Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes
Abstract
1. Introduction
2. Materials and Methods
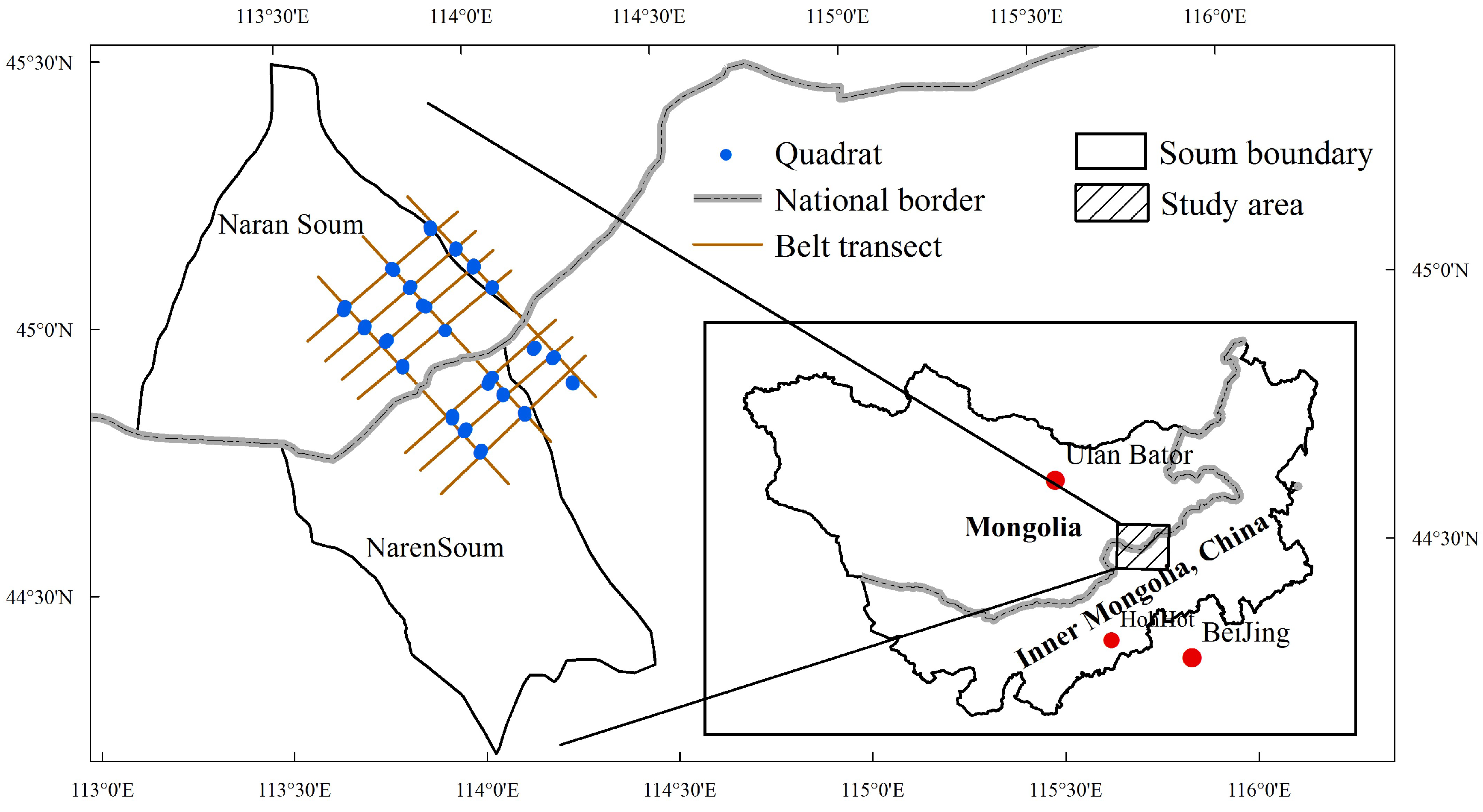
2.1. Study Area
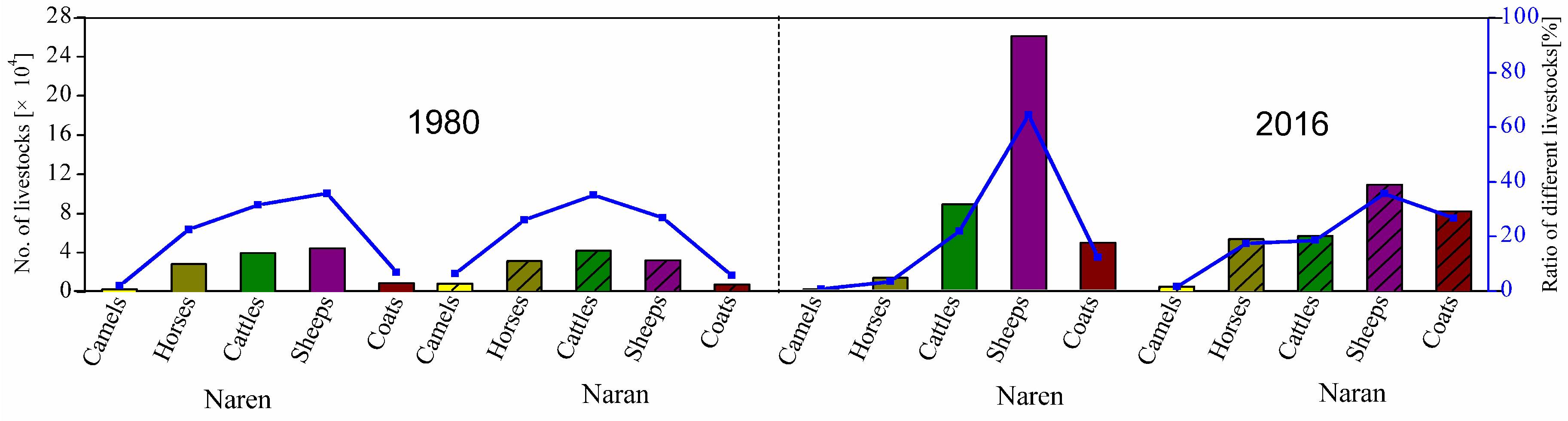
2.2. Evolution of Grazing Systems in Mongolia and Inner Mongolia
2.3. Sampling and Measurement
2.3.1. Field Sampling and Data Collection
2.3.2. Remote Sensing Data Processing
2.4. Analysis Methods
2.4.1. Calculation of Dominance of Major Species
2.4.2. Calculation of SAVI
2.4.3. Correlation between Aboveground Biomass and SAVI
2.5. Statistical Analysis
3. Results
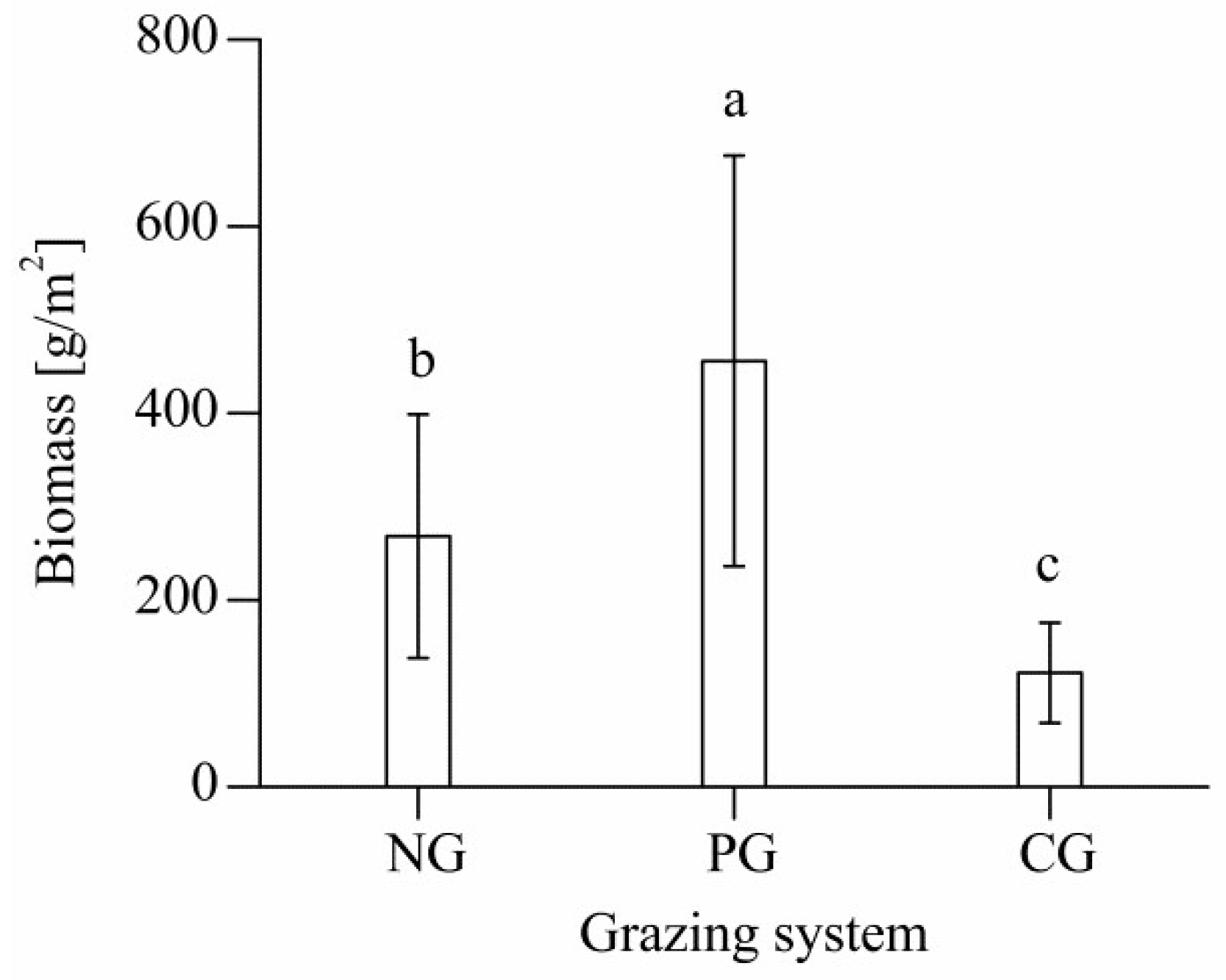
3.1. Aboveground Biomass of Different Grazing Sites
3.2. Dominant Plant Species at Different Grazing Sites
3.2.1. Dominant Species in Non-Degraded Areas
3.2.2. Dominant Species in Lightly Degraded Areas
3.2.3. Dominant Species in Moderately Degraded Areas
3.2.4. Dominant Species in Heavily Degraded Areas
3.3. Changes in SAVI under Different Grazing Systems
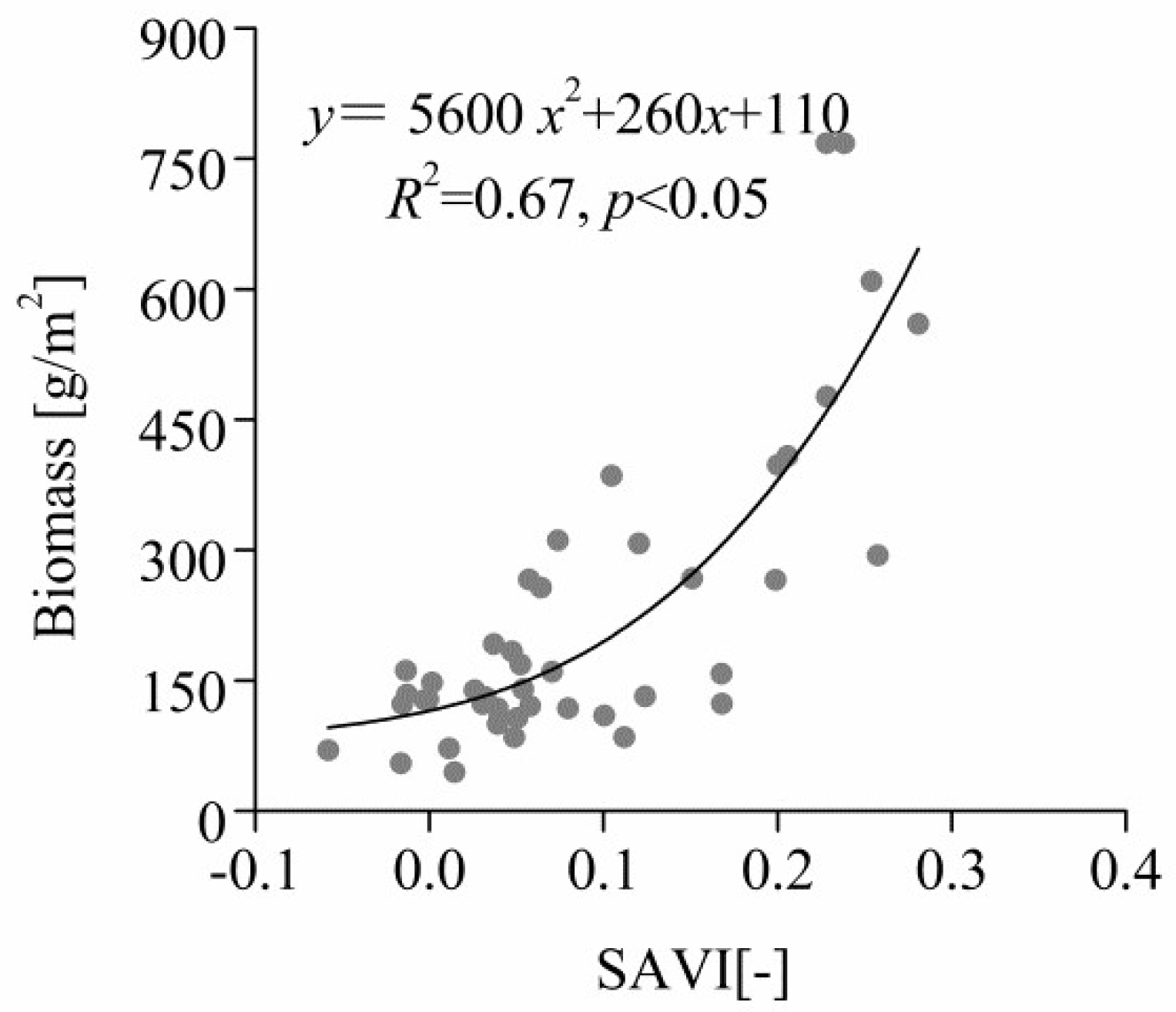
3.4. Correlation between Total Aboveground Biomass and SAVI in Quadrats
4. Discussion
4.1. Effects of Different Grazing Systems on Total Aboveground Biomass in Plant Communities
4.2. Effects of Different Grazing Systems on the Community Dominance of Major Species
4.3. Effects of Different Grazing Systems on Community SAVI Changes
4.4. Correlation between Total Aboveground Biomass and SAVI in Quadrats
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qin, F.Y.; Jia, G.S.; Yang, J.; Na, Y.T.; Hou, M.T.; Narenmandula. Spatiotemporal variability of precipitation during 1961–2014 across the Mongolian Plateau. J. Mt. Sci. 2018, 15, 992–1005. [Google Scholar] [CrossRef]
- Na, Y.T.; Bao, S.; Hashimoto, K.; Mccarthy, C.; Hoshino, B. The Effects of Grazing Systems on Plant Communities in Steppe Lands—A Case Study from Mongolia’s Pastoralists and Inner Mongolian Settlement Areas. Land 2018, 7, 10. [Google Scholar] [CrossRef]
