Biocement Fabrication and Design Application for a Sustainable Urban Area
Abstract
1. Introduction
- (1)
- Study of MICP to improve the strength of biocement through water permeability analysis and compressive strength testing.
- (2)
- Aesthetic and scalability study using biocement.
- (3)
- Design of an eco-friendly building module using biocement.
2. Material and Methods
2.1. Strain and Cultivation
2.2. Sand Characteristics
2.3. Urease Activity Test
2.4. MICP (Microbially Induced Calcite Precipitation)
2.5. Evaluation of Biocement
2.5.1. Water Percolation and Sand Loss
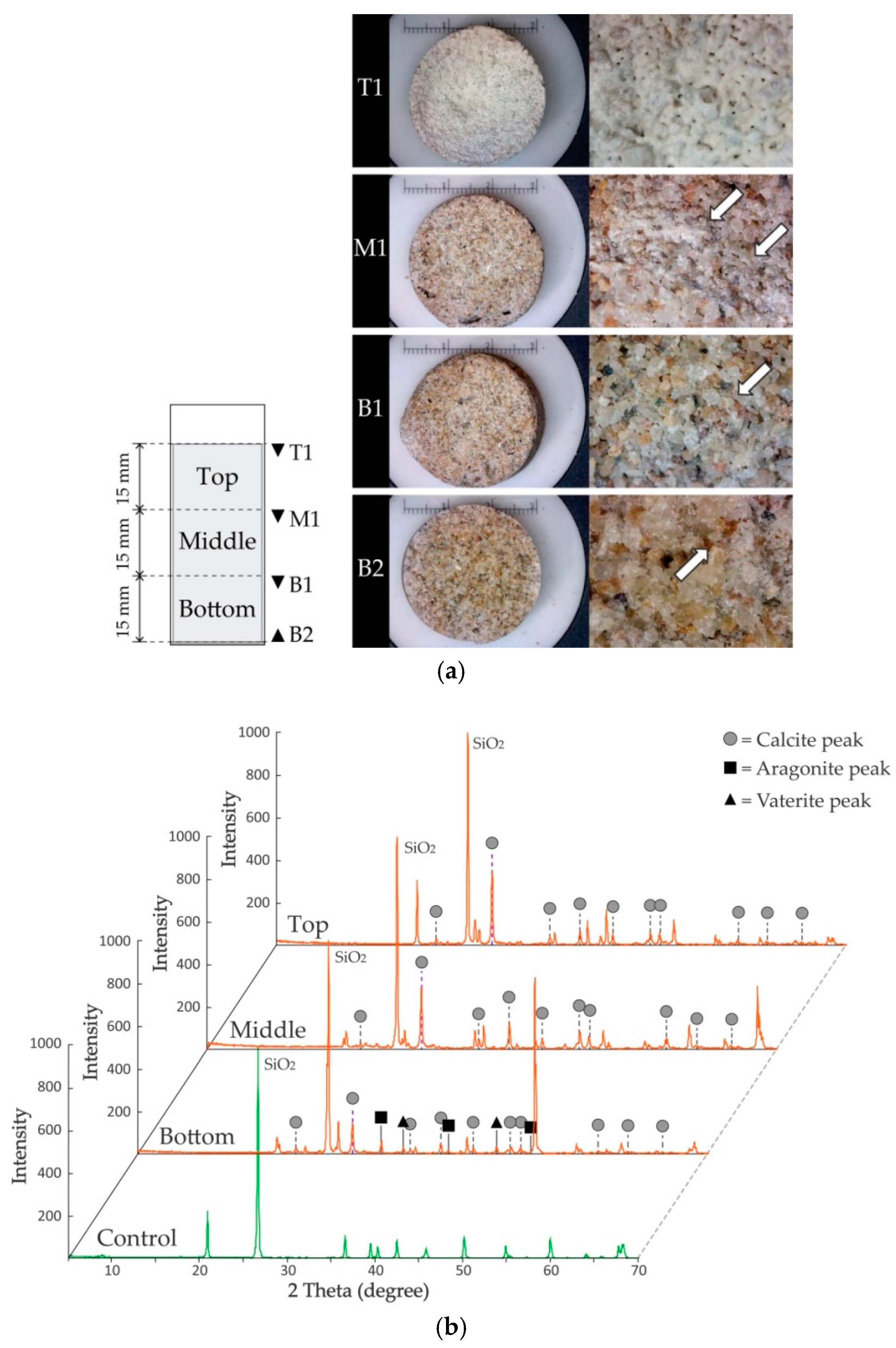
2.5.2. X-ray Diffraction (XRD) Analysis
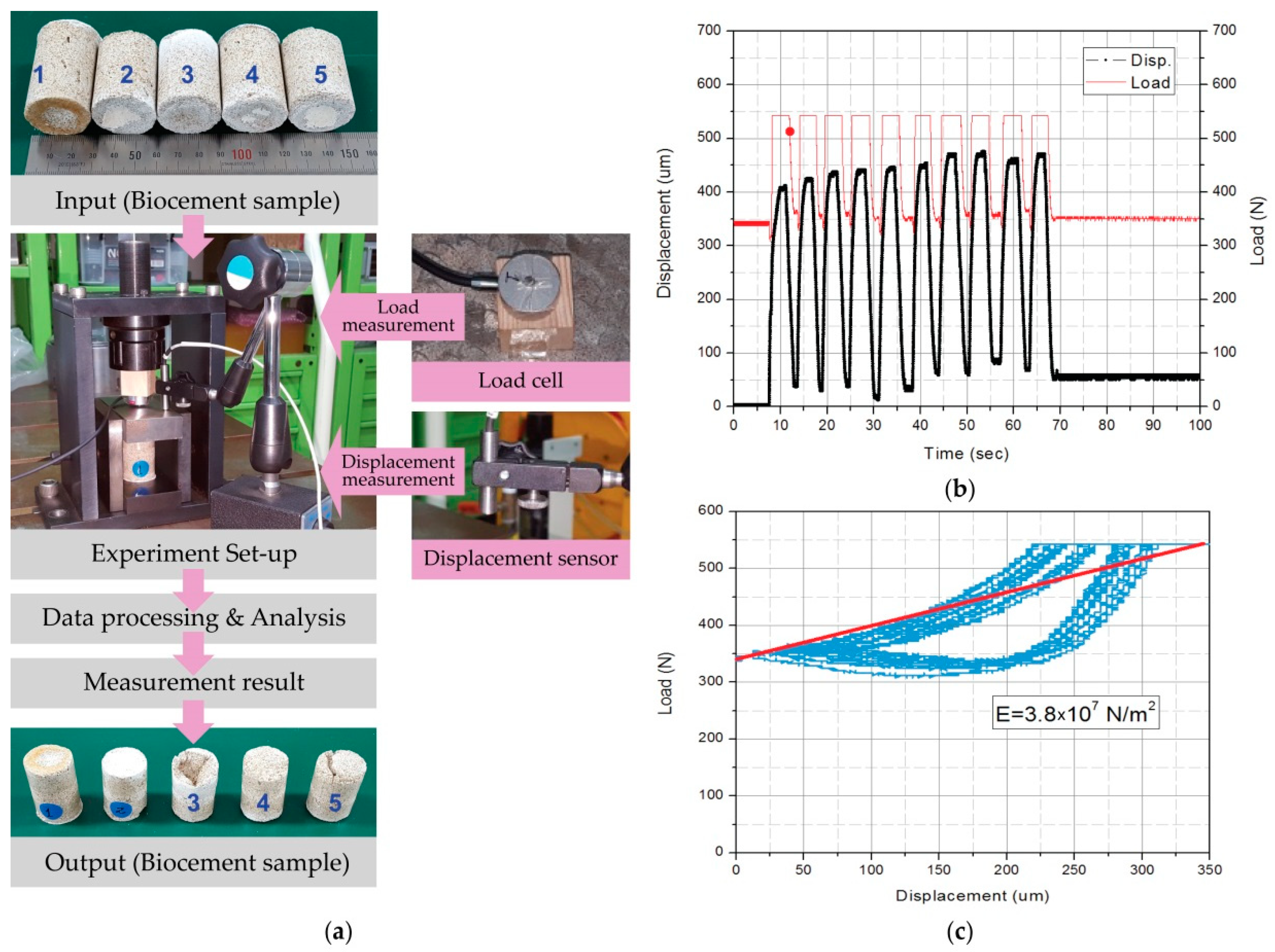
2.5.3. Compressive Strength Test
2.6. Material Studies of Compatibility and Scalability Using the Biocement
2.7. Design Application Using Biocement
3. Results and Discussion
