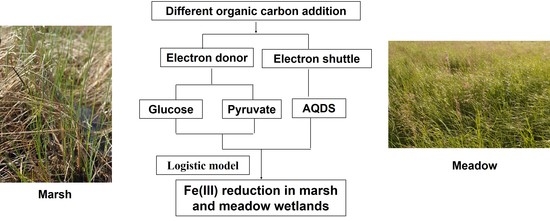
Effects of Carbon Addition on Dissimilatory Fe(III) Reduction in Freshwater Marsh and Meadow Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Anaerobic Slurry Incubation
2.4. Soil Microbial Inoculation Incubation
2.5. Sample Analysis
2.6. Data Analysis
3. Results
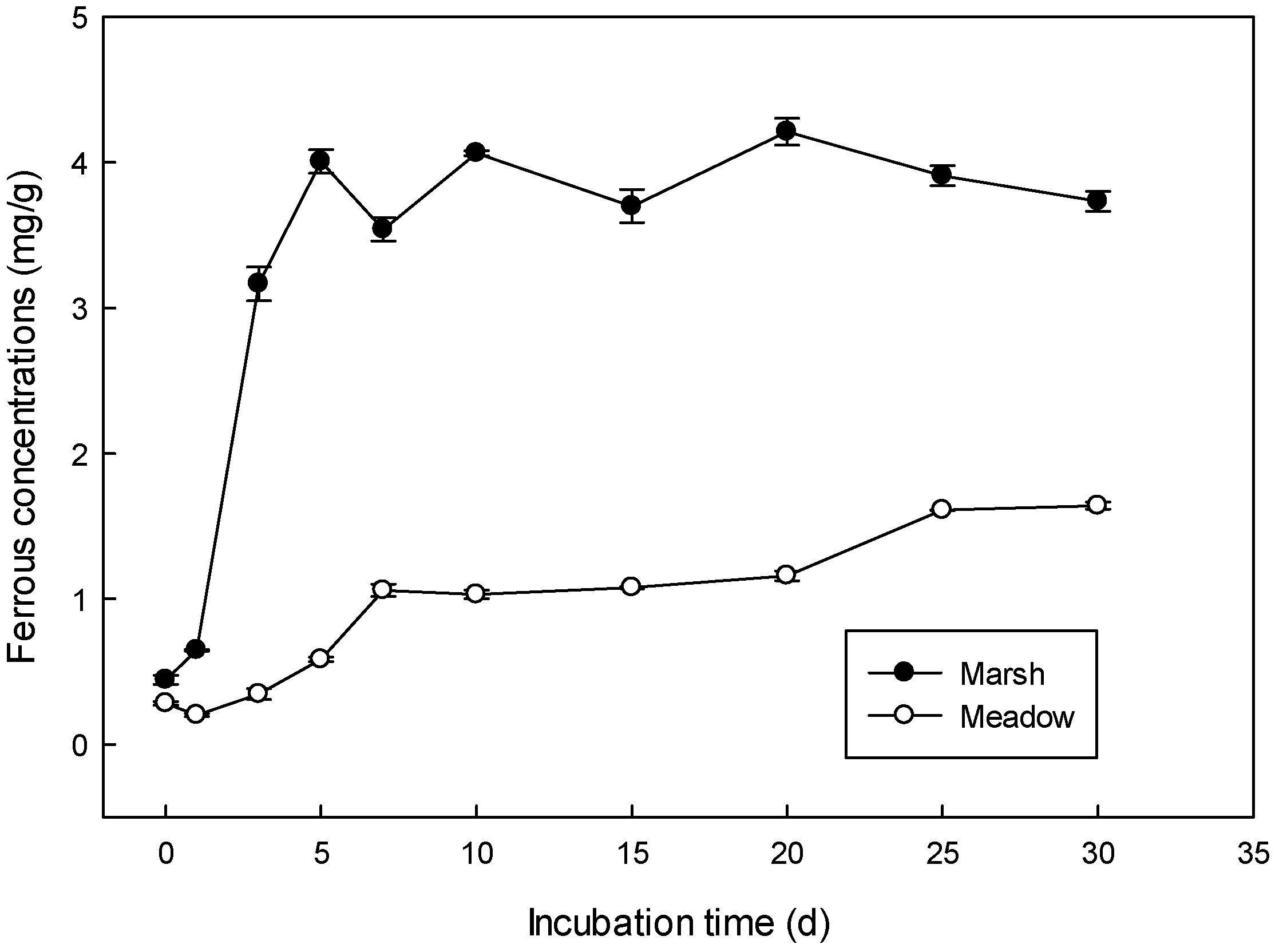
3.1. Anaerobic Slurry Incubation
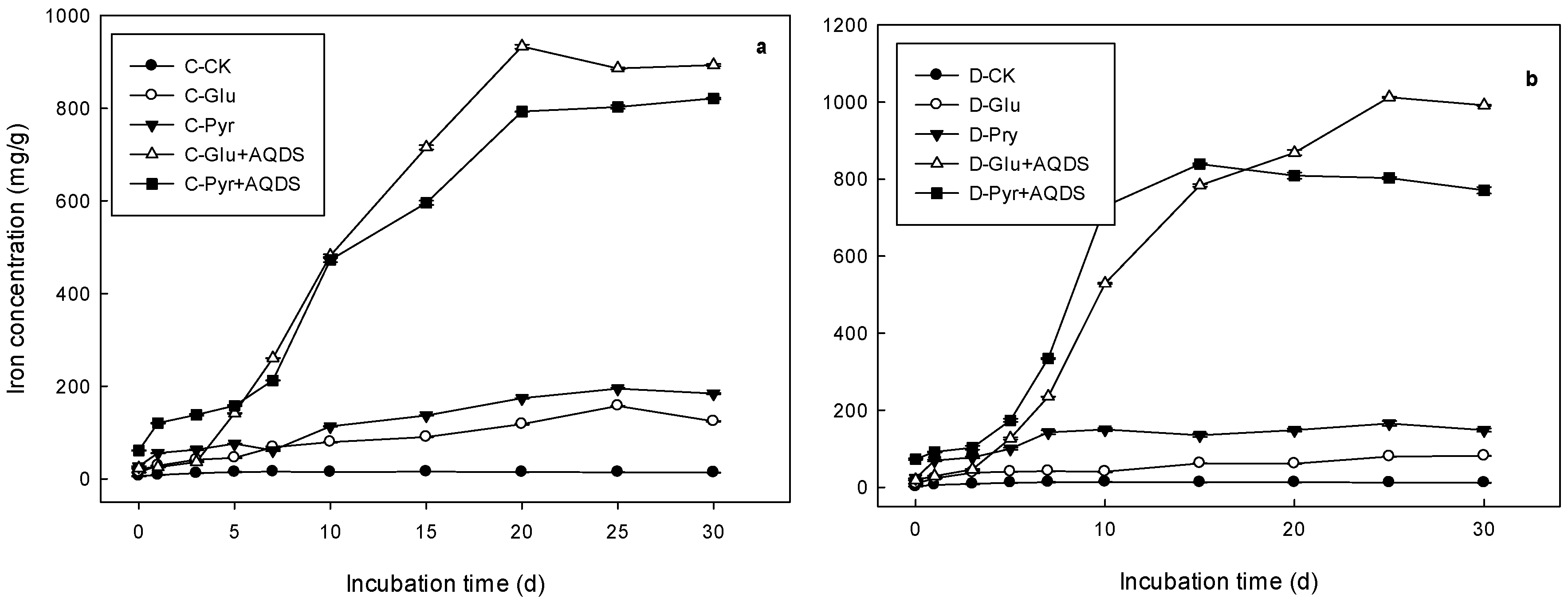
3.2. Soil Microbial Inoculation Incubation
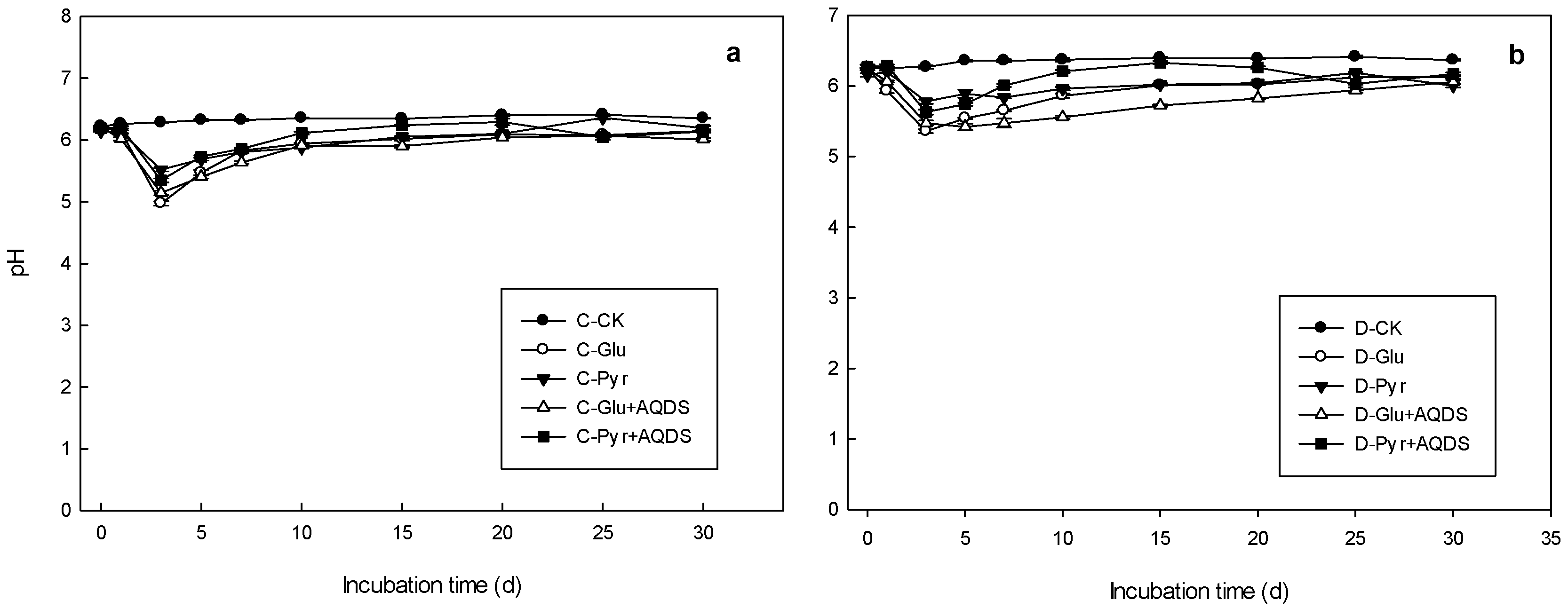
3.3. pH Changes in Microbial Inoculation Incubations
4. Discussion
4.1. Soil Slurry Incubation
4.2. Microbial Inoculation Incubation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, J.M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn (IV) reduction. FEMS Microbiol. Rev. 1991, 55, 259–287. [Google Scholar]
- Myers, C.R.; Nealson, K.H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 1988, 240, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Urrutia, M.M.; Churchill, P.F.; Kukkadapu, R.K.; Roden, E.E. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 2006, 8, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Wetzel, R.G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 1996, 41, 1733–1748. [Google Scholar] [CrossRef]
- Lipson, D.A.; Jha, M.; Raab, T.K.; Oechel, W.C. Reduction of iron(III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. Biogeosciences 2010, 115. [Google Scholar] [CrossRef]
- Luo, M.; Zeng, C.S.; Tong, C.; Huang, J.F.; Chen, K.; Liu, F.Q. Iron reduction along an inundation gradient in a tidal sedge (Cyperus malaccensis) marsh: The rates, pathways, and contributions to anaerobic organic matter mineralization. Estuar. Coast. 2015, 39, 1679–1693. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445. [Google Scholar] [CrossRef]
- Keller, J.K.; Weisenhorn, P.B.; Megonigal, J.P. Humic acids as electron acceptors in wetland decomposition. Soil Biol. Biochem. 2009, 41, 1518–1522. [Google Scholar] [CrossRef]