- Fang, J.; Bai, Y.; Wu, J. Towards a better understanding of landscape patterns and ecosystem processes of the Mongolian Plateau. Landsc. Ecol. 2015, 30, 1573–1578. [Google Scholar] [CrossRef]
- Jun, L.W.; Ali, S.H.; Zhang, Q. Property rights and grassland degradation: A study of the Xilingol pasture, Inner Mongolia, China. J. Environ. Manag. 2007, 85, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Batoyun, T.; Shinoda, M.; Cheng, Y.; Purevdorj, Y. Effects of grazing and precipitation variability on vegetation dynamics in a Mongolian dry steppe. J. Plant Ecol. 2016, 9, 508–519. [Google Scholar] [CrossRef]
- Li, W.; Huntsinger, L. China’s Grassland Contract Policy and its Impacts on Herder Ability to Benefit in Inner Mongolia: Tragic Feedbacks. Ecol. Soc. 2011, 16, 1–13. [Google Scholar] [CrossRef]
- Zhang, M.D.A.; Borjigin, E.; Zhang, H. Mongolian nomadic culture and ecological culture: On the ecological reconstruction in the agro-pastoral mosaic zone in Northern China. Ecol. Econ. 2007, 62, 19–26. [Google Scholar] [CrossRef]
- Na, Y.; Wulan, T.; Qin, F. Dynamic monitoring of Horqin sandy land desertification based on 3S techniques—A case study in Horqin Left Wing Banner. J. Arid Land Resour. Environ. 2010, 24, 50–54. (In Chinese) [Google Scholar]
- Kang, S.; Lee, G.; Togtokh, C.; Jang, K. Characterizing regional precipitation-driven lake area change in Mongolia. J. Arid Land 2015, 7, 146–158. [Google Scholar] [CrossRef]
- Jiang, L.G.; Yao, Z.J.; Huang, H.Q. Climate variability and change on the Mongolian Plateau: Historical variation and future predictions. Clim. Res. 2016, 67, 1–14. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Qi, X.; Quan, S.; Yu, J.; Mei, S. Studies on matter dynamics and plant compensatory growth under differrent grazing systems on meadow steppe. Grassl. China 2004, 26, 26–31. (In Chinese) [Google Scholar]