3.1. Study of MICP
3.1.1. Cultivation of S. pasteurii for MICP
3.1.2. Study of Bacterial Solution
3.1.3. Study of Cementation Solution
3.1.4. Study of the Cementation Process
3.1.5. Study of Mold
3.2. Mechanical and Chemical Evaluation of Biocement as an Alternative Concrete
3.2.1. Compressive Strength Test
3.2.2. XRD (X-ray Diffraction) Analysis
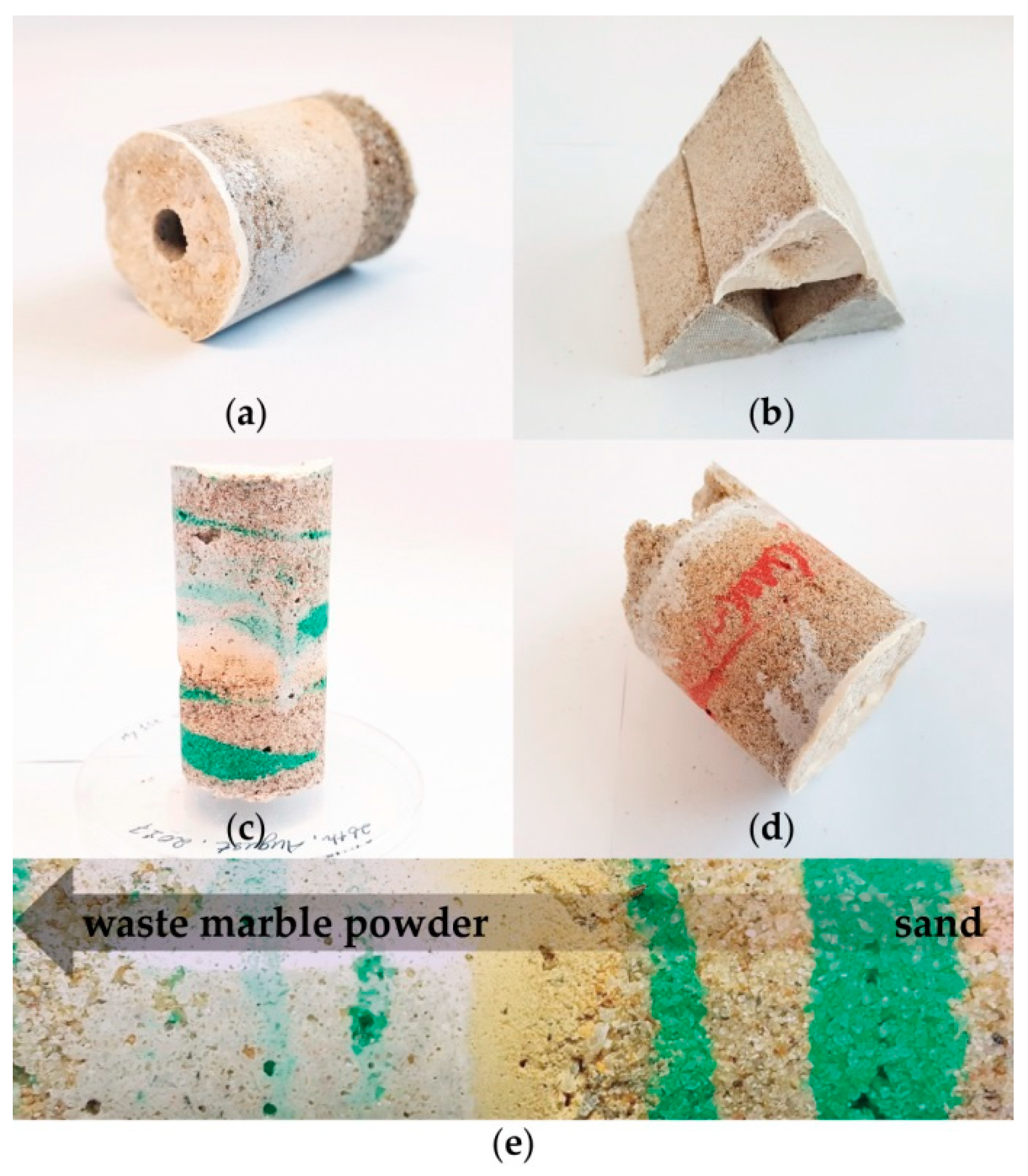
3.3. Compatibility Study of Biocement with Other Minerals and Various Formation Study
3.3.1. Compatibility of Biocement
3.3.2. Improvement of the Finish Quality of Biocement
3.4. Large-Scale Test
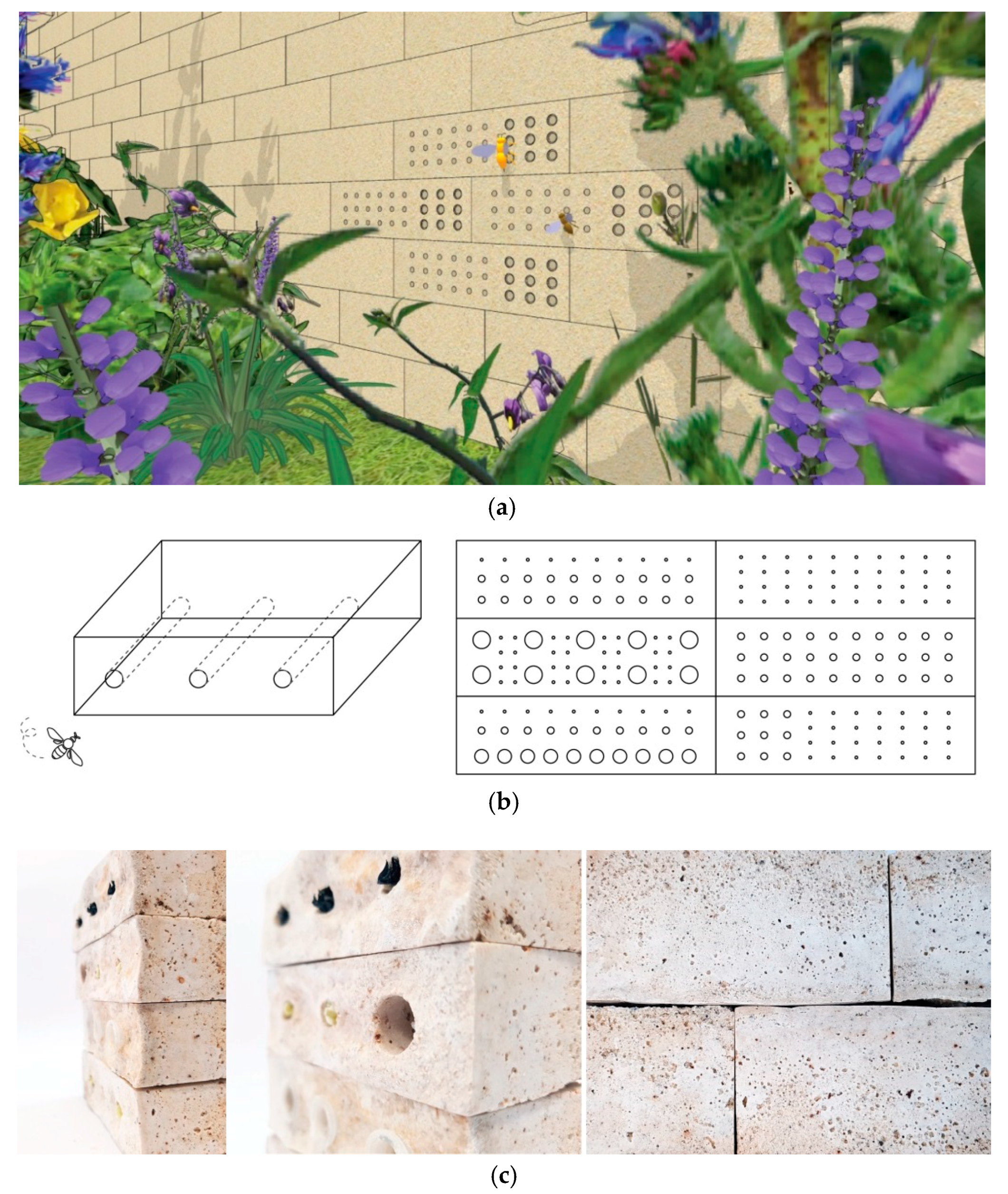
3.5. Potential of Using Biocement in the Design Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzini, E. New design knowledge. Des. Stud. 2009, 30, 4–12. [Google Scholar] [CrossRef]
- Ehrenfeld, J. Sustainability by Design: A Subversive Strategy for Transforming Our Consumer Culture; Yale University: New Haven, CT, USA, 2008. [Google Scholar]
- Myers, W. Bio Design: Nature, Science, Creativity; Museum of Modern Art: New York, NY, USA, 2012. [Google Scholar]
- Camere, S.; Karana, E. Fabricating materials from living organisms: An emerging design practice. J. Clean. Prod. 2018, 186, 570–584. [Google Scholar] [CrossRef]
- Mogas-Soldevila, L.; Duro-Royo, J.; Lizardo, D.; Kayser, M.; Patrick, W.; Sharma, S.; Keating, S.; Klein, J.; Inamura, C.; Oxman, N. Designing the ocean pavilion: Biomaterial templating of structural, manufacturing, and environmental performance. In Proceedings of the International Association for Shell and Spatial Structures, Amsterdam, The Netherland, 20 August 2015; Available online: http://neri.media.mit.edu/assets/pdf/IASS2015_MediatedMatter_small.pdf (accessed on 30 October 2018).
