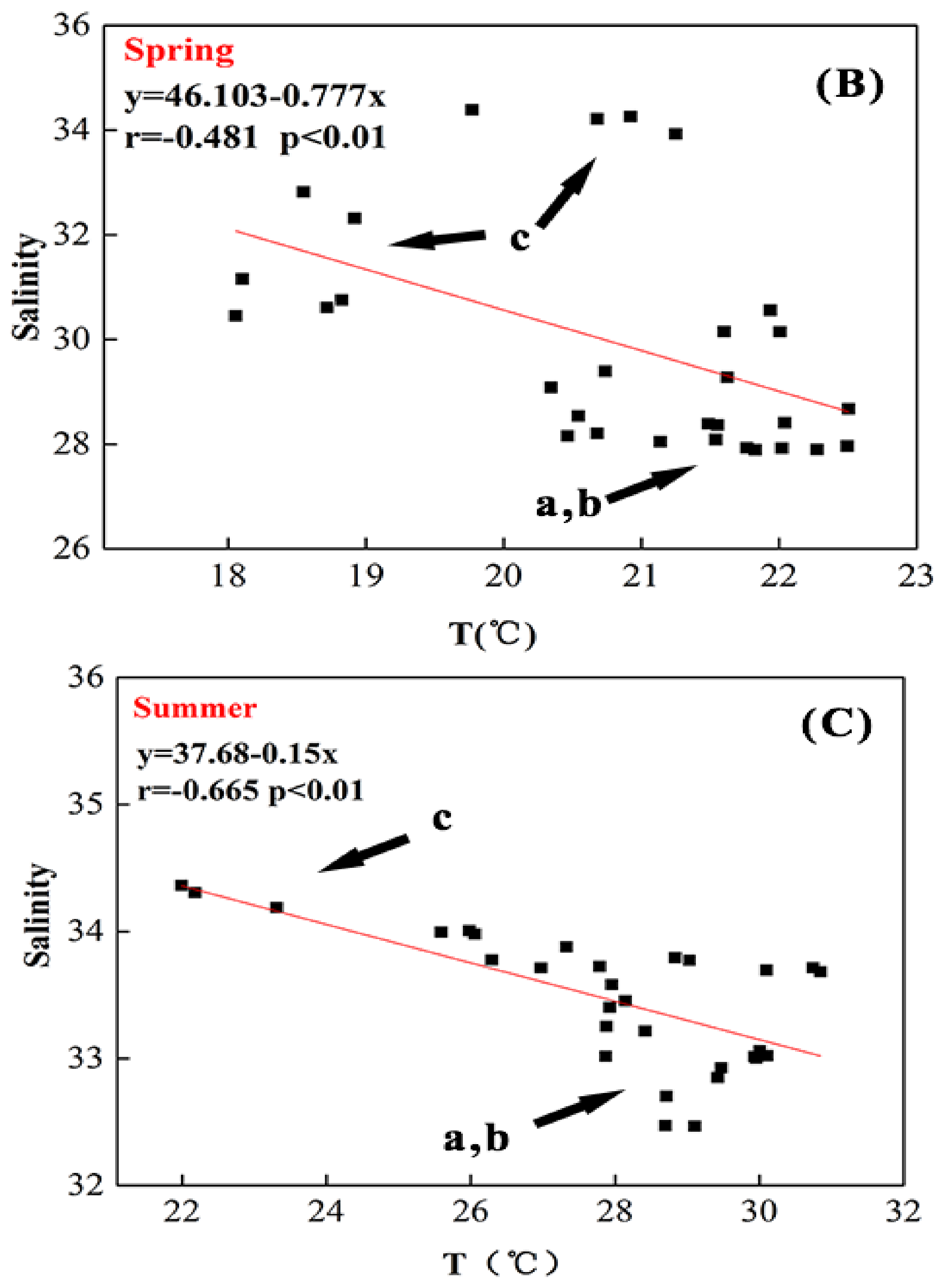
3.1. Temperature and Salinity
3.2. Concentrations of Nutrients
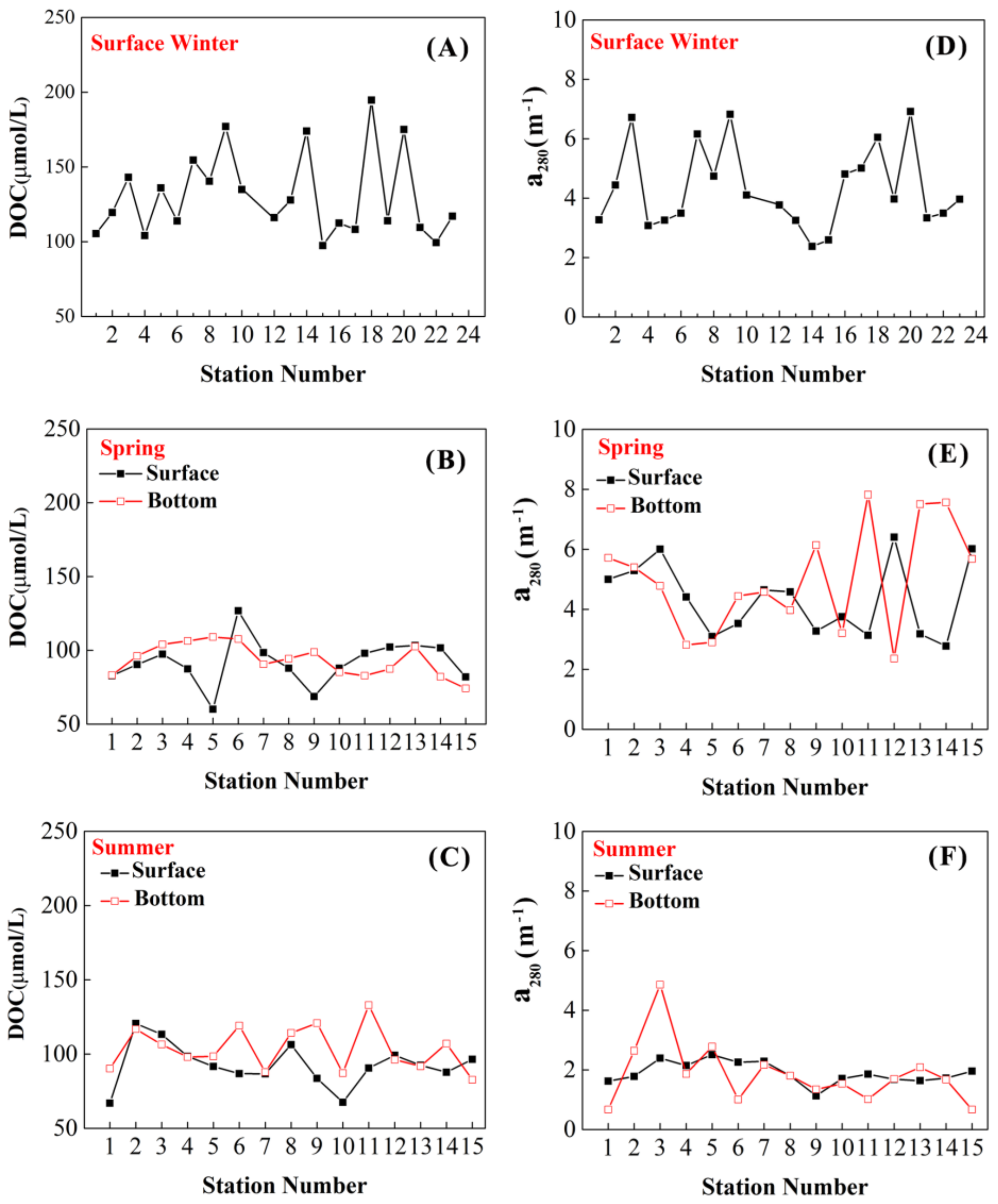
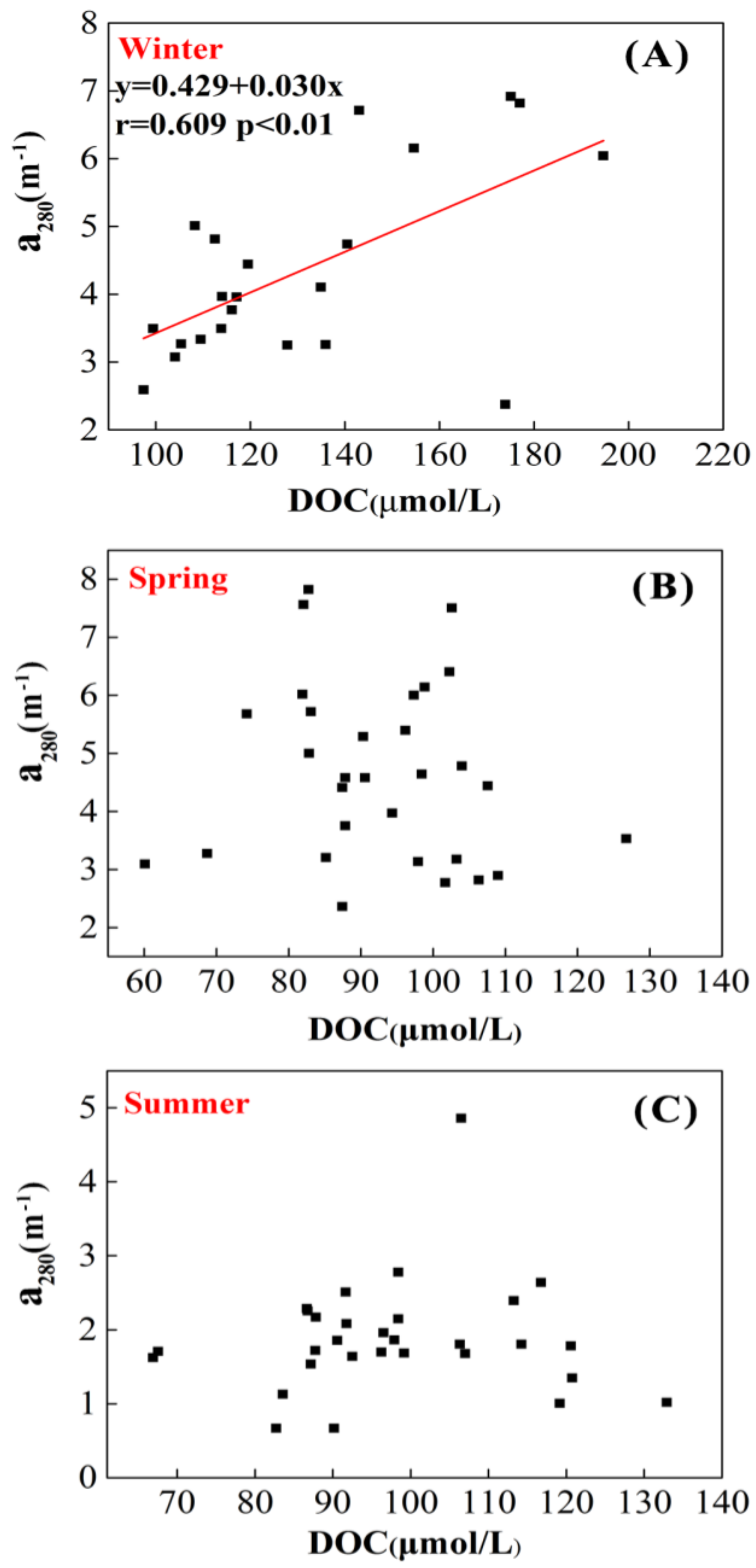
3.3. DOC and Absorption Coefficient
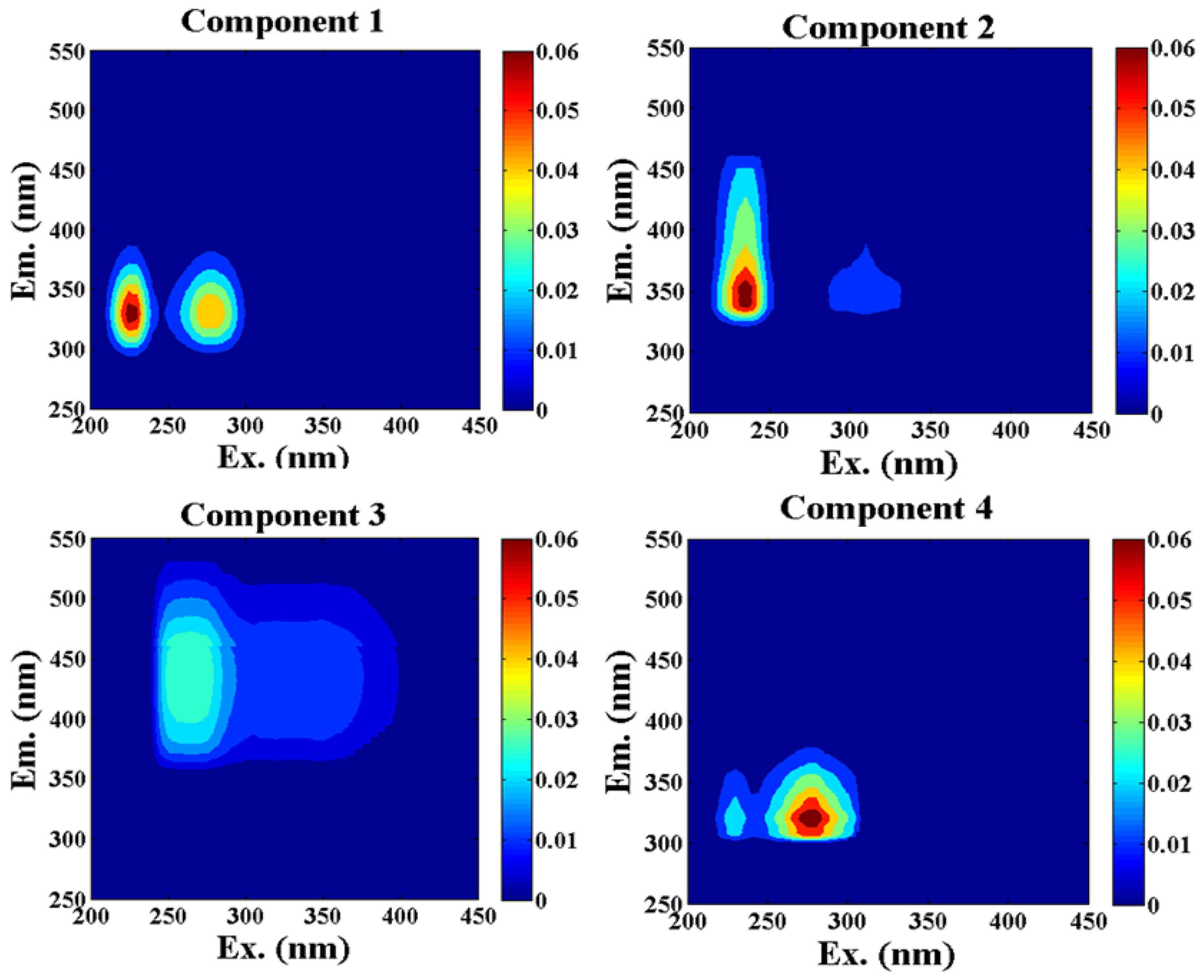
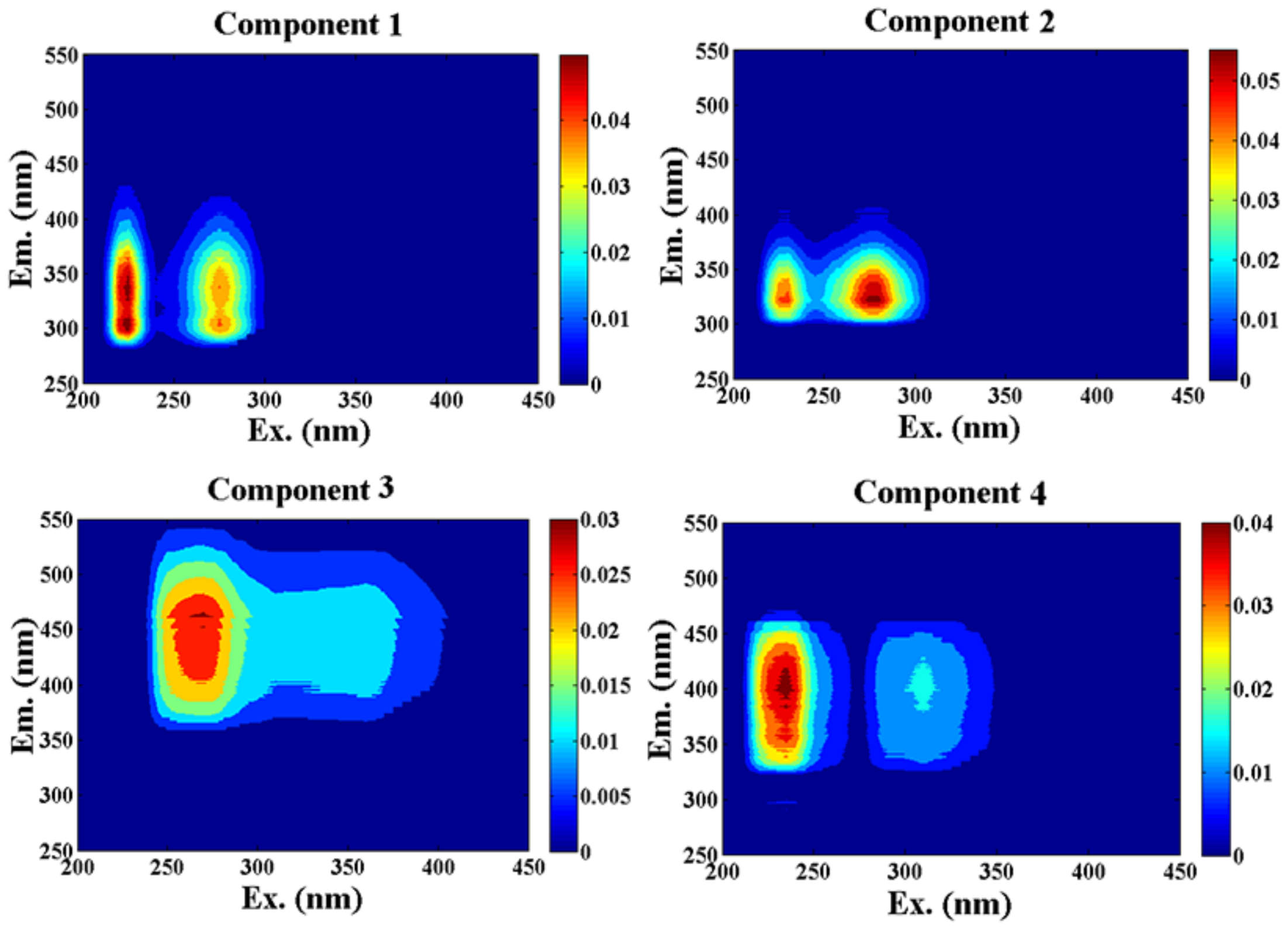
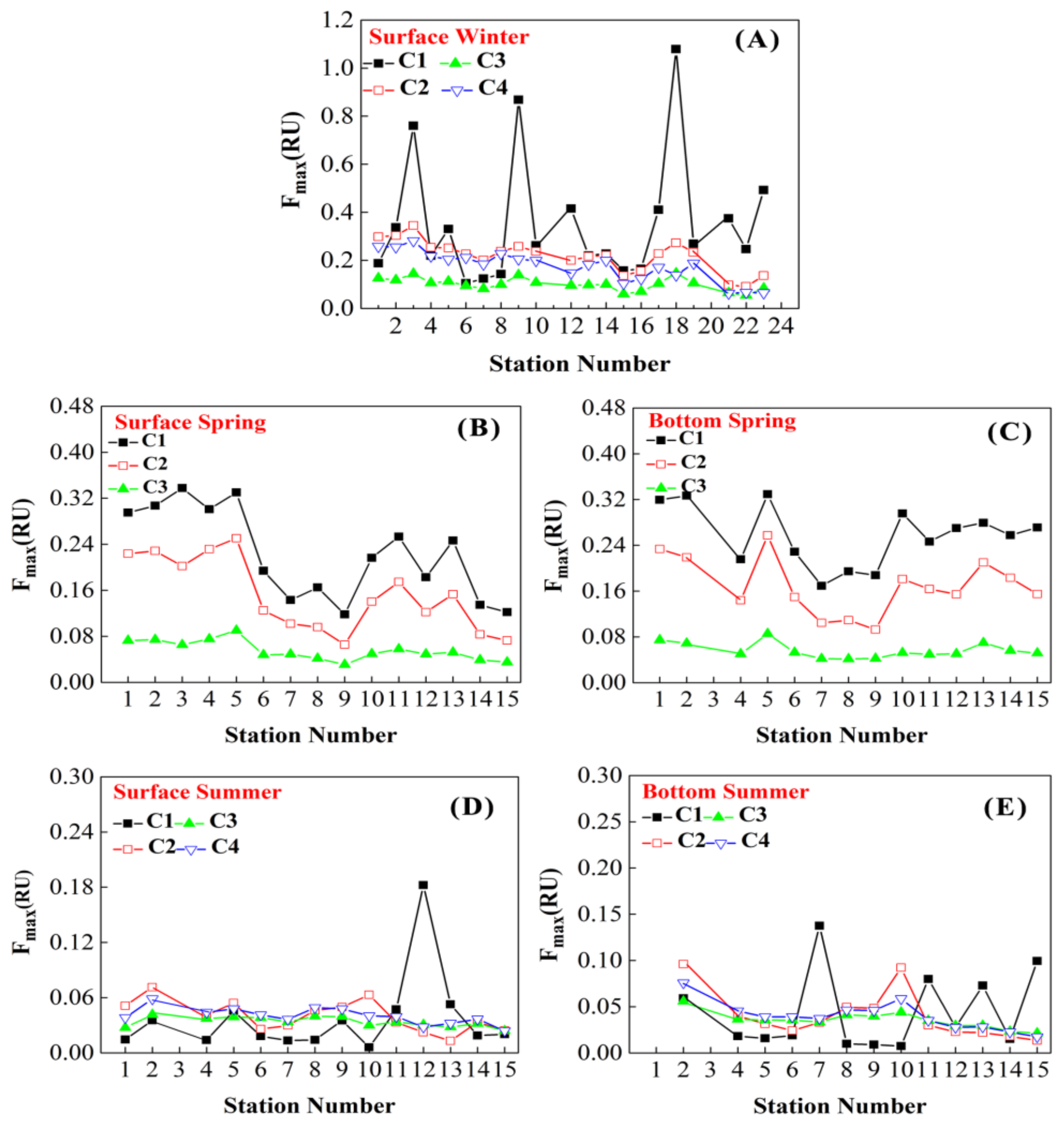
3.4. PARAFAC Components
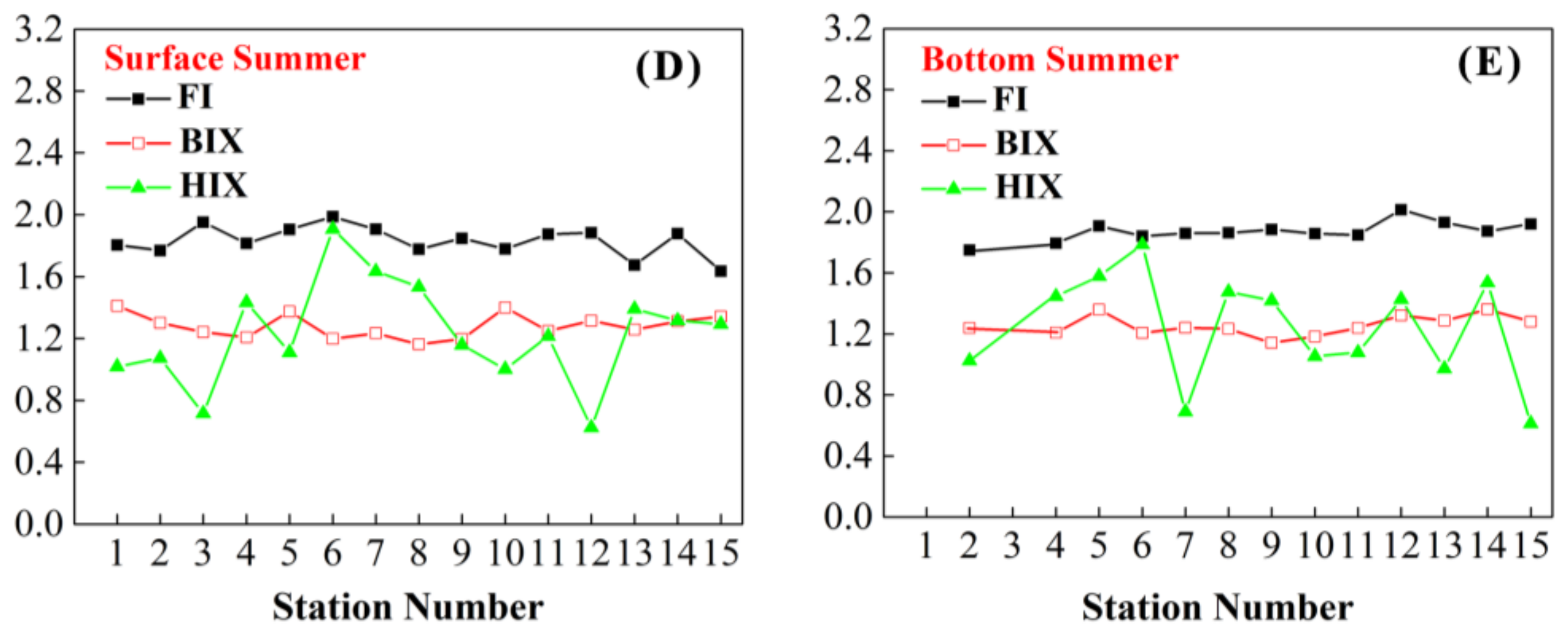
3.5. FI, BIX and HIX
4. Discussion
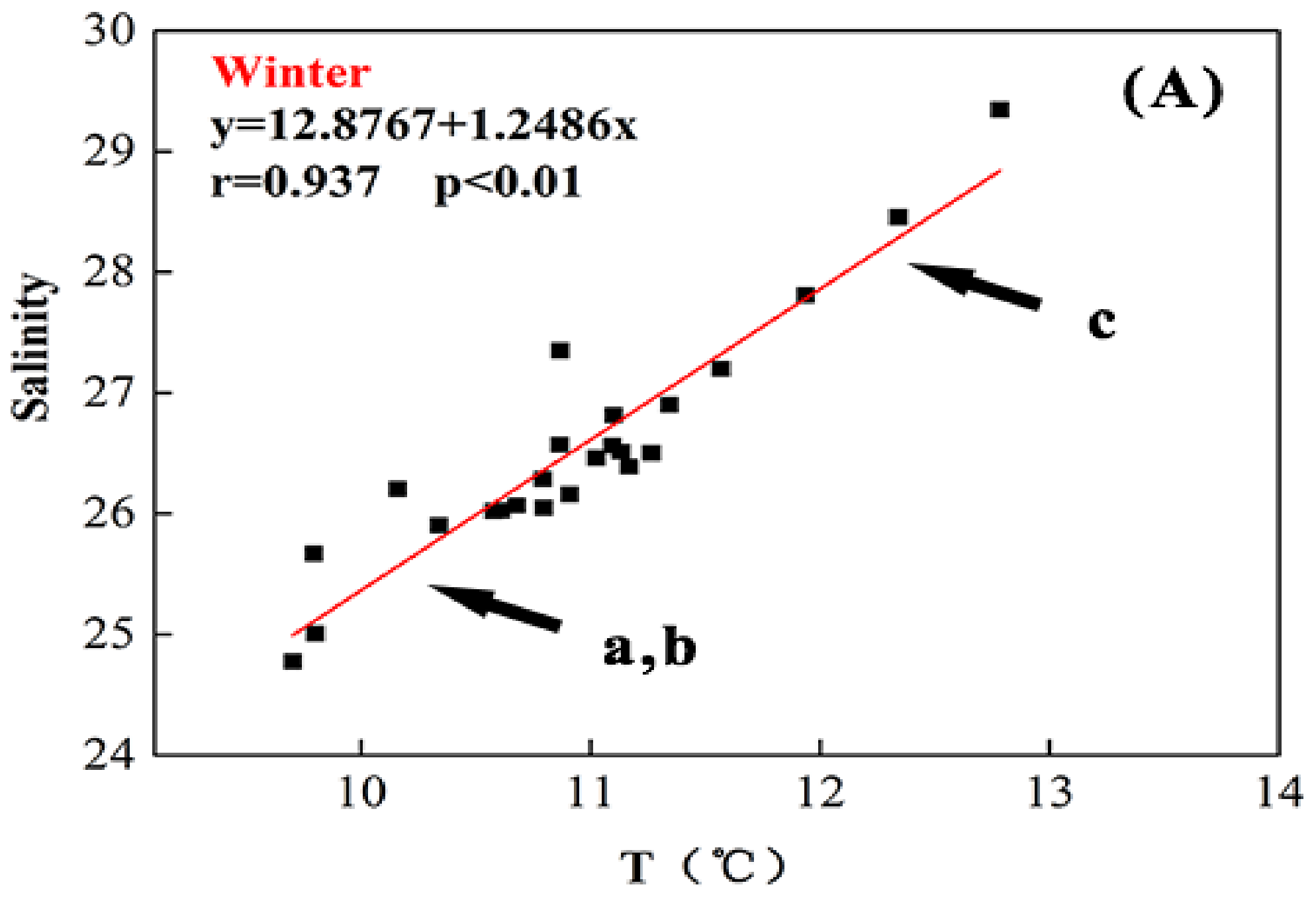
4.1. Seasonal Variations of Temperature and Salinity
4.2. Seasonal Variations of DOC and Absorption Coefficient
4.3. Analysis of Seasonal Changes in CDOM
4.4. Correlations between Concentration of Nutrients and DOM Parameters
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Yang, K.; Du, J.; Zhang, F.; Dong, Y.; Li, W. Chemical characterization of fractions of dissolved humic substances from a marginal sea—A case from the Southern Yellow Sea. Chin. J. Oceanol. Limnol. 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Maizel, A.C.; Remucal, C.K. The effect of advanced secondary municipal wastewater treatment on the molecular composition of dissolved organic matter. Water Res. 2017, 122, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Piccolo, A. Molecular characterization of dissolved organic matter (DOM): A critical review. Anal. Bioanal. Chem. 2013, 405, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, C.T.A.; Lui, H.-K.; Zhuang, W.-E.; Wang, B.-J. Effects of microbial transformation on dissolved organic matter in the east Taiwan Strait and implications for carbon and nutrient cycling. Estuar. Coast. Shelf Sci. 2016, 180, 59–68. [Google Scholar] [CrossRef]
- Lou, T.; Xie, H.; Chen, G.; Gagné, J.P. Effects of photodegradation of dissolved organic matter on the binding of benzo(a)pyrene. Chemosphere 2006, 64, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; He, X.; Xi, B.; Gao, R.; Li, D.; Zhang, H.; Cui, D.; Yuan, Z. Composition, Evolution, and Complexation of Dissolved Organic Matter with Heavy Metals in Landfills. Environ. Sci. 2017, 9, 3705–3712. [Google Scholar]
