The Relationship between POC Export Efficiency and Primary Production: Opposite on the Shelf and Basin of the Northern South China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Areas
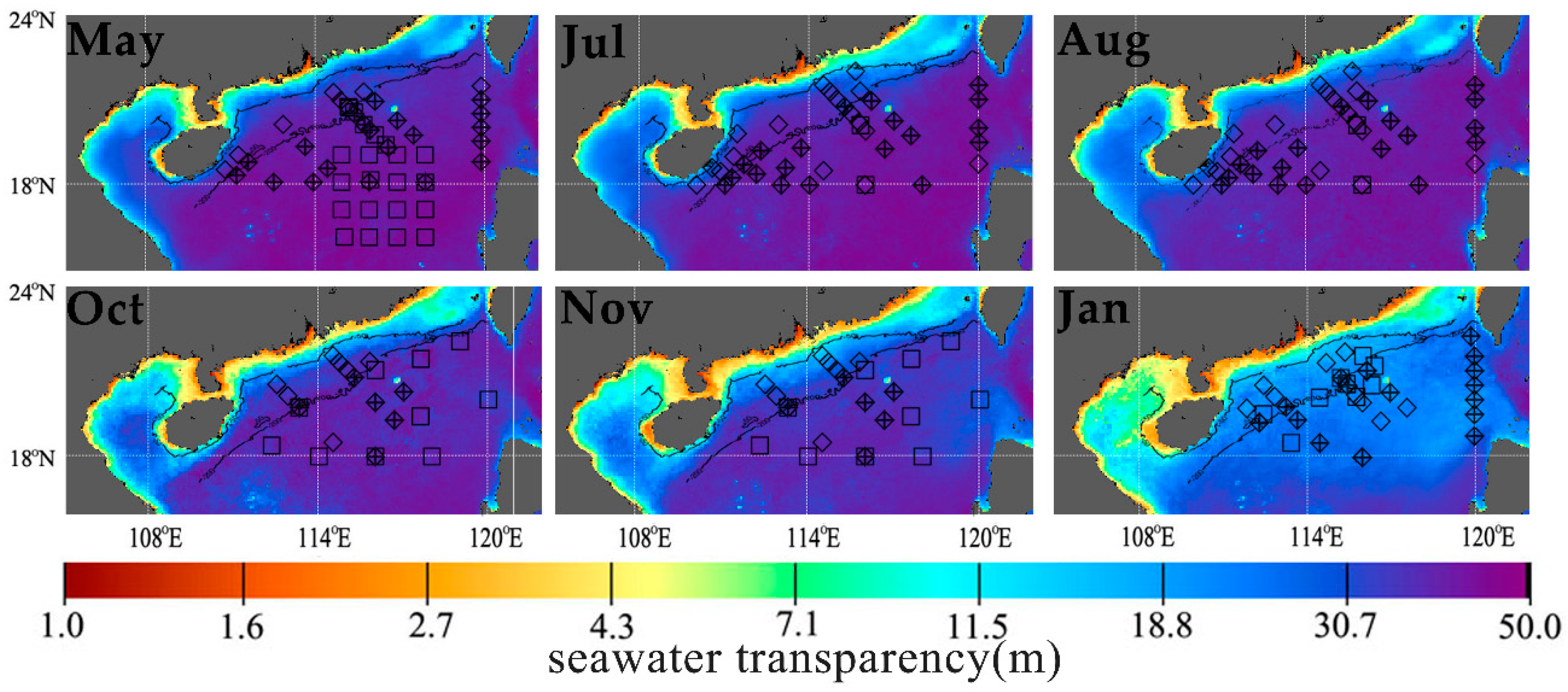
2.2. In Situ POC Export Flux and Chla Data
2.3. Data of Particle Size Distribution
2.4. Satellite Data
2.5. Data of POC Export Efficiency in Euphotic Layer
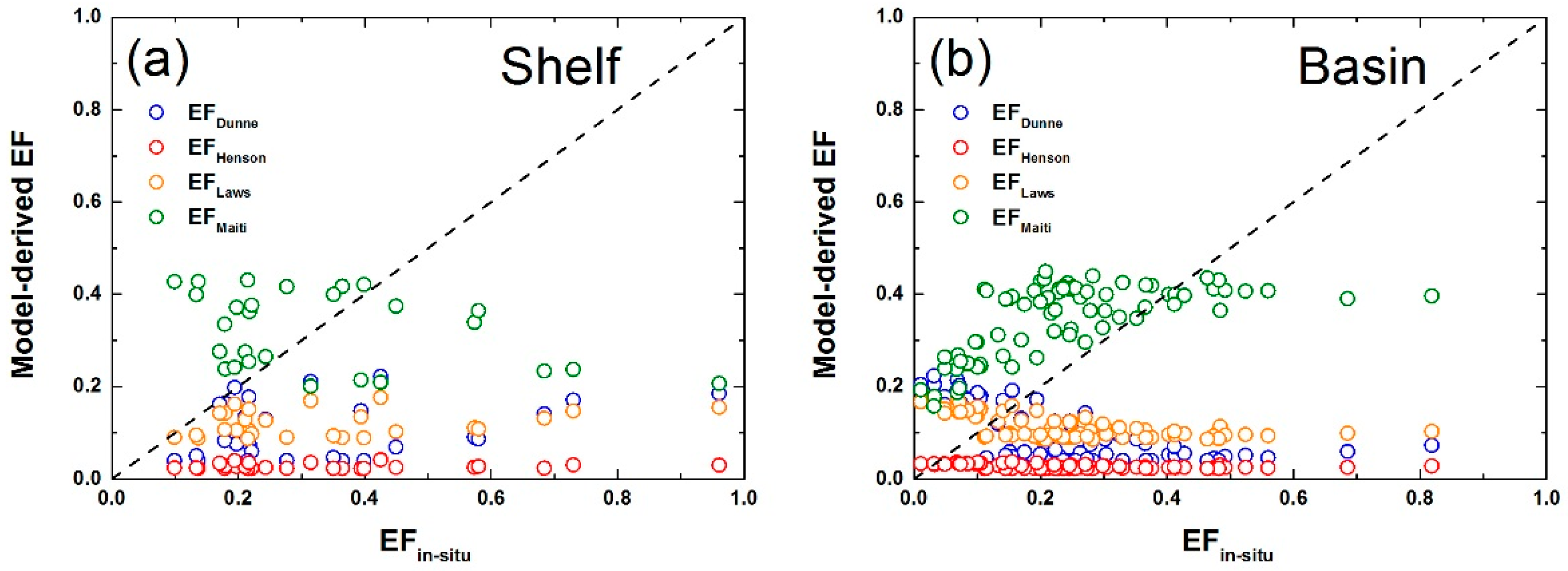
2.6. Models of POC Export Efficiency
3. Results
3.1. Spatiotemporal Distribution of POC Export Efficiency in the NSCS
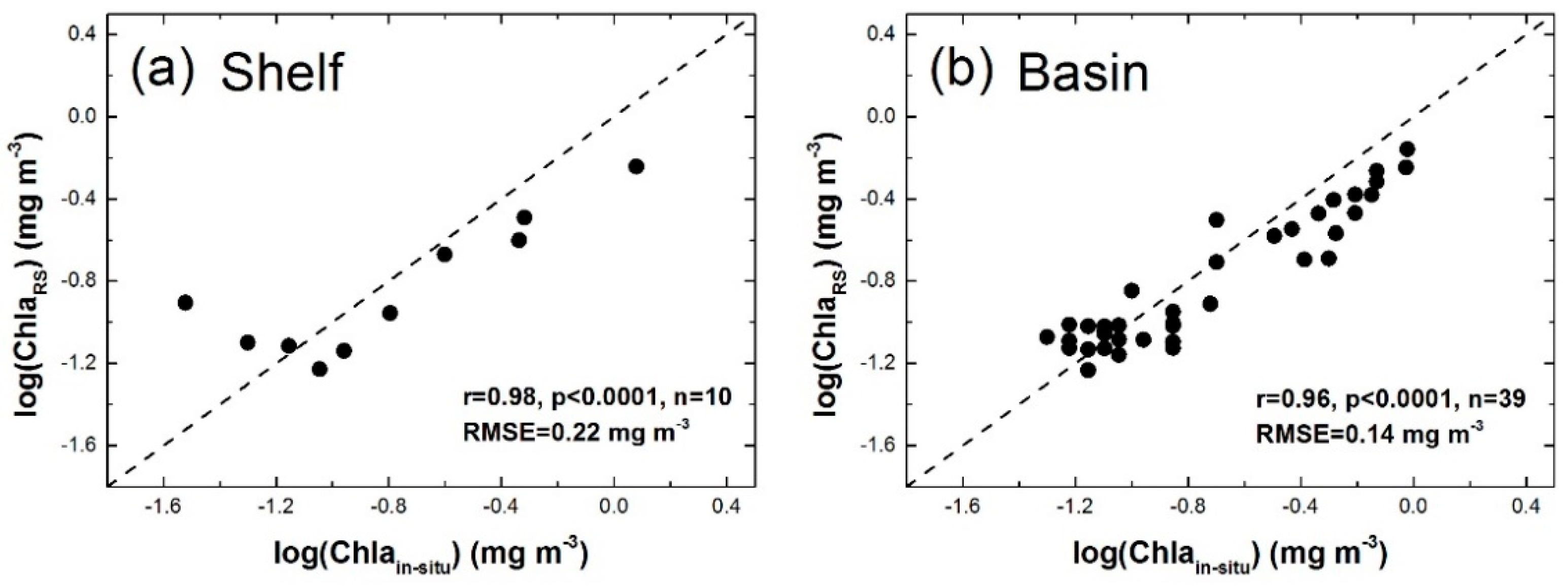
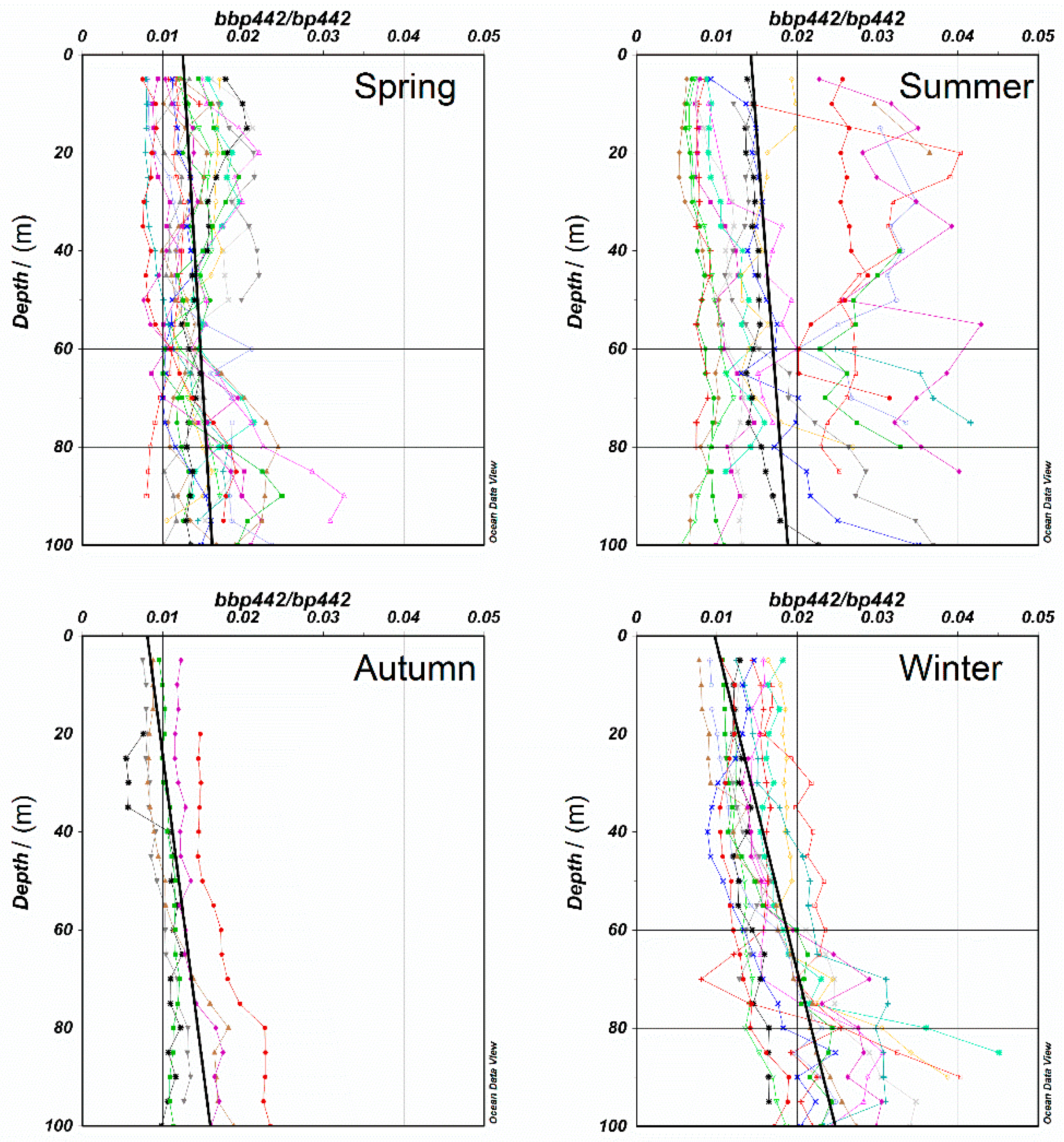
3.1.1. Applicability of VGPM Products in the NSCS
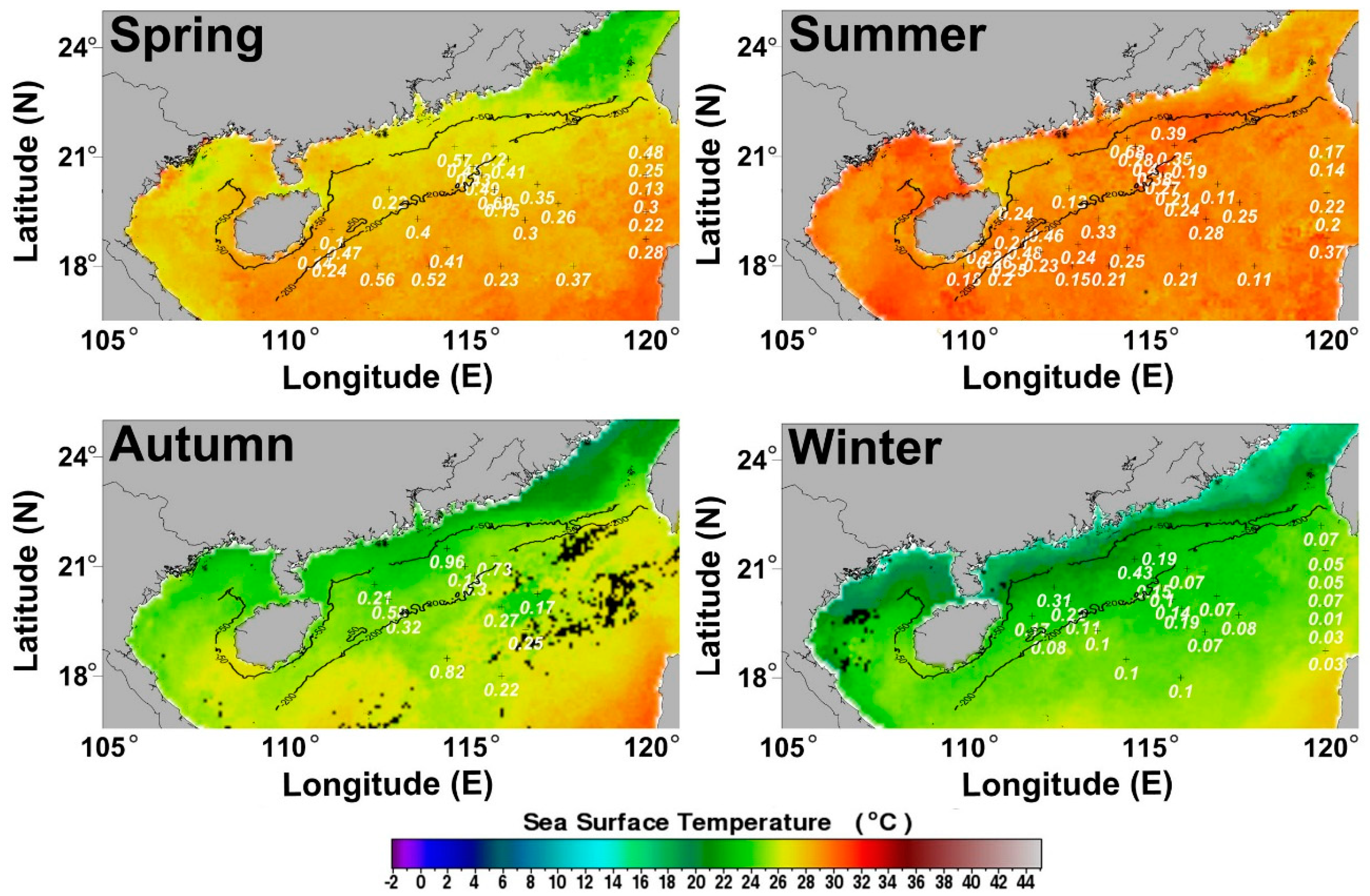
3.1.2. Seasonal Variation of POC Export Efficiency in Euphotic Layer of the NSCS
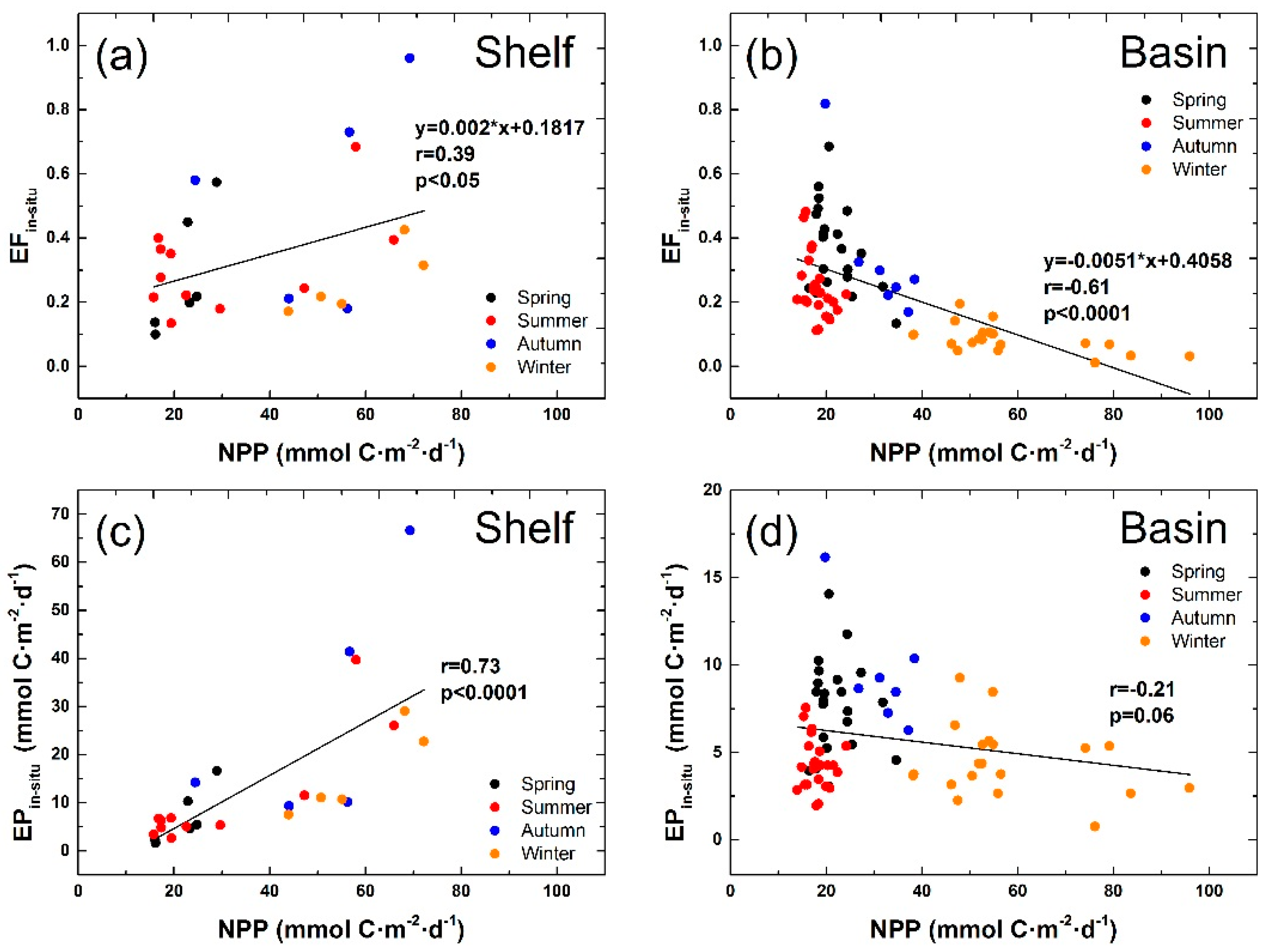
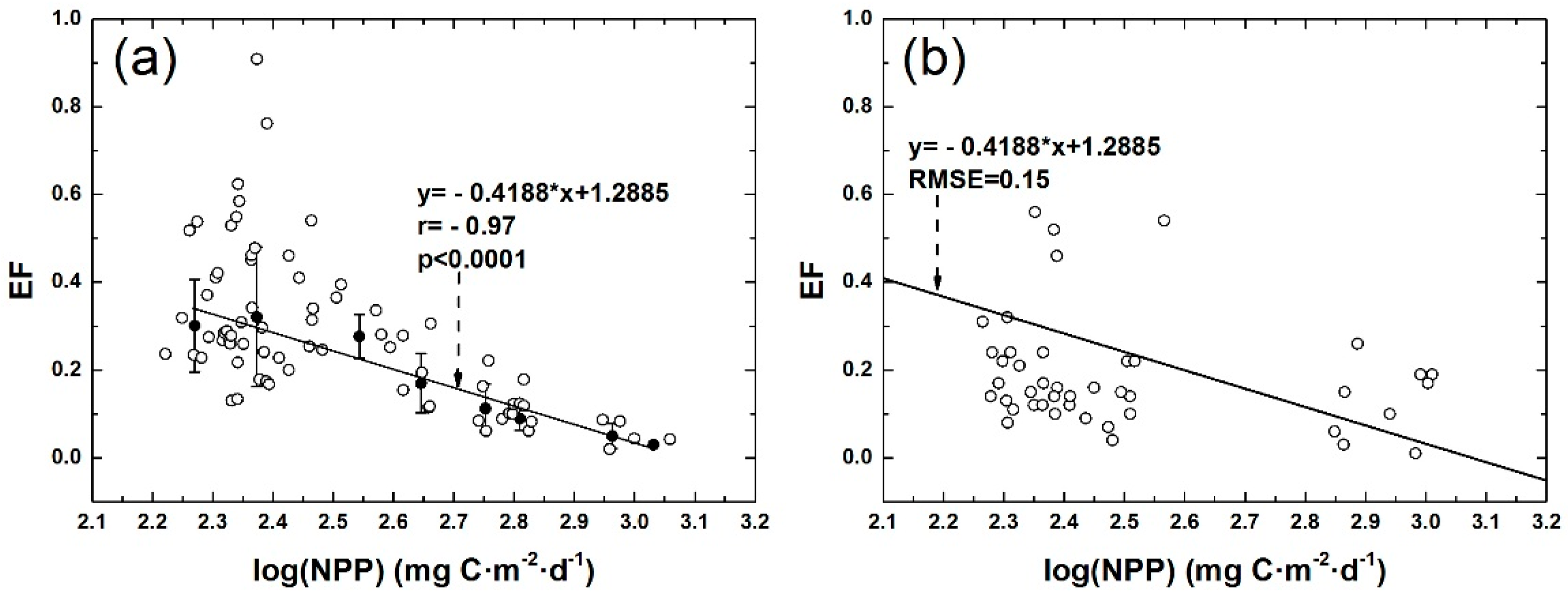
3.2. Correlation between POC Export Efficiency and NPP in Euphotic Layer of the NSCS
3.3. Comparison of Different Models of POC Export Efficiency
4. Discussion
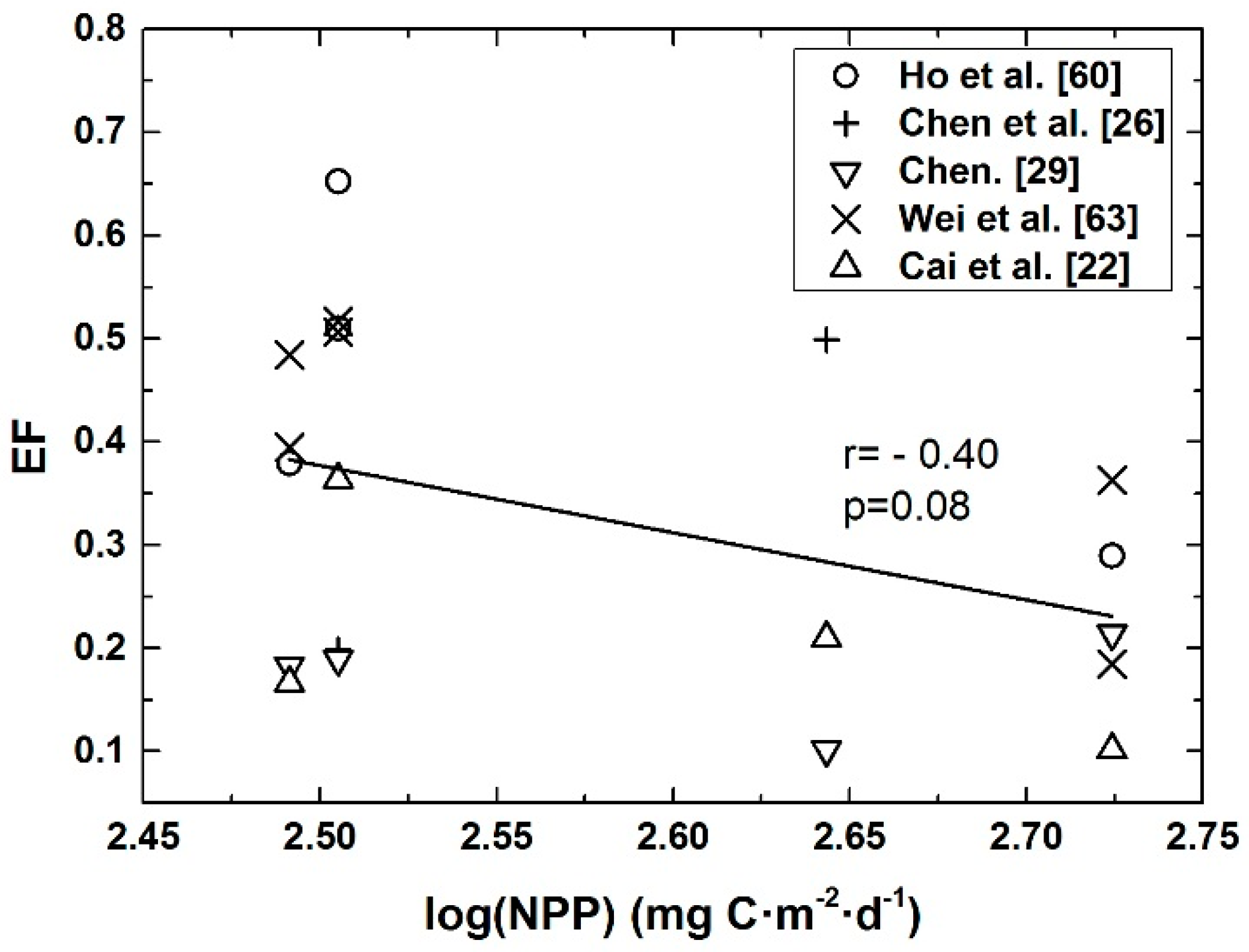
4.1. Seasonal Mean POC Export Efficiency and NPP in Euphotic Layer of the Basin Regions
4.2. Effect of Phytoplankton Community Structure
4.3. Factors in Driving the Inverse Relationship between POC Export Efficiency and NPP in Euphotic Layer of the Basin Regions
4.3.1. Effect of Zooplankton Grazing
4.3.2. Effect of DOC Export in the Basin
4.3.3. Seasonal Decoupling between Primary Production and POC Export
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Stations | Lat | Lon | Export Depth | Chlain-situ | EPin_situ | SSTrs | PARrs | 3d-Chlars | 16d-Chlars | NPPrs | EFin_situ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring 1–21 May 2011 | |||||||||||
| E401 | 21.50 | 120.00 | 100 | 0.1 | 11.8 | 25.18 | 42.86 | 0.09 | 24.38 | 0.48 | |
| E402 | 21.00 | 120.00 | 100 | 0.1 | 7.9 | 25.16 | 41.52 | 0.14 | 0.13 | 31.85 | 0.25 |
| E403 | 20.50 | 120.00 | 100 | 0.08 | 4.6 | 25.74 | 42.16 | 0.15 | 34.62 | 0.13 | |
| E404 | 20.00 | 120.00 | 100 | 0.1 | 7.4 | 26.26 | 44.01 | 0.10 | 24.52 | 0.30 | |
| E405 | 19.50 | 120.00 | 100 | 0.07 | 5.5 | 26.89 | 49.47 | 0.10 | 0.11 | 25.43 | 0.22 |
| E406 | 18.75 | 120.00 | 100 | 0.07 | 6.8 | 27.70 | 50.97 | 0.07 | 0.12 | 24.43 | 0.28 |
| LE09 | 18.00 | 118.00 | 100 | 0.09 | 8.5 | 26.82 | 55.78 | 0.10 | 0.10 | 23.26 | 0.37 |
| SEATS | 18.00 | 116.00 | 100 | 0.06 | 4.1 | 27.49 | 52.37 | 0.07 | 17.89 | 0.23 | |
| LE05 | 18.00 | 114.00 | 100 | 0.06 | 9.7 | 27.57 | 55.07 | 0.07 | 0.07 | 18.51 | 0.52 |
| LE04a | 18.00 | 112.58 | 100 | 0.08 | 10.3 | 28.20 | 55.90 | 0.10 | 0.08 | 18.41 | 0.56 |
| D104 | 18.73 | 111.67 | 100 | 0.06 | 8.5 | 28.07 | 51.46 | 0.08 | 0.08 | 17.95 | 0.47 |
| D103 | 19.00 | 111.32 | 50 | 0.05 | 1.6 | 28.15 | 53.55 | 0.08 | 0.07 | 16.16 | 0.10 |
| DD202 | 18.46 | 110.85 | 50 | 0.07 | 2.2 | 28.58 | 52.99 | 0.07 | 16.10 | 0.14 | |
| DD203 | 18.25 | 111.25 | 100 | 0.09 | 4 | 28.49 | 54.47 | 0.07 | 16.47 | 0.24 | |
| E603 | 20.11 | 112.92 | 50 | 0.1 | 5.4 | 27.65 | 53.63 | 0.11 | 24.83 | 0.22 | |
| E605 | 19.30 | 113.70 | 100 | 0.11 | 7.8 | 27.93 | 55.19 | 0.08 | 19.38 | 0.40 | |
| E607 | 18.50 | 114.50 | 100 | 0.08 | 8 | 28.06 | 55.19 | 0.07 | 0.09 | 19.40 | 0.41 |
| A6 | 21.27 | 114.72 | 50 | 0.17 | 16.6 | 27.45 | 46.47 | 0.14 | 28.94 | 0.57 | |
| A5 | 20.98 | 114.98 | 50 | 0.18 | 10.3 | 27.61 | 50.02 | 0.10 | 22.92 | 0.45 | |
| A4 | 20.73 | 115.23 | 100 | 0.16 | 8.4 | 27.63 | 51.57 | 0.08 | 19.66 | 0.43 | |
| A2 | 20.49 | 115.47 | 100 | 0.15 | 9 | 27.75 | 51.70 | 0.07 | 18.30 | 0.49 | |
| A1a | 20.10 | 115.83 | 100 | 0.15 | 14.1 | 27.65 | 52.93 | 0.09 | 20.58 | 0.69 | |