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Ann. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J.; Stabnikov, V. Basics of construction microbial biotechnology. In Biotechnologies and Biomimetics for Civil Engineering; Springer: Berlin, Germany, 2015; pp. 21–56. [Google Scholar]
- Monks, K. Would you live in a house made of sand and bacteria? It’s a surprisingly good idea. CNN, 20 May 2014. [Google Scholar]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Sarda, D.; Choonia, H.S.; Sarode, D.; Lele, S. Biocalcification by Bacillus pasteurii urease: A novel application. J. Ind. Microbiol. Biotechnol. 2009, 36, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Hamed Khodadadi, T.; Kavazanjian, E.; Van Paassen, L.; DeJong, J. Bio-grout materials: A review. Geotech. Spec. Publ. 2017, 288, 1–12. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Improvement in strength properties of ash bricks by bacterial calcite. Ecol. Eng. 2012, 39, 31–35. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Boling, J. Bioprecipitation of Calcite by Sporosarcina pasteurii: Developing Efficient Methodologies for Microbially Indurated Rammed Earth. Ph.D. Thesis, University of Kansas, Lawrence, KS, USA, 2015. [Google Scholar]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, S. High Strength In-Situ Biocementation of Soil by Calcite Precipitating Locally Isolated Ureolytic Bacteria. Ph.D. Thesis, Murdoch University, Perth, Australia, 2008. [Google Scholar]
- Fortin, D.; Ferris, F.; Beveridge, T. Surface-mediated mineral development by bacteria. Rev. Mineral. Geochem. 1997, 35, 161–180. [Google Scholar]