- Porcal, P.; Dillon, P.J.; Molot, L.A. Interaction of extrinsic chemical factors affecting photodegradation of dissolved organic matter in aquatic ecosystems. Photochem. Photobiol. Sci. 2014, 13, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.I. Global biogeochemical cycles: Progress and problems. Mar. Chem. 1992, 39, 67–93. [Google Scholar] [CrossRef]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Rix, L.; De Goeij, J.M.; Van Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M.S. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct. Ecol. 2017, 31, 778–789. [Google Scholar] [CrossRef]
- Casas-Ruiz, J.P.; Catalán, N.; Von Schiller, D.; Obrador, B.; Kothawala, D.N.; López, P.; Sabater, S.; Marcé, R. A tale of pipes and reactors: Controls on the in-stream dynamics of dissolved organic matter in rivers. Limnol. Oceanogr. 2017, 62, 85–94. [Google Scholar] [CrossRef]
- Su, R.; Bai, Y.; Zhang, C.; Shi, X. The assessment of the spatial and seasonal variability of chromophoric dissolved organic matter in the Southern Yellow Sea and the East China Sea. Mar. Pollut. Bull. 2015, 100, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, W.; Hong, H.; Wang, G. Non-conservative behaviors of chromophoric dissolved organic matter in a turbid estuary: Roles of multiple biogeochemical processes. Estuar. Coast. Shelf Sci. 2013, 133, 285–292. [Google Scholar] [CrossRef]
- Remucal, C.K.; Cory, R.M.; Sander, M.; McNeill, K. Low molecular weight components in an aquatic humic substance as characterized by membrane dialysis and Orbitrap mass spectrometry. Environ. Sci. Technol. 2012, 46, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Reemtsma, T. Determination of molecular formulas of natural organic matter molecules by (ultra-) high-resolution mass spectrometry. Status and needs. J. Chromatogr. A 2009, 1216, 3687–3701. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 649, 631–649. [Google Scholar] [CrossRef]