- Weiss, J.V.; Emerson, D.; Megonigal, J.P. Geochemical control of microbial Fe(III) reduction potential in wetlands: Comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol. Ecol. 2004, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Uchimiya, M.; Sposito, G. Iron(III) bioreduction in soil in the presence of added humic substances. Soil Sci. Soc. Am. J. 2009, 73, 65–71. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2009, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, A.; Behrens, S.; Kappler, A. Comparison of humic substance-and Fe(III)-reducing microbial communities in anoxic aquifers. Geomicrobiol. J. 2014, 31, 917–928. [Google Scholar] [CrossRef]
- Li, L.; Wenbing, T.; Guoan, W. Electron transfer mechanisms of humic substances and their environmental implications: A review. Environ. Chem. 2016, 35, 254–266. [Google Scholar]
- Liptzin, D.; Silver, W.L. Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol. Biochem. 2009, 41, 1696–1702. [Google Scholar] [CrossRef]
- Song, C.; Xu, X.; Tian, H.; Wang, Y. Ecosystem–atmosphere exchange of CH4 and N2O and ecosystem respiration in wetlands in the Sanjiang Plain, Northeastern China. Glob. Chang. Biol. 2009, 15, 692–705. [Google Scholar] [CrossRef]
- Song, C.; Liu, D.; Song, Y.; Yang, G.; Wan, Z.; Li, Y.; Xu, X. Effect of exogenous phosphorus addition on soil respiration in Calamagrostis angustifolia freshwater marshes of Northeast China. Atmos. Environ. 2011, 45, 1402–1406. [Google Scholar] [CrossRef]
- Hou, C. Effects of Hydrological Changes on Soil Carbon Sequestration of Marsh in the Sanjiang Plain. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences, Changchun, China, 2012. [Google Scholar]
- Lovley, D.R.; Phillips, E.J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [PubMed]
- Mao, R.; Song, C.; Zhang, X.; Wang, X.; Zhang, Z. Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 2013, 364, 385–394. [Google Scholar] [CrossRef]