- Han, G.; Wei, Z. Experiment on rotational grazing system in stipa breviflora desert steppe. J. Inn. Mong. Inst. Agric. Anim. Husb. 2001, 22, 60–67. (In Chinese) [Google Scholar]
- John, R.; Chen, J.; Kim, Y.; Ou-Yang, Z.T.; Xiao, J.; Park, H.; Shao, C.; Zhang, Y.; Amarjargal, A.; Batkhshig, O. Differentiating anthropogenic modification and precipitation-driven change on vegetation productivity on the Mongolian Plateau. Landsc. Ecol. 2016, 31, 547–566. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Xin, X.; Ding, Y.; Hou, X.; Sarula; Li, X.; Chen, H.; Yin, Y.; Hu, J. Response of the annual biomass production of a typical steppe plant community to precipitation fluctuations. Rangel. J. 2014, 36, 527–534. [Google Scholar] [CrossRef]
- Hai, S. Study on the Relationship between Human and Land in Inner Mongolia Pasture, 1st ed.; Inner Mongolian Education Press: Hohhot, China, 2013; pp. 131–143. [Google Scholar]
- Fujita, N.; Amartuvshin, N.; Yamada, Y.; Matsui, K.; Sakai, S.; Yamamura, N. Positive and negative effects of livestock grazing on plant diversity of Mongolian nomadic pasturelands along a slope with soil moisture gradient. Grassl. Sci. 2009, 55, 126–134. [Google Scholar] [CrossRef]
- Zhang, J.T. Sampling Methods and Community Characterization; Sciences Publisher: Beijing, China, 2011; pp. 20–35. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ulrich, W.; Maestre, F.T. Randomization tests for quantifying species importance to ecosystem function. Methods Ecol. Evol. 2011, 2, 634–642. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, W.; Hao, D.Y.; Liang, C.Z. Probes on the Degeneration and Recovery Succession Mechanisms of Inner Mongolia Steppe. J. Arid Land Resour. Environ. 2002, 16, 84–91. (In Chinese) [Google Scholar]