- Coble, P.G. Marine optical biogeochemistry: The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Mousavi, M.F.; Nemati, A. Kinetic spectrophotometric determination of trace amounts of nitrite by its reaction with molybdosilicic acid blue. Microchem. J. 2000, 65, 159–163. [Google Scholar] [CrossRef]
- Boyd, T.J.; Osburn, C.L. Changes in CDOM fluorescence from allochthonous and autochthonous sources during tidal mixing and bacterial degradation in two coastal estuaries. Mar. Chem. 2004, 89, 189–210. [Google Scholar] [CrossRef]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation–emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhuang, W.E.; Chen, C.T.A.; Wang, B.J.; Kuo, F.W. Unveiling the transformation and bioavailability of dissolved organic matter in contrasting hydrothermal vents using fluorescence EEM-PARAFAC. Water Res. 2017, 111, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Jeppesen, E.; Murphy, K.R.; Shi, K.; Liu, M.; Liu, X.; Zhu, G. Inflow rate-driven changes in the composition and dynamics of chromophoric dissolved organic matter in a large drinking water lake. Water Res. 2016, 100, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hong, H.; Chen, C.T.A.; Guo, W.; Huang, T. Chromophoric dissolved organic matter in the estuaries of populated and mountainous Taiwan. Mar. Chem. 2013, 157, 12–23. [Google Scholar] [CrossRef]
- Guo, W.; Huang, J.P.; Hong, H.; Xu, J.; Deng, X. Resolving excitation emission matrix spectroscopy of estuarine CDOM with parallel factor analysis and its application in organic pollution monitoring. Huanjing Kexue/Environ. Sci. 2010, 31, 1419–1427. [Google Scholar]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Waite, T.D.; Ruiz, G.M. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar. Chem. 2008, 108, 40–58. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Tranvik, L.; Kronberg, L.; Slätis, T.; Martinsen, W. Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Mar. Chem. 2007, 104, 227–240. [Google Scholar] [CrossRef]