| A0 | 19.90 | 116.02 | 100 | 0.25 | 3.1 | 27.63 | 49.30 | 0.09 | 20.52 | 0.15 | |
| A10 | 19.27 | 116.67 | 100 | 0.26 | 5.9 | 27.83 | 49.16 | 0.08 | 19.43 | 0.30 | |
| S504 | 19.72 | 117.58 | 100 | 0.24 | 5.3 | 27.88 | 44.34 | 0.09 | 20.19 | 0.26 | |
| S503 | 20.25 | 117.00 | 100 | 0.51 | 9.6 | 27.29 | 46.64 | 0.13 | 27.31 | 0.35 | |
| S501 | 20.95 | 116.20 | 100 | 0.31 | 9.2 | 27.17 | 46.18 | 0.09 | 22.36 | 0.41 | |
| S209 | 21.30 | 115.80 | 50 | 0.15 | 4.6 | 26.93 | 44.25 | 0.10 | 23.33 | 0.20 | |
| Summer 20 July to 15 August 2009 | |||||||||||
| LE01 | 18.00 | 110.00 | 50 | 0.16 | 5.3 | 28.64 | 44.21 | 0.17 | 29.68 | 0.18 | |
| LE02 | 18.00 | 111.00 | 100 | 0.08 | 3.2 | 29.05 | 40.44 | 0.09 | 0.07 | 16.02 | 0.20 |
| DD203 | 18.25 | 111.25 | 100 | 0.08 | 4.4 | 29.00 | 40.87 | 0.09 | 0.08 | 17.93 | 0.25 |
| DD202 | 18.47 | 110.85 | 50 | 0.07 | 6.3 | 28.85 | 42.06 | 0.08 | 0.08 | 17.28 | 0.36 |
| DD201 | 18.60 | 110.55 | 50 | 0.16 | 5 | 28.65 | 46.56 | 0.11 | 0.11 | 22.61 | 0.22 |
| D103 | 19.00 | 111.32 | 50 | 0.09 | 3.4 | 28.78 | 41.87 | 0.06 | 0.07 | 15.82 | 0.21 |
| D104 | 18.72 | 111.67 | 100 | 0.07 | 7.6 | 28.81 | 44.95 | 0.06 | 0.07 | 15.77 | 0.48 |
| D105 | 18.38 | 112.12 | 100 | 0.08 | 4.3 | 28.81 | 43.99 | 0.09 | 18.81 | 0.23 | |
| LE04 | 18.00 | 113.00 | 100 | 0.14 | 3.1 | 28.77 | 44.23 | 0.10 | 0.10 | 20.00 | 0.15 |
| LE05 | 18.00 | 114.00 | 100 | 0.09 | 3.2 | 29.17 | 48.90 | 0.08 | 0.07 | 15.55 | 0.21 |
| E505 | 18.60 | 113.15 | 100 | 0.14 | 4.1 | 28.94 | 46.03 | 0.08 | 0.08 | 17.38 | 0.24 |
| E503 | 19.20 | 112.27 | 100 | 0.05 | 7.1 | 28.84 | 47.86 | 0.08 | 0.06 | 15.31 | 0.46 |
| E501 | 19.80 | 111.43 | 50 | 0.03 | 11.5 | 27.78 | 48.17 | 0.12 | 0.30 | 47.29 | 0.24 |
| E603 | 20.12 | 112.90 | 50 | 0.06 | 2.6 | 28.18 | 51.06 | 0.09 | 19.46 | 0.13 | |
| E605 | 19.30 | 113.70 | 100 | 0.06 | 5.4 | 28.39 | 50.98 | 0.07 | 16.37 | 0.33 | |
| E607 | 18.50 | 114.50 | 100 | 0.09 | 4.4 | 28.59 | 48.80 | 0.08 | 17.48 | 0.25 | |
| S206 | 22.00 | 115.63 | 50 | 1.2 | 26 | 28.93 | 50.56 | 0.57 | 0.54 | 65.99 | 0.39 |
| S209 | 21.30 | 115.80 | 50 | 0.11 | 6.8 | 28.61 | 51.84 | 0.07 | 0.09 | 19.40 | 0.35 |
| S501 | 20.95 | 116.20 | 100 | 0.14 | 3.5 | 28.49 | 49.63 | 0.08 | 0.08 | 18.40 | 0.19 |
| S503 | 20.25 | 117.00 | 100 | 0.05 | 2 | 28.83 | 44.37 | 0.08 | 17.97 | 0.11 | |
| S504 | 19.73 | 117.60 | 100 | 0.1 | 4.5 | 28.78 | 44.86 | 0.08 | 17.65 | 0.25 | |
| A10 | 19.27 | 116.67 | 100 | 0.07 | 4.2 | 28.89 | 44.66 | 0.06 | 14.87 | 0.28 | |
| A0 | 19.90 | 116.00 | 100 | 0.12 | 4.2 | 28.71 | 49.99 | 0.08 | 17.83 | 0.24 | |
| A1 | 20.20 | 115.75 | 100 | 0.1 | 4.3 | 28.68 | 49.71 | 0.10 | 20.33 | 0.21 | |
| A2 | 20.50 | 115.48 | 100 | 0.14 | 5.1 | 28.64 | 52.65 | 0.09 | 18.64 | 0.27 | |
| A4 | 20.75 | 115.25 | 100 | 0.11 | 6.4 | 28.65 | 52.60 | 0.07 | 17.04 | 0.38 | |
| A7 | 21.50 | 114.50 | 50 | 0.25 | 39.7 | 28.57 | 50.29 | 0.21 | 0.45 | 58.04 | 0.68 |
| A6 | 21.27 | 114.73 | 50 | 0.18 | 4.8 | 28.67 | 48.26 | 0.08 | 17.35 | 0.28 | |
| A5 | 21.00 | 114.98 | 50 | 0.18 | 6.7 | 28.74 | 46.07 | 0.07 | 16.80 | 0.40 | |
| SEATS | 18.00 | 115.97 | 100 | 0.14 | 2.9 | 28.42 | 39.80 | 0.10 | 0.06 | 13.95 | 0.21 |
| LE09 | 18.00 | 118.00 | 100 | 0.09 | 2.1 | 28.35 | 41.00 | 0.07 | 0.09 | 18.38 | 0.11 |
| E406 | 18.75 | 120.00 | 100 | 0.11 | 6.2 | 28.65 | 43.31 | 0.08 | 0.08 | 16.92 | 0.37 |
| E405 | 19.50 | 120.02 | 100 | 0.06 | 4.3 | 28.87 | 41.05 | 0.10 | 0.11 | 21.54 | 0.20 |
| E404 | 20.00 | 120.00 | 100 | 0.13 | 5.4 | 28.92 | 44.87 | 0.13 | 24.19 | 0.22 | |
| E402 | 21.00 | 120.00 | 100 | 0.11 | 3 | 28.82 | 47.31 | 0.10 | 20.74 | 0.14 | |