- Todorova, S.G.; Siegel, D.I.; Costello, A.M. Microbial Fe(III) reduction in a minerotrophic wetland–geochemical controls and involvement in organic matter decomposition. Appl. Geochem. 2005, 20, 1120–1130. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Reddy, K.R. Biogeochemistry of Wetlands: Science and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 405–443. [Google Scholar]
- Shi, F.; Song, C.; Zhang, X.; Mao, R.; Guo, Y.; Gao, F. Plant zonation patterns reflected by the differences in plant growth, biomass partitioning and root traits along a water level gradient among four common vascular plants in freshwater marshes of the Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 81, 158–164. [Google Scholar] [CrossRef]
- Zhang, X.; Song, C.; Mao, R.; Yang, G.; Tao, B.; Shi, F.; Zhu, X.; Hou, A. Litter mass loss and nutrient dynamics of four emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang plain, Northeast China. Plant Soil 2014, 385, 139–147. [Google Scholar] [CrossRef]
- Yi, W.; Wang, B.; Qu, D. Diversity of isolates performing Fe(III) reduction from paddy soil fed by different organic carbon sources. Afr. J. Biotechnol. 2012, 11, 4407–4417. [Google Scholar]
- Balashova, V.V.; Zavarzin, G.A. Anaerobic reduction of ferric iron by hydrogen bacteria. Mikrobiologiia 1979, 48, 773–778. [Google Scholar] [PubMed]
- Li, L.; Qu, Z.; Wang, B.; Qu, D. Dynamics of the abundance and structure of metabolically active Clostridium community in response to glucose additions in flooded paddy soils: Closely correlated with hydrogen production and Fe(III) reduction. J. Soil Sediments 2017, 17, 1727–1740. [Google Scholar] [CrossRef]
- Lüdemann, H.; Arth, I.; Liesack, W. Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores. Appl. Environ. Microbiol. 2000, 66, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Coates, J.D. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 2000, 3, 252–256. [Google Scholar] [CrossRef]