- Qiao, S.; Yang, Z.; Liu, J.; Sun, X.; Xiang, R.; Shi, X.; Fan, D.; Saito, Y. Records of late-Holocene East Asian winter monsoon in the East China Sea: Key grain-size component of quartz versus bulk sediments. Quat. Int. 2011, 230, 106–114. [Google Scholar] [CrossRef]
- Zhang, G.L.; Zhang, J.; Kang, Y.B.; Liu, S.M. Distributions and fluxes of methane in the East China Sea and the Yellow Sea in spring. J. Geophys. Res. Oceans 2004, 109, 1–10. [Google Scholar] [CrossRef]
- Gao, X.; Song, J. Main Geochemical Characteristics and Key Biogeochemical Carbon Processes in the East China Sea. J. Coast. Res. 2006, 22, 1330–1339. [Google Scholar] [CrossRef]
- Chen, C.T.A. Distributions of Nutrients in the East China Sea and the South China Sea Connection. J. Oceanogr. 2008, 64, 737–751. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, C.; Wang, P.; Hou, J.; Ao, Y. Absorption and fluorescence characteristics of chromophoric dissolved organic matter in the Yangtze Estuary. Environ. Sci. Pollut. Res. 2014, 21, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fan, D.; Li, D.; Cai, J. Fluorescence characteristics of chromophoric dissolved organic matter in shallow water along the Zhejiang coasts, southeast China. Mar. Environ. Res. 2010, 69, 187–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.; Chen, X.; Deng, H.; Du, Y.; Jin, H.Y. Absorption features of chromophoric dissolved organic matter (CDOM) and tracing implication for dissolved organic carbon (DOC) in Changjiang Estuary, China. Biogeosci. Discuss. 2013, 10, 12217–12250. [Google Scholar] [CrossRef]
- Gan, S.; Wu, Y.; Zhang, J. Bioavailability of dissolved organic carbon linked with the regional carbon cycle in the East China Sea. Deep Res. Part II Top. Stud. Oceanogr. 2016, 124, 19–28. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; Volume 167, p. 185.