- Wang, W.; Liu, Z.L.; Hao, D.Y.; Liang, C.Z. Research on the restoring succession of the degenerated grassland in Inner Mongolia—I. Basic characteristics and driving force for restoration of the degenerated grassland. Acta Phytoecol. Sin. 1996, 20, 449–459. (In Chinese) [Google Scholar]
- Wang, R.Z. Photosynthetic pathways, life forms, and reproductive types for forage species along the desertification gradient on hunshandake desert, north china. Photosynthetica 2002, 40, 321–329. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K.; Chapman, P.L.; Kazempour, M.K. Effects of grazing, topography, and precipitation on the structure of a semiarid grassland. Vegetatio 1989, 80, 11–23. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Q.J.; Jing, Z.C.; Wang, W.Y. The effects of protective enclosure on vegetation diversity, and productivity of degraded alpine Kobresia meadow (Qinghai-Tibetan Plateau). Pol. J. Ecol. 2009, 57, 495–502. [Google Scholar]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative Effects of Grazing on Vegetation and Soils Over a Global Range of Environments. Ecol. Monogr. 1993, 63, 328–366. [Google Scholar] [CrossRef]
- Metera, E.; Sakowski, T.; Słoniewski, K.; Romanowicz, B. Grazing as a tool to maintain biodiversity of grassland—A review. Anim. Sci. Pap. Rep. 2010, 28, 315–334. [Google Scholar]
- Li, X.U.; Gao, Q.; Wang, Y. Species richness within a six-year slope exclosure in a temperate grassland and its relationship with aboveground biomass. Ecol. Environ. Sci. 2014, 23, 398–405. (In Chinese) [Google Scholar]
- Casasus, I.; Bernues, A.; Sanz, A.; Villalba, D.; Riedel, J.L.; Revilla, R. Vegetation dynamics in Mediterranean forest pastures as affected by beef cattle grazing. Agr. Ecosyst. Environ. 2007, 121, 365–370. [Google Scholar] [CrossRef]