- Dosier, G.K. Methods for Making Construction Material Using Enzyme Producing Bacteria. Patent 8,728,365, 20 May 2014. [Google Scholar]
- Brownell, B.E.; Swackhamer, M.T. Hypernatural: Architecture’s New Relationship with Nature; Princeton Architectural Press: New York City, NY, USA, 2015. [Google Scholar]
- Hammes, F.; Verstraete, W. Key roles of ph and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. Eng. 2016, 143, 04016083. [Google Scholar] [CrossRef]
- Khodadadi Tirkolaei, H.; Bilsel, H. Estimation on ureolysis-based microbially induced calcium carbonate precipitation progress for geotechnical applications. Mar. Georesour. Geotechnol. 2017, 35, 34–41. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szentgyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Baldock, K.C.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N. Where Is the UK’s Pollinator Biodiversity? The Importance of Urban Areas for Flower-Visiting Insects. Proc. R. Soc. B 2015, 282, 20142849. [Google Scholar] [CrossRef] [PubMed]
- Glaum, P.; Simao, M.-C.; Vaidya, C.; Fitch, G.; Iulinao, B. Big city bombus: Using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. Roy. Soc. Open Sci. 2017, 4, 170156. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.L.; Frankie, G.W.; Thorp, R.W. Ecology of urban bees: A review of current knowledge and directions for future study. CATE 2009, 2, 3. [Google Scholar] [CrossRef]