- Dai, M.; Wang, L.; Guo, X.; Zhai, W.; Li, Q.; He, B.; Kao, S.J. Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: The Pearl River Estuary, China. Biogeosciences 2008, 5, 1227–1244. [Google Scholar] [CrossRef]
- Arthur Chen, C.T.; Wang, S.L. A salinity front in the southern East China Sea separating the Chinese coastal and Taiwan Strait waters from Kuroshio waters. Cont. Shelf Res. 2006, 26, 1636–1653. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Bai, Y.; Su, R.; Han, X.; Zhang, C.; Shi, X. Investigation of seasonal variability of CDOM fluorescence in the southern changjiang river estuary by EEM-PARAFAC. Acta Oceanol. Sin. 2015, 34, 1–12. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Shen, Z.; Chen, J.; Feng, C. Characterization and spacial distribution variability of chromophoric dissolved organic matter (CDOM) in the Yangtze Estuary. Chemosphere 2014, 95, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jeppesen, E.; Zhang, Y.; Shi, K.; Liu, X.; Zhu, G. Dissolved organic matter fluorescence at wavelength 275/342 nm as a key indicator for detection of point-source contamination in a large Chinese drinking water lake. Chemosphere 2016, 144, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, C.; Yang, L.; Chen, J.; Chen, C.T.A.; Wang, B.J. Diurnal variations of dissolved organic matter in the hydrothermal system of Green Island, Taiwan. Mar. Chem. 2017, 195, 61–69. [Google Scholar] [CrossRef]
- Yang, L.; Guo, W.; Chen, N.; Hong, H.; Huang, J.; Xu, J.; Huang, S. Influence of a summer storm event on the flux and composition of dissolved organic matter in a subtropical river, China. Appl. Geochem. 2013, 28, 164–171. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Durako, M.J.; Young, H.; Kahn, A.E.; Cooper, W.J.; Gonsior, M. Characterization of dissolved organic matter fluorescence in the South Atlantic Bight with use of PARAFAC model: Interannual variability. Mar. Chem. 2009, 113, 182–196. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, A.; Guo, W.; Yang, L.; Chen, D. Optical properties of estuarine dissolved organic matter isolated using cross-flow ultrafiltration. Acta Oceanol. Sin. 2014, 33, 22–29. [Google Scholar] [CrossRef]