| E401 | 21.50 | 120.00 | 100 | 0.14 | 3.9 | 28.69 | 46.58 | 0.11 | 0.11 | 22.35 | 0.17 |
| Autumn 31 October to 22 November 2010 | |||||||||||
| E602 | 20.51 | 112.50 | 50 | 0.93 | 9.3 | 26.82 | 33.52 | 0.30 | 44.07 | 0.21 | |
| E603 | 20.11 | 112.90 | 50 | 0.5 | 14.2 | 26.65 | 28.89 | 0.13 | 24.48 | 0.58 | |
| E604 | 19.71 | 113.30 | 100 | 0.55 | 8.7 | 26.63 | 27.83 | 0.15 | 26.81 | 0.32 | |
| A7 | 21.50 | 114.50 | 50 | 66.6 | 25.49 | 27.77 | 1.30 | 0.53 | 69.32 | 0.96 | |
| A5 | 21.00 | 115.00 | 50 | 0.44 | 10.1 | 26.04 | 20.66 | 0.44 | 56.31 | 0.18 | |
| A4 | 20.75 | 115.25 | 100 | 0.52 | 9.3 | 26.00 | 24.18 | 0.39 | 0.18 | 31.20 | 0.30 |
| E607 | 18.49 | 114.50 | 100 | 16.2 | 26.25 | 22.42 | 0.10 | 19.79 | 0.82 | ||
| S209 | 21.30 | 115.80 | 50 | 0.31 | 41.4 | 25.24 | 24.52 | 0.40 | 56.71 | 0.73 | |
| SEATS | 18.00 | 116.00 | 100 | 0.34 | 7.3 | 25.19 | 22.12 | 0.18 | 32.93 | 0.22 | |
| A0 | 19.89 | 116.00 | 100 | 0.19 | 10.4 | 24.71 | 19.93 | 0.12 | 0.23 | 38.44 | 0.27 |
| A10 | 19.27 | 116.67 | 100 | 0.2 | 8.5 | 25.83 | 19.72 | 0.20 | 0.22 | 34.58 | 0.25 |
| S503 | 20.25 | 117.00 | 100 | 0.41 | 6.3 | 25.77 | 23.25 | 0.20 | 0.23 | 37.18 | 0.17 |
| Winter 4–30 January 2010 | |||||||||||
| E603 | 20.11 | 112.90 | 50 | 0.57 | 11 | 23.44 | 24.03 | 0.30 | 50.74 | 0.22 | |
| E602 | 20.50 | 112.50 | 50 | 0.5 | 22.7 | 23.20 | 16.64 | 0.55 | 72.20 | 0.31 | |
| E501a | 19.70 | 111.90 | 50 | 0.46 | 7.5 | 23.80 | 23.00 | 0.25 | 0.25 | 43.95 | 0.17 |
| E502a | 19.20 | 112.35 | 100 | 0.46 | 4.4 | 24.00 | 28.54 | 0.34 | 0.32 | 52.55 | 0.08 |
| E604 | 19.75 | 113.30 | 100 | 0.37 | 5.7 | 23.77 | 24.17 | 0.28 | 0.34 | 54.06 | 0.11 |
| E605 | 19.30 | 113.70 | 100 | 0.2 | 5.5 | 23.90 | 30.51 | 0.31 | 0.31 | 52.73 | 0.10 |
| E607 | 18.50 | 114.50 | 100 | 0.5 | 3.8 | 24.20 | 32.66 | 0.20 | 0.20 | 38.30 | 0.10 |
| SEATS | 18.00 | 116.00 | 100 | 3.7 | 24.32 | 32.60 | 0.20 | 38.20 | 0.10 | ||
| A10 | 19.26 | 116.67 | 100 | 0.72 | 3.2 | 23.64 | 32.11 | 0.25 | 46.17 | 0.07 | |
| A0 | 19.90 | 116.02 | 100 | 9.3 | 23.51 | 24.21 | 0.28 | 47.96 | 0.19 | ||
| A1 | 20.16 | 115.77 | 100 | 0.59 | 6.6 | 23.50 | 22.82 | 0.27 | 46.92 | 0.14 | |
| A2 | 20.50 | 115.48 | 100 | 0.61 | 5.5 | 23.11 | 22.41 | 0.33 | 54.86 | 0.10 | |
| A4 | 20.75 | 115.25 | 100 | 0.59 | 8.5 | 22.62 | 21.05 | 0.33 | 54.86 | 0.15 | |
| A6 | 21.27 | 114.73 | 50 | 0.37 | 29 | 21.33 | 22.31 | 0.42 | 68.23 | 0.43 | |
| S103 | 21.65 | 115.40 | 50 | 0.48 | 10.7 | 21.98 | 20.38 | 0.32 | 0.32 | 55.13 | 0.19 |
| S501a | 21.00 | 116.17 | 100 | 0.74 | 3.8 | 23.15 | 21.60 | 0.54 | 0.35 | 56.50 | 0.07 |
| S503 | 20.25 | 117.00 | 100 | 0.71 | 5.4 | 23.47 | 24.69 | 0.42 | 0.57 | 79.19 | 0.07 |
| S504 | 19.73 | 117.60 | 100 | 0.62 | 4.4 | 24.26 | 29.74 | 0.34 | 0.32 | 51.88 | 0.08 |
| E406 | 18.75 | 120.00 | 100 | 0.32 | 2.7 | 25.01 | 36.36 | 0.26 | 0.65 | 83.63 | 0.03 |
| E405 | 19.49 | 120.00 | 100 | 0.95 | 3 | 24.21 | 34.30 | 0.70 | 0.75 | 95.91 | 0.03 |
| E404 | 20.00 | 120.00 | 100 | 0.74 | 0.8 | 24.17 | 29.98 | 0.48 | 0.54 | 76.16 | 0.01 |
| E403 | 20.50 | 120.00 | 100 | 0.94 | 5.3 | 24.17 | 28.32 | 0.57 | 0.53 | 74.18 | 0.07 |
| E402 | 21.00 | 120.00 | 100 | 0.62 | 2.7 | 24.20 | 27.79 | 0.42 | 0.35 | 55.90 | 0.05 |
| E401 | 21.50 | 120.00 | 100 | 0.53 | 2.3 | 24.57 | 27.19 | 0.27 | 0.29 | 47.50 | 0.05 |
| E400 | 22.19 | 119.87 | 100 | 0.19 | 3.7 | 24.61 | 32.26 | 0.12 | 0.31 | 50.52 | 0.07 |
| Stations | Lat | Lon | 3D_EPin_situ | EPin_situ | SSTrs | PARrs | Chlars | NPPrs | EFin_situ | EFrs |
|---|---|---|---|---|---|---|---|---|---|---|
| Spring 29 April to 11 May | ||||||||||