- Jia, R.; Li, L.; Qu, D. pH shift-mediated dehydrogenation and hydrogen production are responsible for microbial iron(III) reduction in submerged paddy soils. J. Soil Sediments 2015, 15, 1178–1190. [Google Scholar] [CrossRef]
- Weber, S.; Stubner, S.; Conrad, R. Bacterial populations colonizing and degrading rice straw in anoxic paddy soil. Appl. Environ. Microbiol. 2001, 67, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qu, D. Dissimilatory Fe(III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids. J. Environ. Sci. 2008, 20, 1103–1108. [Google Scholar] [CrossRef]
- Kappler, A.; Benz, M.; Schink, B.; Brune, A. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 2004, 47, 85–92. [Google Scholar] [CrossRef]
- Chen, M.; Shih, K.; Hu, M.; Li, F.; Liu, C.; Wu, W.; Tong, H. Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of southern China. J. Agric. Food Chem. 2012, 60, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; Van Der Velde, S.; Lettinga, G.; Field, J.A. Quinones as terminal electron acceptors for anaerobic microbial oxidation of phenolic compounds. Biodegradation 2000, 11, 313–321. [Google Scholar] [CrossRef] [PubMed]
| Soil Characteristics | Gley Marsh | Meadow |
|---|---|---|
| pH | 5.3 ± 0.01 | 5.4 ± 0.06 |
| TN (g/kg) | 9.8 ± 0.9 | 4.2 ± 0.3 |
| TOC (g/kg) | 102.5 ± 1.8 | 41.1 ± 0.8 |
| Bulk density (g/cm3) | 0.12 ± 0.02 | 0.77 ± 0.06 |
| Feox (g/kg) | 1302.61 ± 30.2 | 929.37 ± 27.4 |
| Fef (g/kg) | 5564.95 ± 61.3 | 3043.93 ± 47.5 |
| Fea (g/kg) | 4340 ± 52.8 | 2832.74 ± 35.7 |
| Parameters | Gley Marsh | Meadow | |
|---|---|---|---|
| Logistic Model Parameter | a | 3.89 a | 1.54 b |
| b | 17.62 a | 4.07 b | |
| k | 1.44 a | 0.19 b | |
| R2 | 0.97 | 0.86 | |
| Vmax (mg/L*d) | 1.4 a | 0.073 b | |
| Tvmax (d) | 2.5 a | 3.06 b | |
| Reduction rate of iron (%) | 76% | 30% | |
| Treatments | Logistic Model Parameter | R2 | Vmax | Tvmax | |||
|---|---|---|---|---|---|---|---|
| a | b | k | (mg/L*d) | (d) | |||