- Oesterheld, M.; Mcnaughton, S.J. Interactive Effect of Flooding and Grazing on the Growth of Serengeti Grasses. Oecologia 1991, 88, 153–156. [Google Scholar] [CrossRef]
- Bai, Y.; Li, D.; Xu, Z.; Wei, Z. Growth and reproduction of Stipa krylovii population on a grazing gradient. Acta Ecol. Sin. 1999, 19, 479–484. (In Chinese) [Google Scholar]
- Martin, S.C. Responses of semidesert grasses to seasonal rest. J. Range Manag. 1973, 26, 165–170. [Google Scholar] [CrossRef]
- Holechek, J.L.; Hilton, G.; Francisco, M.; Dee, G. Grazing studies: What we’ve learned. Rangelands 1999, 21, 12–16. [Google Scholar]
- Li, C.L.; Hao, X.Y.; Zhao, M.L.; Han, G.D.; Willms, W.D. Influence of historic sheep grazing on vegetation and soil properties of a Desert Steppe in Inner Mongolia. Agr. Ecosyst. Environ. 2008, 128, 109–116. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Taylor, C.A.J.; Brooks, T.D.; Garza, N.E. Effects of short duration and high-intensity, low-frequency grazing systems on forage production and composition. J. Range Manag. 1993, 46, 118–121. [Google Scholar] [CrossRef]
- Hyder, D.N.; Sawyer, W.A. Rotation-Deferred Grazing as Compared to Season-Long Grazing on Sagebrush-Bunchgrass Ranges in Oregon. J. Range Manag. 1951, 4, 30–34. [Google Scholar] [CrossRef]
- Carter, J.; Catlin, J.C.; Hurwitz, N.; Jones, A.L.; Ratner, J. Upland Water and Deferred Rotation Effects on Cattle Use in Riparian and Upland Areas. Rangelands 2017, 39, 112–118. [Google Scholar] [CrossRef]
- Schönbach, P.; Wan, H.; Schiborra, A.; Gierus, M.; Bai, Y.; Müller, K.; Glindemann, T.; Wang, C.; Susenbeth, A.; Taube, F. Short-term management and stocking rate effects of grazing sheep on herbage quality and productivity of Inner Mongolia steppe. Crop Pasture Sci. 2009, 60, 963–974. [Google Scholar] [CrossRef]