- Chen, C.T.A. Chemical and physical fronts in the Bohai, Yellow and East China seas. J. Mar. Syst. 2009, 78, 394–410. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, D.L.; Bai, Y. Estimating dissolved organic carbon inventories in the East China Sea using remote-sensing data. J. Geophys. Res. Oceans 2014, 119, 6557–6574. [Google Scholar] [CrossRef]
- Chen, C.T.A. Rare northward flow in the Taiwan Strait in winter: A note. Cont. Shelf Res. 2003, 23, 387–391. [Google Scholar] [CrossRef]
- Lin, H.; Guo, W.; Hu, M.; Cai, L.; Ji, W. Spatial and temporal variability of colored dissolved organic matter absorption properties in the Taiwan Strait. ACTA Ocean. 2012, 31, 98–106. [Google Scholar] [CrossRef][Green Version]
- Yamashita, Y.; Maie, N.; Briceño, H.; Jaffé, R. Optical characterization of dissolved organic matter in tropical rivers of the Guayana Shield, Venezuela. J. Geophys. Res. Biogeosci. 2010, 115, 1–15. [Google Scholar] [CrossRef]
- Hung, J.J.; Chen, C.H.; Gong, G.C.; Sheu, D.D.; Shiah, F.K. Distributions, stoichiometric patterns and cross-shelf exports of dissolved organic matter in the East China Sea. Deep Res. Part II 2003, 50, 1127–1145. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Liu, L.; Zhang, P.; Leng, W. Sources, behaviors and degradation of dissolved organic matter in the East China Sea. J. Mar. Syst. 2016, 155, 84–97. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Pan, J. Distributions of colored dissolved organic matter and dissolved organic carbon in the Pearl River Estuary, China. Cont. Shelf Res. 2004, 24, 1845–1856. [Google Scholar] [CrossRef]
- Vodacek, A.; Blough, N.V.; DeGrandpre, M.D.; DeGrandpre, M.D.; Nelson, R.K. Seasonal variation of CDOM and DOC in the Middle Atlantic Bight: Terrestrial inputs and photooxidation. Limnol. Oceanogr. 1997, 42, 674–686. [Google Scholar] [CrossRef]