| S23 | 20.75 | 115.25 | 6.6 | 9.27 | 24.77 | 44.02 | 0.06 | 20.35 | 0.46 | 0.29 |
| S22 | 20.71 | 115.29 | 1.6 | 2.59 | 24.84 | 47.20 | 0.10 | 26.94 | 0.10 | 0.24 |
| S21 | 20.55 | 115.46 | 2.5 | 3.72 | 24.84 | 47.20 | 0.10 | 26.93 | 0.14 | 0.24 |
| S19 | 20.1 | 115.8 | 1 | 1.07 | 25.78 | 51.23 | 0.10 | 25.19 | 0.04 | 0.25 |
| S18 | 19.73 | 116.2 | 4.2 | 4.02 | 25.98 | 53.50 | 0.10 | 26.03 | 0.15 | 0.24 |
| S17 | 19.37 | 116.6 | 6 | 5.95 | 26.24 | 54.67 | 0.11 | 26.63 | 0.22 | 0.24 |
| S14 | 19 | 117 | 1.9 | 2.00 | 26.88 | 55.11 | 0.09 | 22.73 | 0.09 | 0.27 |
| S13 | 19 | 117.99 | 3.6 | 3.74 | 27.15 | 54.96 | 0.10 | 23.46 | 0.16 | 0.26 |
| S12 | 18 | 118 | 4.1 | 4.16 | 27.78 | 55.00 | 0.07 | 17.05 | 0.24 | 0.32 |
| S05 | 17.01 | 118 | 3.9 | 3.79 | 28.21 | 56.24 | 0.08 | 17.65 | 0.21 | 0.31 |
| S04 | 16 | 118 | 1.5 | 1.37 | 28.92 | 55.79 | 0.08 | 16.87 | 0.08 | 0.32 |
| S03 | 16 | 117 | 3.9 | 3.77 | 28.77 | 55.62 | 0.07 | 15.90 | 0.24 | 0.33 |
| S02 | 16 | 116 | 3.8 | 3.59 | 28.45 | 55.61 | 0.07 | 16.55 | 0.22 | 0.33 |
| S01 | 16.01 | 115.11 | 3.5 | 3.34 | 28.22 | 55.52 | 0.09 | 19.33 | 0.17 | 0.30 |
| S08 | 17 | 115 | 11 | 10.50 | 27.71 | 55.68 | 0.09 | 20.13 | 0.52 | 0.29 |
| S07 | 17 | 116 | 2.2 | 2.15 | 28.08 | 55.85 | 0.08 | 18.65 | 0.12 | 0.30 |
| S06 | 17 | 117 | 5.4 | 5.32 | 28.61 | 55.74 | 0.08 | 16.84 | 0.32 | 0.32 |
| S11 | 18 | 117 | 2 | 2.16 | 28.26 | 55.51 | 0.07 | 16.79 | 0.13 | 0.32 |
| S10 | 18.09 | 116.05 | 1.8 | 1.82 | 28.15 | 55.44 | 0.07 | 17.24 | 0.11 | 0.32 |
| S09 | 18 | 115 | 3.5 | 3.31 | 28.15 | 54.40 | 0.09 | 20.38 | 0.16 | 0.29 |
| S16 | 19 | 115 | 2.8 | 2.66 | 27.72 | 51.70 | 0.09 | 21.37 | 0.12 | 0.28 |
| S15 | 19 | 116 | 1.9 | 1.85 | 27.46 | 51.54 | 0.11 | 24.76 | 0.07 | 0.25 |
| Summer 10–13 July | ||||||||||
| A1 | 20.1 | 115.81 | 4.70 | 28.69 | 48.76 | 0.09 | 19.31 | 0.24 | 0.30 | |
| S1 | 18 | 116 | 4.76 | 29.32 | 47.98 | 0.06 | 15.34 | 0.31 | 0.34 | |
| Autumn 19 September to 2 October | ||||||||||
| E104 | 21 | 116 | 15.7 | 16.43 | 28.66 | 33.50 | 0.21 | 30.67 | 0.54 | 0.21 |
| E108 | 19.4 | 117.6 | 2.6 | 2.81 | 28.63 | 38.07 | 0.10 | 18.43 | 0.15 | 0.31 |
| E205 | 21.4 | 117.61 | 9.4 | 10.43 | 28.79 | 33.62 | 0.10 | 18.73 | 0.56 | 0.30 |
| E306 | 22.01 | 119 | 6.3 | 5.91 | 27.87 | 38.81 | 0.15 | 27.40 | 0.22 | 0.23 |
| E404 | 19.98 | 120.01 | 2.6 | 2.75 | 28.60 | 39.20 | 0.11 | 20.15 | 0.14 | 0.29 |
| E408 | 18 | 118 | 2.3 | 2.17 | 28.58 | 41.46 | 0.08 | 15.82 | 0.14 | 0.33 |
| E410 | 18 | 115.99 | 2.9 | 2.85 | 28.33 | 38.60 | 0.08 | 16.30 | 0.17 | 0.33 |
| E412 | 18 | 114 | 2.3 | 2.24 | 28.43 | 40.73 | 0.10 | 19.27 | 0.12 | 0.30 |
| E501 | 18.38 | 112.32 | 2.1 | 2.11 | 28.58 | 48.58 | 0.11 | 20.22 | 0.10 | 0.29 |
| E606 | 19.7 | 113.3 | 2.8 | 2.94 | 28.58 | 44.08 | 0.12 | 21.42 | 0.14 | 0.28 |
| Winter 18 February to 1 March | ||||||||||
| D3 | 19.5 | 112.5 | 15.9 | 15.80 | 22.37 | 24.40 | 0.56 | 85.27 | 0.19 | 0.03 |
| D1 | 18.5 | 113.49 | 16.6 | 16.42 | 22.55 | 29.70 | 0.36 | 64.16 | 0.26 | 0.08 |
| C2 | 20.08 | 114.49 | 1.2 | 1.14 | 21.24 | 28.90 | 0.47 | 80.07 | 0.01 | 0.04 |
| A4 | 20.75 | 115.24 | 16.3 | 15.50 | 22.30 | 36.22 | 0.48 | 81.59 | 0.19 | 0.04 |
| A3 | 20.54 | 115.46 | 9.9 | 9.18 | 22.54 | 38.40 | 0.32 | 61.11 | 0.15 | 0.09 |
| A1 | 20.1 | 115.8 | 8 | 7.46 | 22.70 | 41.29 | 0.41 | 72.60 | 0.10 | 0.06 |
| B1 | 20.5 | 116.34 | 4 | 3.70 | 23.11 | 40.76 | 0.31 | 58.80 | 0.06 | 0.10 |
| B2 | 21.17 | 116.45 | 1.9 | 1.91 | 22.69 | 36.78 | 0.32 | 60.82 | 0.03 | 0.09 |
| B3 | 21.51 | 116.04 | 13.5 | 13.98 | 22.29 | 34.22 | 0.50 | 83.97 | 0.17 | 0.03 |
| Season | Time | Number of Stations | NPP g C·m−2·day−1 |
|---|---|---|---|