- Kellert, S.R.; Heerwagen, J.; Mador, M. Biophilic Design: The Theory, Science and Practice of Bringing Buildings to Life; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Matteson, K.C.; Ascher, J.S.; Langellotto, G.A. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 2008, 101, 140–150. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331. [Google Scholar] [CrossRef]
- Kim, G.; Youn, H. Microbially induced calcite precipitation employing environmental isolates. Materials 2016, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J.-Am. Concr. Inst. 2001, 98, 3–9. [Google Scholar]
- Chunxiang, Q.; Jianyun, W.; Ruixing, W.; Liang, C. Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater. Sci. Eng. C 2009, 29, 1273–1280. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.-H.; Kim, S.-G.; Park, J.-Y.; Kang, C.-H.; Jeong, J.-H.; So, J.-S. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Mobley, H.; Hausinger, R. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [PubMed]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Kucharski, E.S.; Cord-Ruwisch, R.; Whiffin, V.; Al-thawadi, S.M. Microbial Biocementation. Patent 8,182,604, 22 May 2012. [Google Scholar]
- Beer, F.P.; Johnston, E.R.; DeWolf, J.T.; Mazurek, D.F. Mechanics of Materials; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Rashid, M.A.; Mansur, M.A. Considerations in producing high strength concrete. J. Civil Eng. (IEB) 2009, 37, 53–63. [Google Scholar]