- Bailey, D.W.; Thomas, M.G.; Walker, J.W.; Witmore, B.K.; Tolleson, D. Effect of Previous Experience on Grazing Patterns and Diet Selection of Brangus Cows in the Chihuahuan Desert. Rangel. Ecol. Manag. 2010, 63, 223–232. [Google Scholar] [CrossRef]
- Peterson, D.; Brownlee, M.; Kelley, T. Stocking Density Affects Diet Selection. Rangelands 2013, 35, 62–66. [Google Scholar] [CrossRef]
- Angell, D.L.; Mcclaran, M.P. Long-term influences of livestock management and a non-native grass on grass dynamics in the Desert Grassland. J. Arid Environ. 2001, 49, 507–520. [Google Scholar] [CrossRef]
- Ren, H.Y.; Zheng, S.X.; Bai, Y.F. Effects of grazing on foliage biomass allocation of grassland communities in Xilin River Basin, Inner Mongolia. Chin. J. Plant Ecol. 2009, 33, 1065–1074. (In Chinese) [Google Scholar]
- Kogan, F.; Stark, R.; Gitelson, A.; Jargalsaikhan, L.; Dugrajav, C.; Tsooj, S. Derivation of pasture biomass in Mongolia from AVHRR-based vegetation health indices. Int. J. Remote Sens. 2004, 25, 2889–2896. [Google Scholar] [CrossRef]
- Flynn, E.S.; Dougherty, C.T.; Wendroth, O. Assessment of Pasture Biomass with the Normalized Difference Vegetation Index from Active Ground-Based Sensors. Agron. J. 2008, 100, 114–121. [Google Scholar] [CrossRef]
- Todd, S.W.; Hoffer, R.M.; Milchunas, D.G. Biomass estimation on grazed and ungrazed rangelands using spectral indices. Int. J. Remote Sens. 1998, 19, 427–438. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, X.B.; Ying, G.; Fu, N. Vegetation indexes-biomass models for typical semi-arid steppe: A case study for Xilinhot in Northern China. Chin. J. Plant Ecol. 2007, 31, 23–31. (In Chinese) [Google Scholar]