| Spring | March 2001 | 3 | 0.44 ± 0.30 |
| March 2002 | 4 | ||
| Summer | 27 June–4 July 2001 | 5 | 0.31 ± 0.14 |
| 6–19 July 2003 | 4 | ||
| 24 June–6 July 2004 | 2 | ||
| Autumn | October 2001 | 1 | 0.32 ± 0.14 |
| October 2002 | 5 | ||
| Winter | 18–24 January 2003 | 1 | 0.53 ± 0.19 |
| 10–22 February 2004 | 5 |
References
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.E.; Elser, J.; Gruber, N.; Hibbard, K.; Hogberg, P.; Linder, S. The global carbon cycle: A test of our knowledge of the earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- DeVries, T.; Primeau, F.; Deutsch, C. The sequestration efficiency of the biological pump. Geophys. Res. Lett. 2012, 39, L13601. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Steinberg, D.K.; Buesseler, K.O. Upper ocean carbon export and the biological pump. Oceanography 2001, 14, 50–58. [Google Scholar] [CrossRef]
- Henson, S.A.; Sanders, R.; Madsen, E.; Morris, P.J.; Moigne, F.L.; Quartly, G.D. A reduced estimate of the strength of the ocean’s biological carbon pump. Geophys. Res. Lett. 2011, 38, L04606. [Google Scholar] [CrossRef]
- Tsunogai, S.; Watanabe, S.; Sato, T. Is there a “continental shelf pump” for the absorption of atmospheric CO2? Tellus B 1999, 51, 701–712. [Google Scholar] [CrossRef]
- Boyd, P.W.; Trull, T.W. Understanding the export of biogenic particles in oceanic waters: Is there consensus? Prog. Oceanogr. 2007, 72, 276–312. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Maiti, K.; Bosu, S.; D’Sa, E.J.; Adhikari, P.L.; Sutor, M.; Longnecker, K. Export fluxes in northern gulf of mexico-comparative evaluation of direct, indirect and satellite-based estimates. Mar. Chem. 2016, 184, 60–77. [Google Scholar] [CrossRef]
- Bopp, L.; Monfray, P.; Aumont, O.; Dufresne, J.L.; Le Treut, H.; Madec, G.; Terray, L.; Orr, J.C. Potential impact of climate change on marine export production. Glob. Biogeochem. Cycles 2001, 15, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Gregg, W.W.; Conkright, M.E.; Ginoux, P.; Oreilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 30, 1809:1–1809:4. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Steinacher, M.; Joos, F.; Frolicher, T.L.; Bopp, L.; Cadule, P.; Cocco, V.; Doney, S.C.; Gehlen, M.; Lindsay, K.; Moore, J.K. Projected 21st century decrease in marine productivity: A multi-model analysis. Biogeosciences 2010, 7, 979–1005. [Google Scholar] [CrossRef] [Green Version]
- Henson, S.A.; Yool, A.; Sanders, R.J. Variability in efficiency of particulate organic carbon export: A model study. Glob. Biogeochem. Cycles 2015, 29, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Dunne, J.P.; Armstrong, R.A.; Gnanadesikan, A.; Sarmiento, J.L. Empirical and mechanistic models for the particle export ratio. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef] [Green Version]
- Laws, E.A.; Dsa, E.J.; Naik, P. Simple equations to estimate ratios of new or export production to total production from satellite-derived estimates of sea surface temperature and primary production. Limnol. Oceanogr. Methods 2011, 9, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Maiti, K.; Charette, M.A.; Buesseler, K.O.; Kahru, M. An inverse relationship between production and export efficiency in the Southern Ocean. Geophys. Res. Lett. 2013, 40, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Cavan, E.; Henson, S.A.; Belcher, A.; Sanders, R.J. Role of zooplankton in determining the efficiency of the biological carbon pump. Biogeosciences 2017, 14, 177–186. [Google Scholar] [CrossRef]