| CK | Marsh | 14.86 ± 0.01 a | 1.59 ± 0.13 a | 0.7 ± 0.07 a | 0.94 ± 0.01 | 2.61 ± 0.26 a | 0.81 ± 0.04 a |
| Meadow | 12.78 ± 0.27 a | 2.81 ± 0.59 a | 0.73 ± 0.15 a | 0.89 ± 0.05 | 2.33 ± 0.51 a | 1.35 ± 0.05 a | |
| Pyr | Marsh | 142.35 ± 1.18 b | 4.7 ± 0.05 b | 0.17 ± 0.0 b | 0.92 ± 0.0 | 6.05 ± 0.05 b | 3.32 ± 0.01 b |
| Meadow | 86.89 ± 0.53 b | 2.88 ± 0.08 a | 0.12 ± 0.0 b | 0.89 ± 0.01 | 2.61 ± 0.02 a | 3.18 ± 0.03 b | |
| Glu | Marsh | 201.61 ± 1.98 c | 4.23 ± 0.02 c | 0.15 ± 0.01 b | 0.95 ± 0.01 | 7.73 ± 0.22 c | 3.32 ± 0.04 b |
| Meadow | 151.54 ± 4.16 c | 3.08 ± 0.12 a | 0.43 ± 0.01 c | 0.91 ± 0.01 | 16.29 ± 0.44 b | 1.97 ± 0.04 c | |
| Glu + AQDS | Marsh | 903.11 ± 0.51 d | 30.27 ± 0.21 d | 0.34 ± 0.01 c | 0.99 ± 0.0 | 77.52 ± 1.52 d | 4.48 ± 0.01 c |
| Meadow | 966.15 ± 3.18 d | 32.91 ± 0.86 b | 0.34 ± 0.01 c | 0.99 ± 0.0 | 82.92 ± 1.13 c | 4.56 ± 0.01 d | |
| Pyr + AQDS | Marsh | 832.22 ± 1.41 e | 11.76 ± 0.05 e | 0.24 ± 0.0 d | 0.98 ± 0.0 | 49.93 ± 0.08 e | 3.81 ± 0.0 d |
| Meadow | 814.22 ± 5.22 e | 59.2 ± 5.91 c | 0.57 ± 0.02 d | 0.98 ± 0.0 | 116 ± 2.84 d | 4.64 ± 0.07 e | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yuan, Y.; Jiang, M. Effects of Carbon Addition on Dissimilatory Fe(III) Reduction in Freshwater Marsh and Meadow Wetlands. Sustainability 2018, 10, 4309. https://doi.org/10.3390/su10114309
Zhu X, Yuan Y, Jiang M. Effects of Carbon Addition on Dissimilatory Fe(III) Reduction in Freshwater Marsh and Meadow Wetlands. Sustainability. 2018; 10(11):4309. https://doi.org/10.3390/su10114309
Chicago/Turabian StyleZhu, Xiaoyan, Yuxiang Yuan, and Ming Jiang. 2018. "Effects of Carbon Addition on Dissimilatory Fe(III) Reduction in Freshwater Marsh and Meadow Wetlands" Sustainability 10, no. 11: 4309. https://doi.org/10.3390/su10114309
APA StyleZhu, X., Yuan, Y., & Jiang, M. (2018). Effects of Carbon Addition on Dissimilatory Fe(III) Reduction in Freshwater Marsh and Meadow Wetlands. Sustainability, 10(11), 4309. https://doi.org/10.3390/su10114309