- Wang, H.; Alfredsson, V.; Tropsch, J.; Ettl, R.; Nylander, T. Formation of CaCO3 deposits on hard surfaces effect of bulk solution conditions and surface properties. ACS Appl. Mater. Interfaces 2013, 5, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.C.; DeJong, J.T. Bio-mediated soil improvement: Load transfer mechanisms at the micro-and macro-scales. In Proceedings of the U.S.-China Workshop on Ground Improvement Technologies, Orlando, FL, USA, 14 March 2009. [Google Scholar]
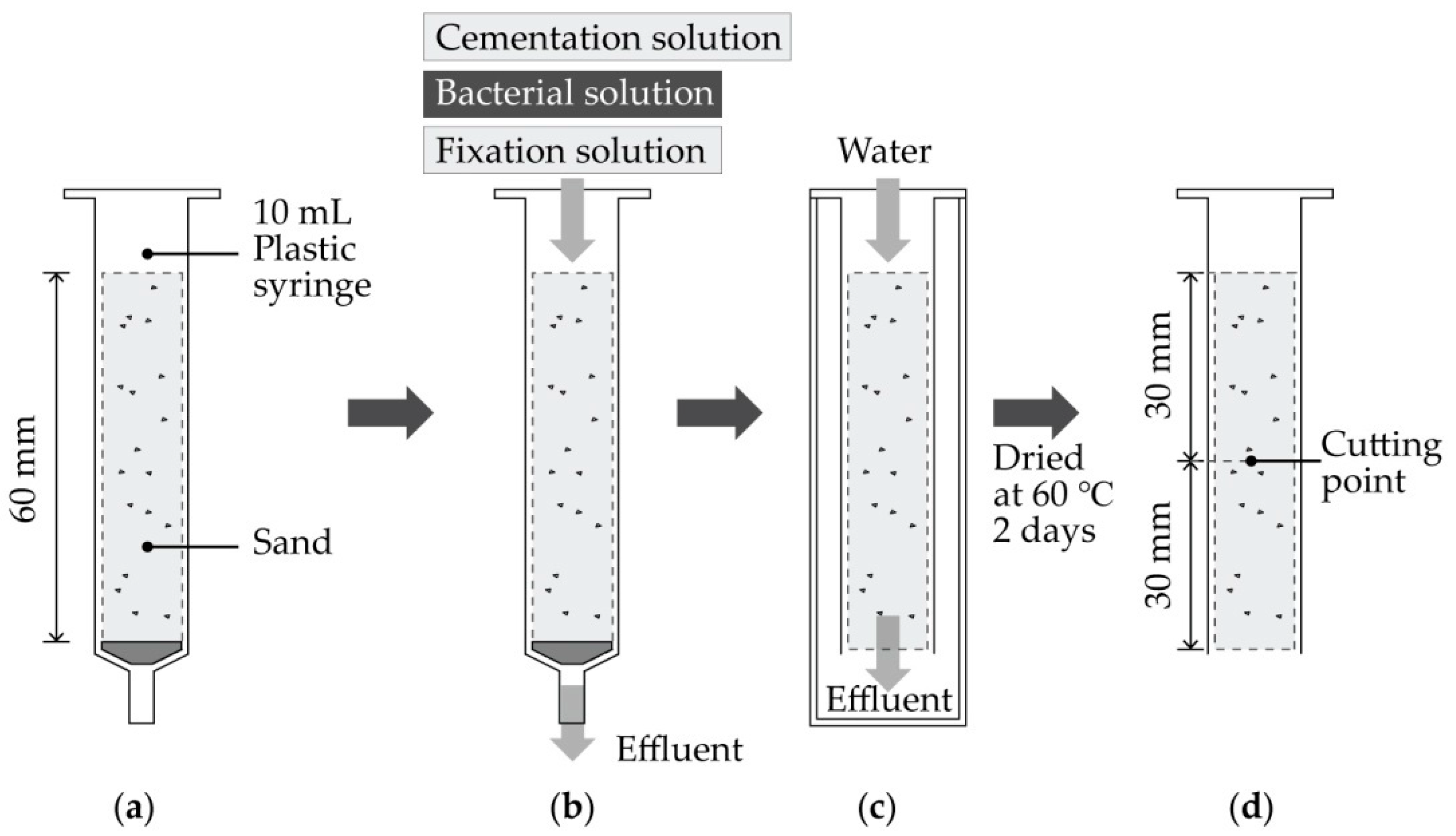
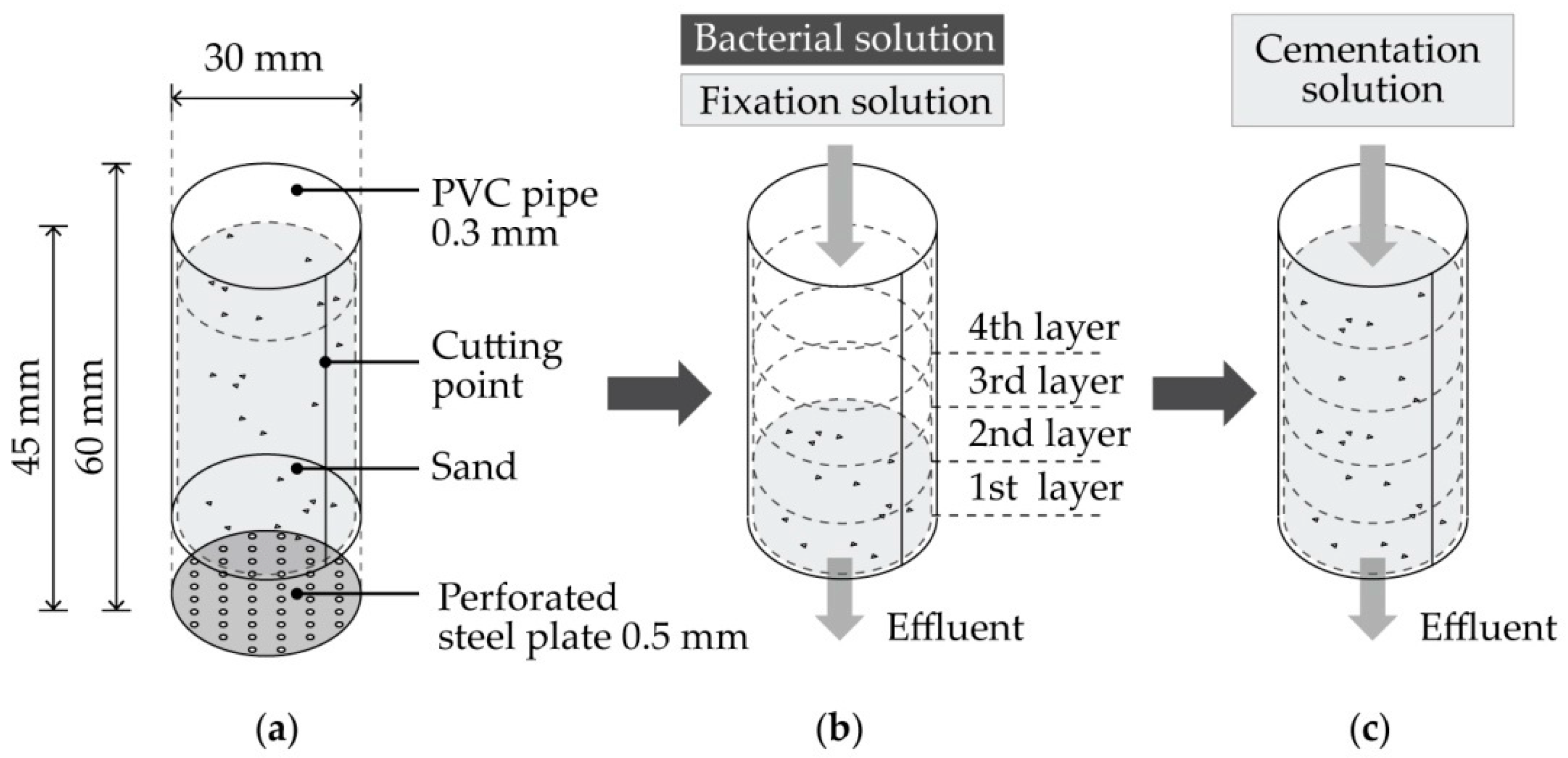
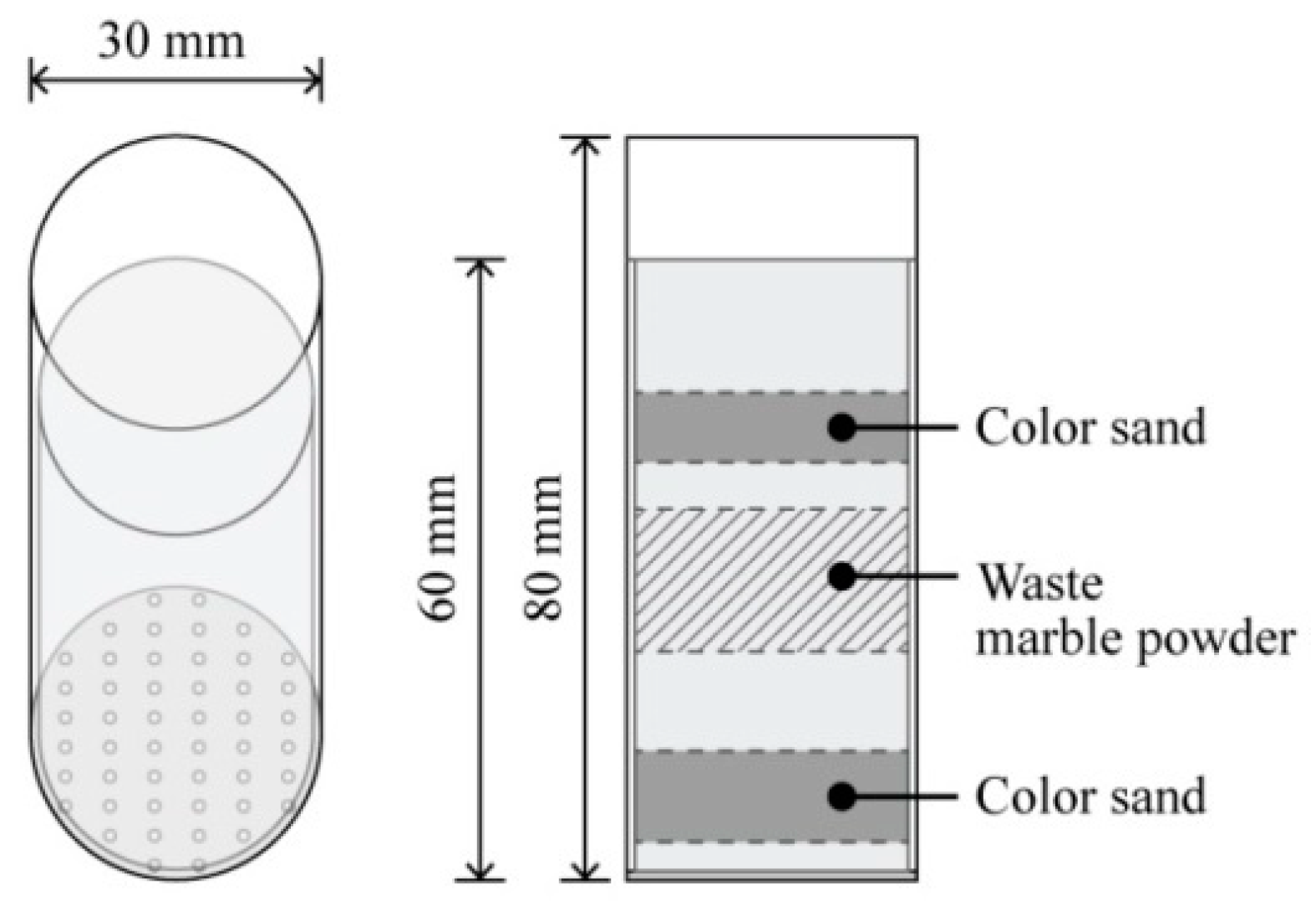
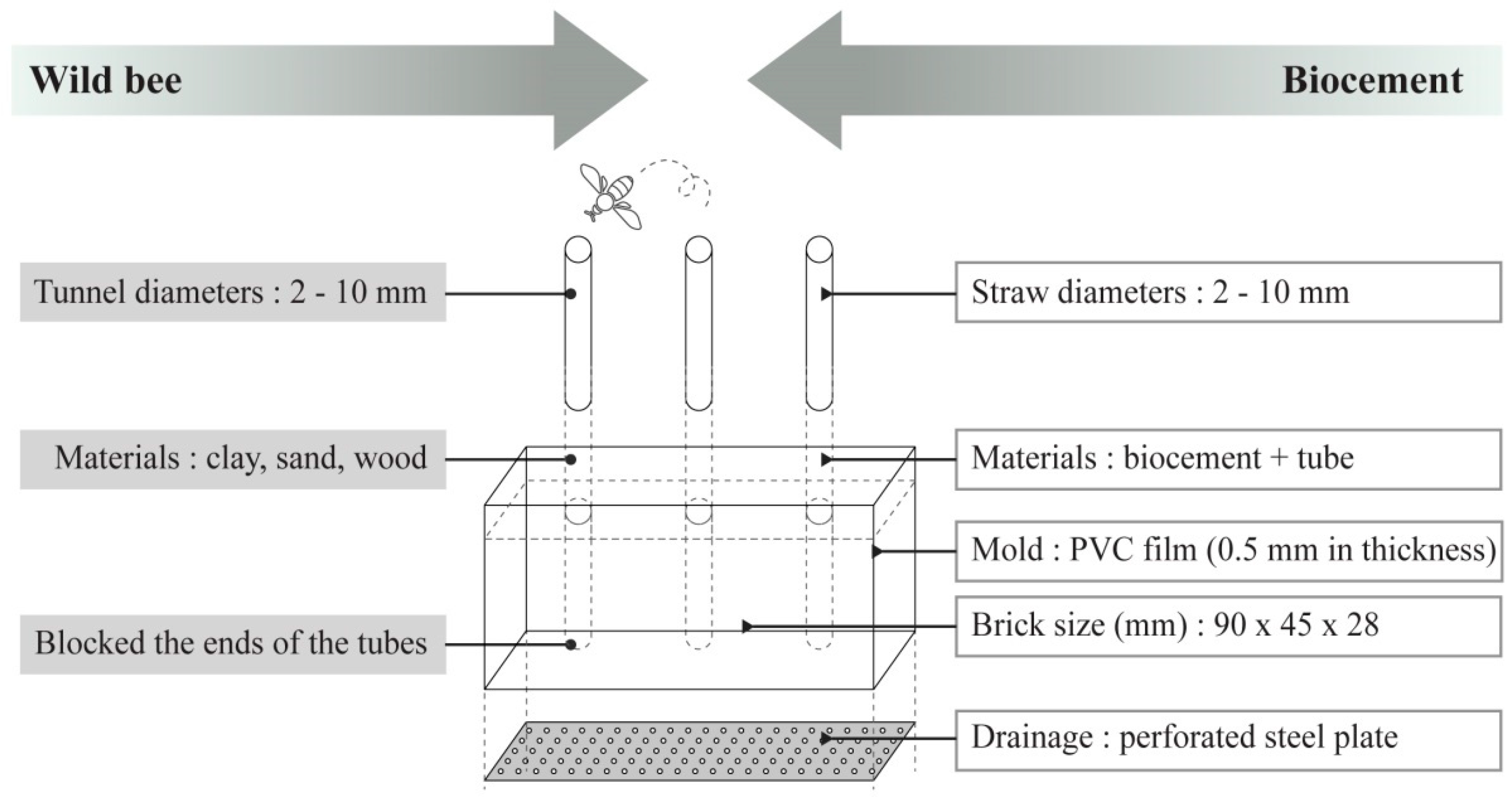
| Control Variable | Range | References | |
|---|---|---|---|
| Sand type | Grain size | 250–500 μm | [33] |
| Sterilization | Non sterilization | This study | |
| Bacterial solution | Microorganism | Sporosarcina pasteurii | [12] |
| Bacterial density | OD600 2.4–2.7 | [35] | |
| Carbon source | Yeast extract | [15] | |
| Cementation solution | Calcium source | CaCl2 | [36] |
| Nitrogen source | Urea | [37] | |
| Added substances | 25 mM NaHCO3 150 mM NH4Cl | [15] | |
| pH | 7.0 | [15] | |
| Sterilization | Non-sterilization | This study | |
| Fixation solution | Attaching bacteria to sand particles | 55 mM CaCl2 | [21] |
| Control Variable | Range | References | |
|---|---|---|---|
| Mold | Material | PVC cylinder pipe (0.3 mm in thickness, 30 mm in diameter, and 60 mm in height), Perforated steel plate (0.5 mm in thickness, 0.5 mm in hole diameter) | This study |
| Bacterial culture Injection | Bacterial placement | Layer by layer 1 | [38] |
| Cementation solution Injection | Injection frequency | 2 times a day | This study |
| Injection time (days) | 14–21 | ||