- Chen, C.; Shiah, F.K.; Chung, S.W.; Liu, K.K. Winter phytoplankton blooms in the shallow mixed layer of the south china sea enhanced by upwelling. J. Mar. Syst. 2006, 59, 97–110. [Google Scholar] [CrossRef]
- Hu, S.; Cao, W.; Wang, G.; Xu, Z.; Zhao, W.; Lin, J.; Zhou, W.; Yao, L. Empirical ocean color algorithm for estimating particulate organic carbon in the south china sea. Chin. J. Oceanol. Limnol. 2015, 33, 764–778. [Google Scholar] [CrossRef]
- Chen, X.; Pan, D.; Bai, Y.; He, X.; Chen, C.T.A.; Kang, Y.; Tao, B. Estimation of typhoon-enhanced primary production in the south china sea: A comparison with the Western North Pacific. Cont. Shelf Res. 2015, 111, 286–293. [Google Scholar] [CrossRef]
- He, X.; Xu, D.; Bai, Y.; Pan, D.; Chen, C.T.A.; Chen, X.; Gong, F. Eddy-entrained Pearl River plume into the oligotrophic basin of the South China Sea. Cont. Shelf Res. 2016, 124, 117–124. [Google Scholar] [CrossRef]
- Cai, P.; Zhao, D.; Wang, L.; Huang, B.; Dai, M. Role of particle stock and phytoplankton community structure in regulating particulate organic carbon export in a large marginal sea. J. Geophys. Res. Oceans 2015, 120, 2063–2095. [Google Scholar] [CrossRef]
- Liu, G.; Chai, F. Seasonal and interannual variability of primary and export production in the south china sea: A three-dimensional physical–biogeochemical model study. ICES J. Mar. Sci. 2008, 66, 420–431. [Google Scholar] [CrossRef]
- Cai, P.; Chen, W.; Dai, M.; Wan, Z.; Wang, D.; Li, Q.; Tang, T.; Lv, D. A high-resolution study of particle export in the southern south china sea based on 234th:238u disequilibrium. J. Geophys. Res. 2008, 113, C04019. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Y.; Chen, M.; Liu, G.; Qiu, Y. New production in the South China Sea. Sci. China Ser. D Earth Sci. 2002, 45, 103–109. [Google Scholar] [CrossRef]
- Chen, W.; Cai, P.; Dai, M.; Wei, J. 234th/238u disequilibrium and particulate organic carbon export in the northern South China Sea. J. Oceanogr. 2008, 64, 417–428. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, Z.; He, J.; Chen, L.; Yin, M.; Zeng, S.; Zeng, W. Vertical flux of particulate organic carbon in the central South China Sea estimated from 234th-238u disequilibria. Chin. J. Oceanol. Limnol. 2008, 26, 480–485. [Google Scholar] [CrossRef]
- Lui, H.; Chen, K.; Chen, C.T.A.; Wang, B.; Lin, H.; Ho, S.; Tseng, C.; Yang, Y.; Chan, J. Physical forcing-driven productivity and sediment flux to the deep basin of northern South China Sea: A decadal time series study. Sustainability 2018, 10, 971. [Google Scholar] [CrossRef]
- Chen, W. On the Export Fluxes, Seasonality and Controls of Particulate Organic Carbon in the Northern South China Sea. Ph.D. Thesis, Xiamen University, Xiamen, China, 2008. [Google Scholar]
- Hung, J.J.; Wang, S.M.; Chen, Y.L. Biogeochemical controls on distributions and fluxes of dissolved and particulate organic carbon in the northern South China Sea. Deep Sea Res Part 2 Top. Stud. Oceanogr. 2007, 54, 1486–1503. [Google Scholar] [CrossRef]
- Ma, W.; Chai, F.; Xiu, P.; Xue, H.; Tian, J. Simulation of export production and biological pump structure in the South China Sea. Geo-Mar. Lett. 2014, 34, 541–554. [Google Scholar] [CrossRef]
- Hu, J.; Kawamura, H.; Hong, H.; Qi, Y. A review on the currents in the South China Sea: Seasonal circulation, South China Sea warm current and Kuroshio intrusion. J. Oceanogr. 2000, 56, 607–624. [Google Scholar] [CrossRef]
- Shaw, P.; Chao, S. Surface circulation in the South China Sea. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 1994, 41, 1663–1683. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Wang, S.; Lu, X.X.; Zhang, S.; Lui, H.; Tseng, H.; Wang, B.; Huang, H. Hydrogeochemistry and greenhouse gases of the Pearl River, its estuary and beyond. Quat. Int. 2008, 186, 79–90. [Google Scholar] [CrossRef]