- Cao, F.; Medeiros, P.M.; Miller, W.L. Optical characterization of dissolved organic matter in the Amazon River plume and the Adjacent Ocean: Examining the relative role of mixing, photochemistry, and microbial alterations. Mar. Chem. 2016, 186, 178–188. [Google Scholar] [CrossRef]
- Yu, X.; Shen, F.; Liu, Y. Light absorption properties of CDOM in the Changjiang (Yangtze) estuarine and coastal waters: An alternative approach for DOC estimation. Estuar. Coast. Shelf Sci. 2016, 181, 302–311. [Google Scholar] [CrossRef]
- Pitta, E.; Zeri, C.; Tzortziou, M.; Mousdis, G.; Scoullos, M. Seasonal variations in dissolved organic matter composition using absorbance and fluorescence spectroscopy in the Dardanelles Straits—North Aegean Sea mixing zone. Cont. Shelf Res. 2017, 149, 82–95. [Google Scholar] [CrossRef]


| Season | N-(NO2− + NO3−) (μmol/L) | P-(PO43−) (μmol/L) | Si-(SiO32−) (μmol/L) | |
|---|---|---|---|---|
| Winter | Surface | 26.83–59.00 | 0.98–2.04 | 21.40–39.87 |
| Spring | Surface | 4.67–80.04 | 0.04–3.12 | 7.10–53.76 |
| Bottom | 3.77–41.35 | 0.05–1.20 | 6.68–29.42 | |
| Summer | Surface | 6.33–16.11 | 0.55–1.08 | 10.90–24.42 |
| Bottom | 5.28–16.15 | 0.43–1.06 | 6.24–24.19 |
| Season | Components | Ex/Em Maxima (nm) | Traditional Peak [17,18] | Comparison with Other Studies Using PARAFAC | Probable Source |
|---|---|---|---|---|---|
| Winter | C1 | 225(275)/330 | T | C2, 230(280)/340 [43] | Tryptophan-like |
| C2 | 235/350 | T | C2, 275(230)/342 [44] | Tryptophan-like | |
| C3 | 265/441 | A | C2, 255/440 [45] | Humic-like | |
| C4 | 280/320 | T | C6, 280/324 [23] | Tryptophan-like | |
| Summer | C1 | 225(275)/337 | T | C2, 230(280)/340 [43] | Tryptophan-like |
| C2 | 275(230)/322 | T | C2, 275(230)/342 [44] | Tryptophan-like | |
| C3 | 270/461 | A | C3, 260(395)/458 [46] | Humic-like | |
| C4 | 235(310)/399 | A and M | C5, 315/412 [22] | Humic-like | |
| C5, 235(305)/394 [23] | |||||
| Spring | C1 | 275/320 | T | C6, 280/324 [23] | Tyrosine-like |