| Injection strategy | Applied from the top to the bottom by gravity | ||
| Percolation | Percolated by gravity | ||
| Temperature (°C) | 25–30 2 | ||
| Total injection solution per each sample (mL) | 200 |
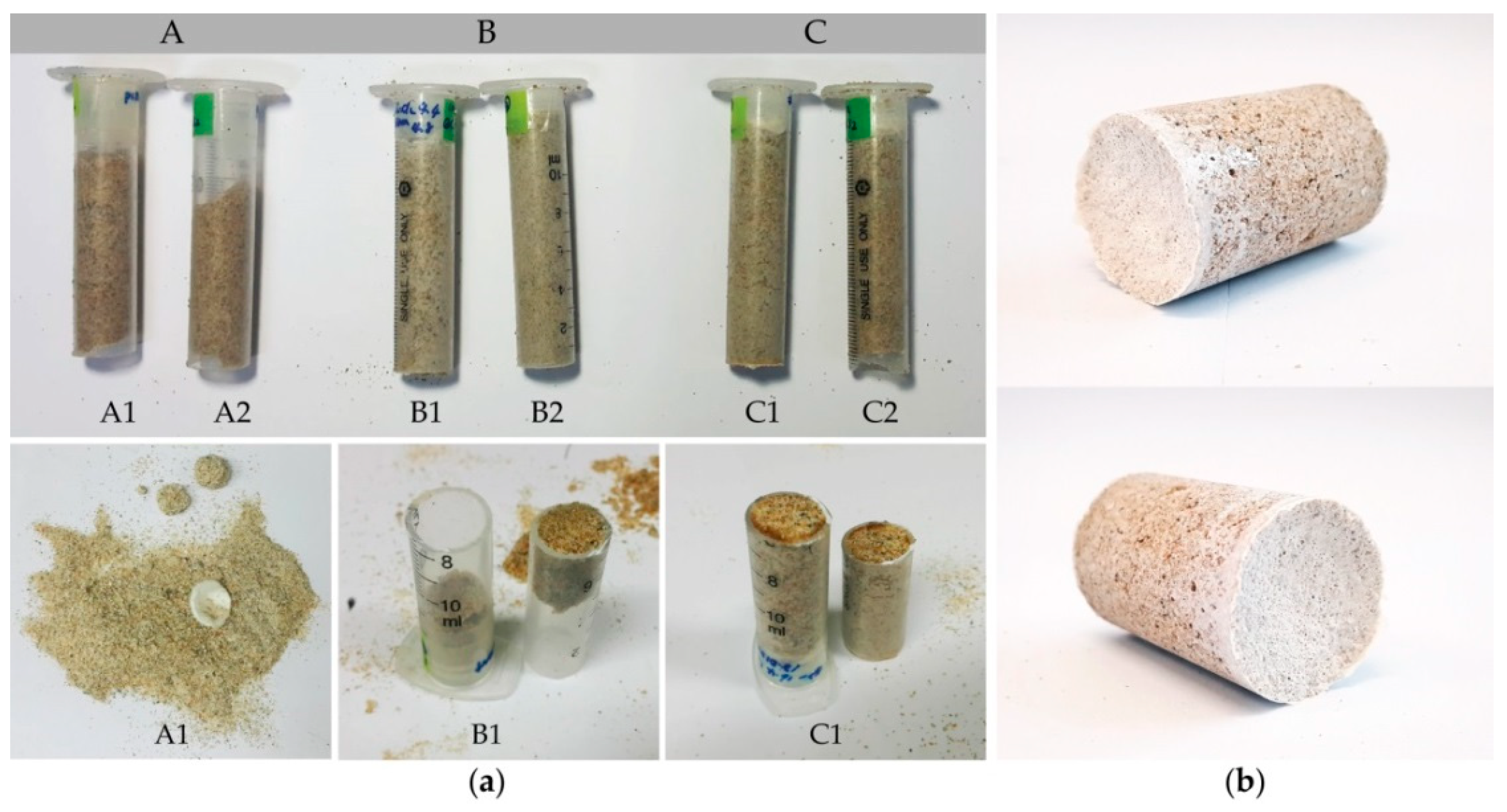
| Name | [Urea] (M) | [CaCl2] (M) | Cell Density (time) | Biocement Sample Weight (g) | Water Percolation Quantity (mL) | Biocement Sample Weight (g) after Water Percolation Test | Loss of Sand Weight (g) | |
|---|---|---|---|---|---|---|---|---|
| A | A1 1 | 0.12 | 0.055 | 1 | 19.3 | 51 | 14.1 | 5.18 |
| A2 | 10 | 17.7 | 32.5 | 16.3 | 1.42 | |||
| B | B1 | 0.4 | 0.2 | 1 | 21.4 | 6.2 | 20.3 | 1.06 |
| B2 | 10 | 24.5 | 10 | 24.1 | 0.4 | |||
| C | C1 2 | 1.5 | 0.75 | 1 | 28.2 | 0 | 27.6 | 0.6 |
| C2 | 10 | 25 | 0 | 25 | 0 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Lee, H.; Kim, O.B. Biocement Fabrication and Design Application for a Sustainable Urban Area. Sustainability 2018, 10, 4079. https://doi.org/10.3390/su10114079
Lee C, Lee H, Kim OB. Biocement Fabrication and Design Application for a Sustainable Urban Area. Sustainability. 2018; 10(11):4079. https://doi.org/10.3390/su10114079
Chicago/Turabian StyleLee, Chungmin, Hyesun Lee, and Ok Bin Kim. 2018. "Biocement Fabrication and Design Application for a Sustainable Urban Area" Sustainability 10, no. 11: 4079. https://doi.org/10.3390/su10114079
APA StyleLee, C., Lee, H., & Kim, O. B. (2018). Biocement Fabrication and Design Application for a Sustainable Urban Area. Sustainability, 10(11), 4079. https://doi.org/10.3390/su10114079