- Chen, Y. Spatial and seasonal variations of nitrate-based new production and primary production in the South China Sea. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2005, 52, 319–340. [Google Scholar] [CrossRef]
- Ning, X.; Chai, F.; Xue, H.; Cai, Y.; Liu, C.; Shi, J. Physical-biological oceanographic coupling influencing phytoplankton and primary production in the South China Sea. J. Geophys. Res. 2004, 109, C10005. [Google Scholar] [CrossRef]
- Liu, K.K.; Chao, S.Y.; Shaw, P.T.; Gong, G.C.; Chen, C.C.; Tang, T.Y. Monsoon-forced chlorophyll distribution and primary production in the South China Sea: Observations and a numerical study. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2002, 49, 1387–1412. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, D.; Wang, Y. Comparison of phytoplankton blooms triggered by two typhoons with different intensities and translation speeds in the South China Sea. Mar. Ecol. Prog. Ser. 2008, 365, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Xian, T.; Sun, L.; Yang, Y.; Fu, Y. Monsoon and eddy forcing of chlorophyll-a variation in the northeast South China Sea. Int. J. Remote Sens. 2012, 33, 7431–7443. [Google Scholar] [CrossRef]
- Pan, X.; Wong, G.T.; Shiah, F.-K.; Ho, T.-Y. Enhancement of biological productivity by internal waves: Observations in the summertime in the northern South China Sea. J. Oceanogr. 2012, 68, 427–437. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H. Seasonal dynamics of primary and new production in the northern South China Sea: The significance of river discharge and nutrient advection. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2006, 53, 971–986. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, G.-S. Seasonal and inter-annual variations of the mixed layer depth in the South China Sea. Mar. Forcasts 2013, 30, 9–17. [Google Scholar]
- Shang, S.; Lee, Z.; Wei, G. Characterization of modis-derived euphotic zone depth: Results for the china sea. Remote Sens. Environ. 2011, 115, 180–186. [Google Scholar] [CrossRef]
- He, X.; Pan, D.; Bai, Y.; Wang, T.; Chen, C.-T.A.; Zhu, Q.; Hao, Z.; Gong, F. Recent changes of global ocean transparency observed by seawifs. Cont. Shelf Res. 2017, 143, 159–166. [Google Scholar] [CrossRef]
- Mueller, J.L.; Fargion, G.S.; McClain, C.R.; Pegau, S.; Zanefeld, J.; Mitchell, B.G.; Kahru, M.; Wieland, J.; Stramska, M. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4, Volume IV: Inherent Optical Properties: Instruments, Characterizations, Field Measurements and Data Analysis Protocols. Available online: https://oceancolor.gsfc.nasa.gov/docs/technical/protocols_ver4_voliv.pdf (accessed on 10 September 2015).
- Morel, A. Optical properties of pure water and pure sea water. Opt. Asp. Oceanogr. 1974, 1, 1–24. [Google Scholar]
- Reynolds, R.A.; Stramski, D.; Wright, V.M.; Woźniak, S.B. Measurements and characterization of particle size distributions in coastal waters. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Stramski, D.; Boss, E.; Bogucki, D.; Voss, K.J. The role of seawater constituents in light backscattering in the ocean. Prog. Oceanogr. 2004, 61, 27–56. [Google Scholar] [CrossRef]
- Ulloa, O.; Sathyendranath, S.; Platt, T. Effect of the particle-size distribution on the backscattering ratio in seawater. Appl. Opt. 1994, 33, 7070–7077. [Google Scholar] [CrossRef] [PubMed]
- Globcolour. Globcolour Product User Guide. Available online: http://hermes.acri.fr (accessed on 17 July 2017).