- Ren, H.R.; Zhou, G.S. Determination of green aboveground biomass in desert steppe using litter-soil-adjusted vegetation index. Eur. Eur. J. Remote Sens. 2014, 47, 611–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.J.; Wang, W.Q.; Wang, C.J.; Lu, W.H.; Sun, S.Z.; Gao, J. Estimation of grassland aboveground biomass using Landsat 8 OLI satellite image in the northern hillside of Tianshan Mountain. Remote Sens. Technol. Appl. 2017, 32, 1012–1021. (In Chinese) [Google Scholar]

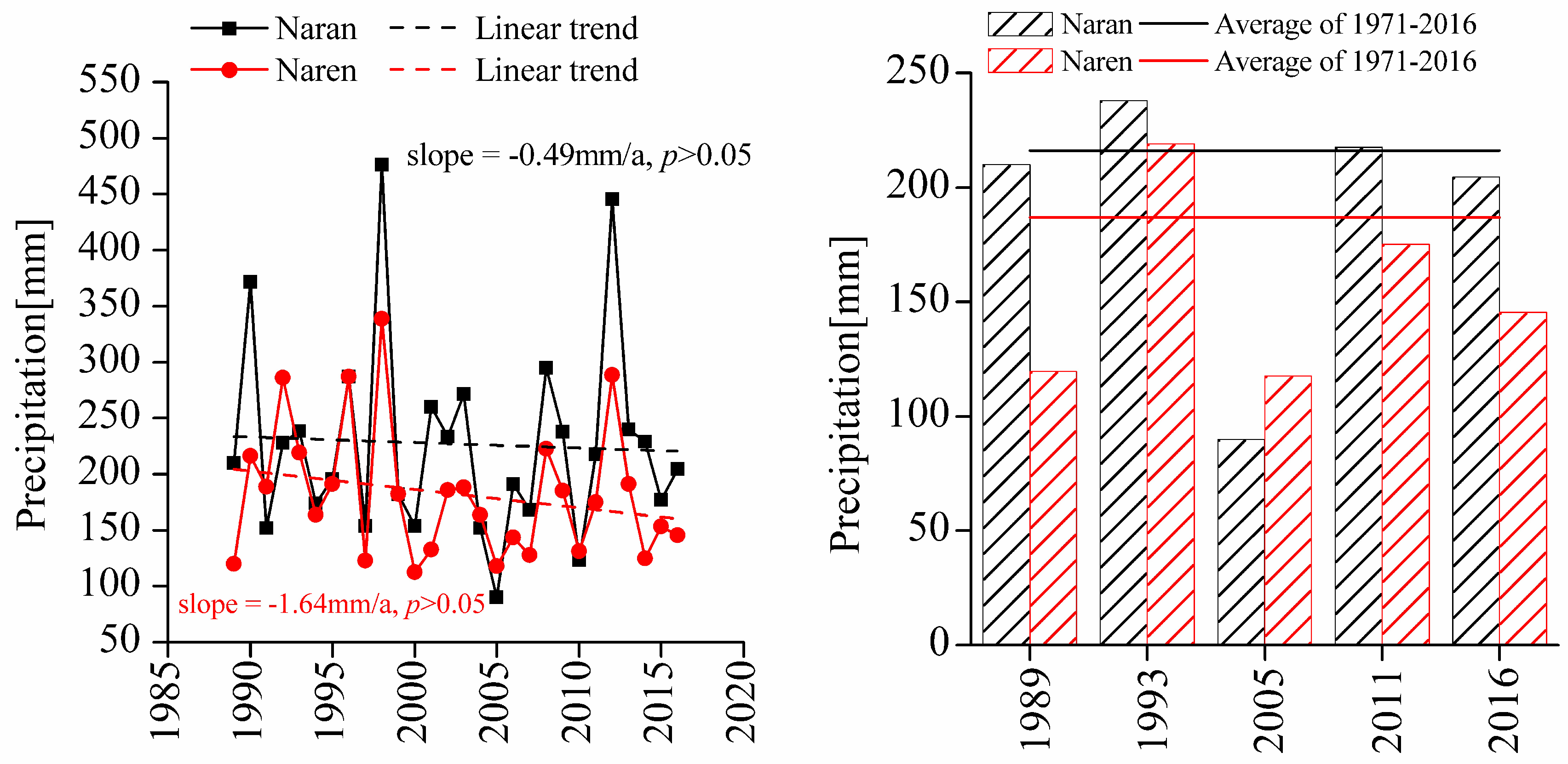
| Ecological Factors | Naran Soum | Naren Soum |
|---|---|---|
| Annual mean temperature (°C) | 1.41a | 1.03a |
| Annual accumulated precipitation (mm) | 216.80a | 187.07b |
| Altitude (m) | 1356.21a | 1346.39a |
| Average annual evaporation (mm) | 1505.14a | 1498.52a |
| Average stocking rate (sheep unit/km2) | 42a | 50a |
| Soil type | Chestnut soil | Chestnut soil |
| Soil volume water content | 7.5%a | 6.9%b |
| Country | Mongolia | Inner Mongolia | |||
|---|---|---|---|---|---|
| Time Period | 1958–1990 | 1990–Today | 1978–1990 | 1990–Today | |
| Livestock Ownership | Common | Private | Private | Private | |
| Pasture Ownership | Common | Common | Common | Private | |
| Grazing Method | Nomadic | Nomadic | Nomadic | Continuous | |
| Species | Dominance (%) | Order | LD | Life form | Family |
|---|---|---|---|---|---|
| Carex duriuscula | 16.1 ± 10.56 | 1 | MD | Perennial weeds | Cyperaceae |
| Leymus chinensis | 10.99 ± 9.94 | 2 | ND | Perennial grass | Gramineae |
| Stipa krylovii | 8.11 ± 9.40 | 3 | LD | Perennial grass | Gramineae |
| Chenopodium acuminatum | 7.36 ± 6.41 | 4 | HD | Annual grass | Chenopodiaceae |
| Cleistogenes squarrosa | 6.62 ± 5.63 | 5 | HD | Perennial grass | Gramineae |
| Artemisia frigida | 6.32 ± 5.30 | 6 | MD | Perennial weeds | Compositae |
| Allium polyrhizum | 5.47 ± 6.26 | 7 | MD | Perennial weeds | Liliaceae |
| Salsola collina | 4.66 ± 4.03 | 8 | HD | Annual grass | Chenopodiaceae |
| Stipa grandis | 3.66 ± 8.34 | 9 | ND | Perennial grass | Gramineae |