| C2 | 240(310)/350 | T and M | C6, 250(290)/356 [47] | Tryptophan-like and humic-like | |
| C3 | 265/430 | A | C2, 265(360)/458 [48] | Humic-like |
| Season | Nutrient | C1 | C2 | C3 | C4 |
|---|---|---|---|---|---|
| Winter | N-(NO3− + NO2−) | 0.135 | 0.679 ** | 0.560 ** | 0.631 ** |
| P-(PO43−) | 0.062 | 0.841 ** | 0.643 ** | 0.845 ** | |
| Si-(SiO32−) | 0.030 | 0.313 | 0.256 | 0.303 | |
| Summer | N-(NO3− + NO2−) | −0.335 | 0.817 ** | 0.661 ** | 0.792 ** |
| P-(PO43−) | −0.227 | 0.488 ** | 0.620 ** | 0.652 ** | |
| Si-(SiO32−) | −0.34 | 0.786 ** | 0.638 ** | 0.779 ** | |
| Spring | N-(NO3− + NO2−) | 0.673 ** | 0.757 ** | 0.731 ** | |
| P-(PO43−) | 0.554 ** | 0.627 ** | 0.611 ** | ||
| Si-(SiO32−) | 0.669 ** | 0.729 ** | 0.668 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yan, Z.; Chen, J.; Jin, H.; Chen, C.-T.A.; Liu, M.; Yan, Z.; Ji, Z. Seasonal Variations of Dissolved Organic Matter in the East China Sea Using EEM-PARAFAC and Implications for Carbon and Nutrient Cycling. Sustainability 2018, 10, 1444. https://doi.org/10.3390/su10051444
Zheng H, Yan Z, Chen J, Jin H, Chen C-TA, Liu M, Yan Z, Ji Z. Seasonal Variations of Dissolved Organic Matter in the East China Sea Using EEM-PARAFAC and Implications for Carbon and Nutrient Cycling. Sustainability. 2018; 10(5):1444. https://doi.org/10.3390/su10051444
Chicago/Turabian StyleZheng, Hao, Zhishen Yan, Jianfang Chen, Haiyan Jin, Chen-Tung Arthur Chen, Maokun Liu, Zupeng Yan, and Zhongqiang Ji. 2018. "Seasonal Variations of Dissolved Organic Matter in the East China Sea Using EEM-PARAFAC and Implications for Carbon and Nutrient Cycling" Sustainability 10, no. 5: 1444. https://doi.org/10.3390/su10051444
APA StyleZheng, H., Yan, Z., Chen, J., Jin, H., Chen, C.-T. A., Liu, M., Yan, Z., & Ji, Z. (2018). Seasonal Variations of Dissolved Organic Matter in the East China Sea Using EEM-PARAFAC and Implications for Carbon and Nutrient Cycling. Sustainability, 10(5), 1444. https://doi.org/10.3390/su10051444