- Morel, A.; Berthon, J.F. Surface pigments, algal biomass profiles, and potential production of the euphotic layer: Relationships reinvestigated in view of remote-sensing applications. Limnol. Oceanogr. 1989, 34, 1545–1562. [Google Scholar] [CrossRef] [Green Version]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Ning, X.; Liu, C.; Cai, Y.; Cai, F. Satellite and in situ observations of primary production in the northern South China Sea. Acta Oceanol. Sin. 2007, 29, 58–68. [Google Scholar]
- Tan, S.C.; Shi, G.Y. Spatiotemporal variability of satellite-derived primary production in the South China Sea, 1998–2006. J. Geophys. Res. 2009, 114, G03015. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, W.; Li, A.; Ji, S. Similarities and differences of oceanic primary productivity product estimated by three models based on modis for the open South China Sea. In Proceedings of the 4th International Conference on Geo-Informatics in Resource Management and Sustainable Ecosystems, Hong Kong, China, 18–20 November 2016; pp. 328–336. [Google Scholar]
- Antoine, D.; Morel, A. Oceanic primary production: 1. Adaptation of a spectral light-photosynthesis model in view of application to satellite chlorophyll observations. Glob. Biogeochem. Cycles 1996, 10, 43–55. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Siegel, D.A.; Shea, D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yang, B.; Chen, B.; Qiu, G.; Wang, H.; Huang, B. Net community production in the South China Sea basin estimated from in situ O2 measurements on an argo profiling float. Deep Sea Res. Part 1 Oceanogr. Res. Pap. 2018, 131, 54–61. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. 234th:238u disequilibria within the Californian current. Limnol. Oceanogr. 1985, 30, 22–33. [Google Scholar] [CrossRef]
- Ho, T.; Chou, W.; Wei, C.; Lin, F.; Wong, G.T.F.; Line, H. Trace metal cycling in the surface water of the South China Sea: Vertical fluxes, composition, and sources. Limnol. Oceanogr. 2010, 55, 1807–1820. [Google Scholar] [CrossRef] [Green Version]
- Moigne, F.A.C.L.; Henson, S.A.; Cavan, E.; Georges, C.; Pabortsava, K.; Achterberg, E.P.; Ceballosromero, E.; Zubkov, M.V.; Sanders, R. What causes the inverse relationship between primary production and export efficiency in the Southern Ocean. Geophys. Res. Lett. 2016, 43, 4457–4466. [Google Scholar] [CrossRef]
- Cael, B.B.; Follows, M.J. On the temperature dependence of oceanic export efficiency. Geophys. Res. Lett. 2016, 43, 5170–5175. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.L.; Lin, S.Y.; Sheu, D.D.; Chou, W.; Yi, M.C.; Santschi, P.H.; Wen, L.S. Particle-reactive radionuclides (234th, 210pb, 210po) as tracers for the estimation of export production in the South China Sea. Biogeosciences 2011, 8, 3793–3808. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K.R.M. Phytoplankton as key mediators of the biological carbon pump: Their responses to a changing climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef]
- Mouw, C.B.; Barnett, A.; McKinley, G.A.; Gloege, L.; Pilcher, D. Phytoplankton size impact on export flux in the global ocean. Glob. Biogeochem. Cycles 2016, 30, 1542–1562. [Google Scholar] [CrossRef]
- Barber, R.T. Picoplankton do some heavy lifting. Science 2007, 315, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Klut, M.E.; Stockner, J.G. Picoplankton associations in an ultra-oligotrophic lake on Vancouver Island, British Columbia. Can. J. Fish. Aquat. Sci. 1991, 48, 1092–1099. [Google Scholar] [CrossRef]
- Richardson, T.L.; Jackson, G.A. Small phytoplankton and carbon export from the surface ocean. Science 2007, 315, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. The Spatio-Temporal Variation of Phytoplankton Community Structure and Its Responses to Mesoscale Physical Processes in the South China Sea. Ph.D. Thesis, Xiamen Univerisity, Xiamen, China, 2012. [Google Scholar]
- Hansell, D.A.; Carlson, C.A.; Repeta, D.J.; Schlitzer, R. Dissolved organic matter in the ocean: A controversy stimulates new insights. Oceanography 2009, 22, 202–211. [Google Scholar] [CrossRef]
- Lampitt, R.S.; Noji, T.T.; Von Bodungen, B. What happens to zooplankton faecal pellets? Implications for material flux. Mar. Biol. 1990, 104, 15–23. [Google Scholar] [CrossRef]
- Berelson, W.M. Particle settling rates increase with depth in the ocean. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2001, 49, 237–251. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Song, S.; Sun, J. Size-fractionated mesozooplankton biomass and grazing impact on phytoplankton in northern South China Sea during four seasons. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2015, 117, 108–118. [Google Scholar] [CrossRef]
- Landry, M.R.; Lorenzen, C.J.; Peterson, W.K. Mesozooplankton grazing in the Southern California bight. Ii. Grazing impact and particulate flux. Mar. Ecol. Prog. Ser. 1994, 115, 73–85. [Google Scholar] [CrossRef]
- Ho, T.; Pan, X.; Yang, H.-H.; Wong, G.T.F.; Shiah, F.-K. Controls on temporal and spatial variations of phytoplankton pigment distribution in the Northern South China Sea. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2015, 117, 65–85. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Chen, J.; Jin, H.; Chen, C.; Liu, M.; Yan, Z.; Ji, Z. Seasonal variations of dissolved organic matter in the east china sea using eem-parafac and implications for carbon and nutrient cycling. Sustainability 2018, 10, 1444. [Google Scholar] [CrossRef]
- Karl, D.M.; Hebel, D.V.; Bjorkman, K.M.; Letelier, R.M. The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 1998, 43, 1270–1286. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Dai, M.; Chen, J.; Meng, F.; Li, X.; Liu, Z.; Du, C.; Gan, J. Dissolved organic carbon in the South China Sea and its exchange with the Western Pacific Ocean. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2015, 122, 41–51. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, L.; Huang, B.; Song, S.; Liu, H. Seasonal and spatial comparisons of phytoplankton growth and mortality rates due to microzooplankton grazing in the northern South China Sea. Biogeosciences 2013, 10, 2775–2785. [Google Scholar] [CrossRef]
| Data Source | Cruise Time | Number of Stations |
|---|---|---|
| Cai et al. [22] | 18 July to 16 August 2009 | 31 stations |
| 6–30 January 2010 | 36 stations | |
| 26 October to 24 November 2010 | 18 stations | |
| 30 April to 24 May 2011 | 27 stations | |
| Chen [29] | 18 February to 1 March 2004 | 22 stations |
| 10–13 July 2004 | 2 stations | |
| 19 September to 2 October 2004 | 10 stations | |
| 29 April to 11 May 2005 | 9 stations |
| Study Areas | Formulas | Data Sources |
|---|---|---|
| Global 1 | EFDunne = −0.0101 × SST + 0.0582 × ln(NPP/Zeu) + 0.419 | 122 field observations of NPP and new production, and sediment traps or 234Th based POC export flux at the euphotic layer depth. |
| Global 2 | EFLaws = 0.04756 × (0.78 − 0.43 × SST/30) × NPP0.307 | 31 field observations of NPP and new production at the euphotic layer depth. |
| Global 3 | EFHenson = 0.23 × exp(−0.08 × SST) | 306 234Th-derived POC export flux data at 100 m depth and 16 days integrated satellite-derived NPP prior to the in situ measurement. |
| Southern Ocean 4 | EFMaiti = −0.3482 × log(NPP) + 1.2239 | ~136 field observations of NPP and sediment traps or 234Th based POC export flux at 100 m depth. |
| Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|
| Shelf areas | ||||
| NPP | 22.05 ± 4.61 | 29.97 ± 17.47 | 50.18 ± 15.13 | 58.05 ± 10.63 |
| EPin_situ | 6.78 ± 5.22 | 10.74 ± 11.04 | 28.32 ± 22.49 | 16.18 ± 8.23 |
| EFin_situ | 0.28 ± 0.17 | 0.31 ± 0.14 | 0.53 ± 0.30 | 0.26 ± 0.10 |
| Basin areas | ||||
| NPP | 22.04 ± 4.53 | 18.06 ± 2.38 | 31.56 ± 5.99 | 57.90 ± 15.13 |
| EPin_situ | 7.70 ± 2.61 | 4.35 ± 1.39 | 9.53 ± 2.99 | 4.52 ± 1.99 |
| EFin_situ | 0.36 ± 0.14 | 0.25 ± 0.09 | 0.34 ± 0.20 | 0.08 ± 0.04 |
| Observation Period | POC Export Flux (mmol C·m−2·day−1) | Method | Reference |
|---|---|---|---|
| 18–24 October 2006 | 17.4 | Free floating sediment traps. Flux at 100 m. | Ho et al. [60] |
| 12–19 January 2007 | 12.8 | ||
| 28 July–3 August 2007 | 9.8 | ||
| 21–30 October 2007 | 13.6 | ||
| November 2002 | 5.3 | 234Th/238U disequilibrium. Flux at 100 m. | Chen et al. [26] |
| May 2001 | 18.3 | ||
| 29 April–11 May 2005 | 3.73 | 234Th/238U disequilibrium. Flux at 100 m. | Chen [29] |
| 10–13 July 2004 | 4.73 | ||
| 19 September to 02 October 2004 | 5.06 | ||
| 18 February–1 March 2004 | 9.46 | ||
| October 2006 | 17.29, 21.04, 8.60, 8.23 | Fluxes were measured by floating sediment trap, 234Th, 210Po and 210Pb proxy respectively. Flux at 100 m. | Wei et al. [63] |
| January 2007 | 12.80, 9.51, 1.65, 8.69 | ||
| July 2007 | 9.60, 11.43, 7.77, 21.22 | ||
| October 2007 | 12.53, 10.52, 14.91, 16.10 | ||
| June 2008 | 15.09, 13.54, 5.12, 7.04 | ||
| December 2008 | 18.29, 13.72, 20.12, 11.98 | ||
| 30 April–24 May 2011 | 7.7 | 234Th/238U disequilibrium. Flux at 100 m. | Cai et al. [22] |
| 18 July–16 August 2009 | 4.3 | ||
| 26 October–24 November 2010 | 9.7 | ||
| 6–30 January 2010 | 4.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Bai, Y.; He, X.; Chen, X.; Chen, C.-T.A.; Tao, B.; Pan, D.; Zhang, X. The Relationship between POC Export Efficiency and Primary Production: Opposite on the Shelf and Basin of the Northern South China Sea. Sustainability 2018, 10, 3634. https://doi.org/10.3390/su10103634
Li T, Bai Y, He X, Chen X, Chen C-TA, Tao B, Pan D, Zhang X. The Relationship between POC Export Efficiency and Primary Production: Opposite on the Shelf and Basin of the Northern South China Sea. Sustainability. 2018; 10(10):3634. https://doi.org/10.3390/su10103634
Chicago/Turabian StyleLi, Teng, Yan Bai, Xianqiang He, Xiaoyan Chen, Chen-Tung Arthur Chen, Bangyi Tao, Delu Pan, and Xuan Zhang. 2018. "The Relationship between POC Export Efficiency and Primary Production: Opposite on the Shelf and Basin of the Northern South China Sea" Sustainability 10, no. 10: 3634. https://doi.org/10.3390/su10103634
APA StyleLi, T., Bai, Y., He, X., Chen, X., Chen, C.-T. A., Tao, B., Pan, D., & Zhang, X. (2018). The Relationship between POC Export Efficiency and Primary Production: Opposite on the Shelf and Basin of the Northern South China Sea. Sustainability, 10(10), 3634. https://doi.org/10.3390/su10103634