| Allium tenuissimum | 3.16 ± 2.90 | 10 | MD | Perennial weeds | Liliaceae |
| 72.46% |
| LD | Species | NG (%) | DLD (%) | FG (%) | DLD (%) | CG (%) | DLD (%) |
|---|---|---|---|---|---|---|---|
| ND | Leymus chinensis | 11.15 ± 13.23a | 15.91 ± 6.44a | 9.56 ± 5.91a | |||
| Stipa grandis | 3.27 ± 6.93b | 14.42 | 12.8 ± 13.68a | 28.71 | 1.68 ± 6.53b | 11.24 | |
| LD | Stipa krylovii | 3.46 ± 5.08b | 3.46 | 2.84 ± 5.07b | 2.84 | 14.13 ± 10.23a | 14.13 |
| MD | Carex duriuscula | 22.27 ± 10.24a | 10.67 ± 10.66b | 11.37 ± 7.45b | |||
| Artemisia frigida | 6.68 ± 6.34a | 5.61 ± 5.37a | 6.15 ± 4.2a | ||||
| Allium polyrhizum | 5.34 ± 7.99a | 5.01 ± 3.84a | 5.71 ± 4.81a | ||||
| Allium tenuissimum | 1.99 ± 2.33b | 36.28 | 3.59 ± 2.5ab | 24.88 | 4.23 ± 3.15a | 27.46 | |
| HD | Chenopodium acuminatum | 6.34 ± 5.65a | 6.58 ± 7.77a | 8.57 ± 6.8a | |||
| Cleistogenes squarrosa | 5.52 ± 4.18b | 3.57 ± 2.5b | 8.51 ± 6.85a | ||||
| Salsola collina | 4.15 ± 3.71a | 16.01 | 2.44 ± 3.1a | 12.59 | 5.75 ± 4.32a | 22.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, Y.; Li, J.; Hoshino, B.; Bao, S.; Qin, F.; Myagmartseren, P. Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes. Sustainability 2018, 10, 4753. https://doi.org/10.3390/su10124753
Na Y, Li J, Hoshino B, Bao S, Qin F, Myagmartseren P. Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes. Sustainability. 2018; 10(12):4753. https://doi.org/10.3390/su10124753
Chicago/Turabian StyleNa, Yintai, Jinxia Li, Buho Hoshino, Saixialt Bao, Fuying Qin, and Purevtseren Myagmartseren. 2018. "Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes" Sustainability 10, no. 12: 4753. https://doi.org/10.3390/su10124753
APA StyleNa, Y., Li, J., Hoshino, B., Bao, S., Qin, F., & Myagmartseren, P. (2018). Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes. Sustainability, 10(12), 4753. https://doi.org/10.3390/su10124753





