Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways
Abstract
1. Introduction
2. Results
2.1. Effect of Osthole on Body Weight and Liver Index
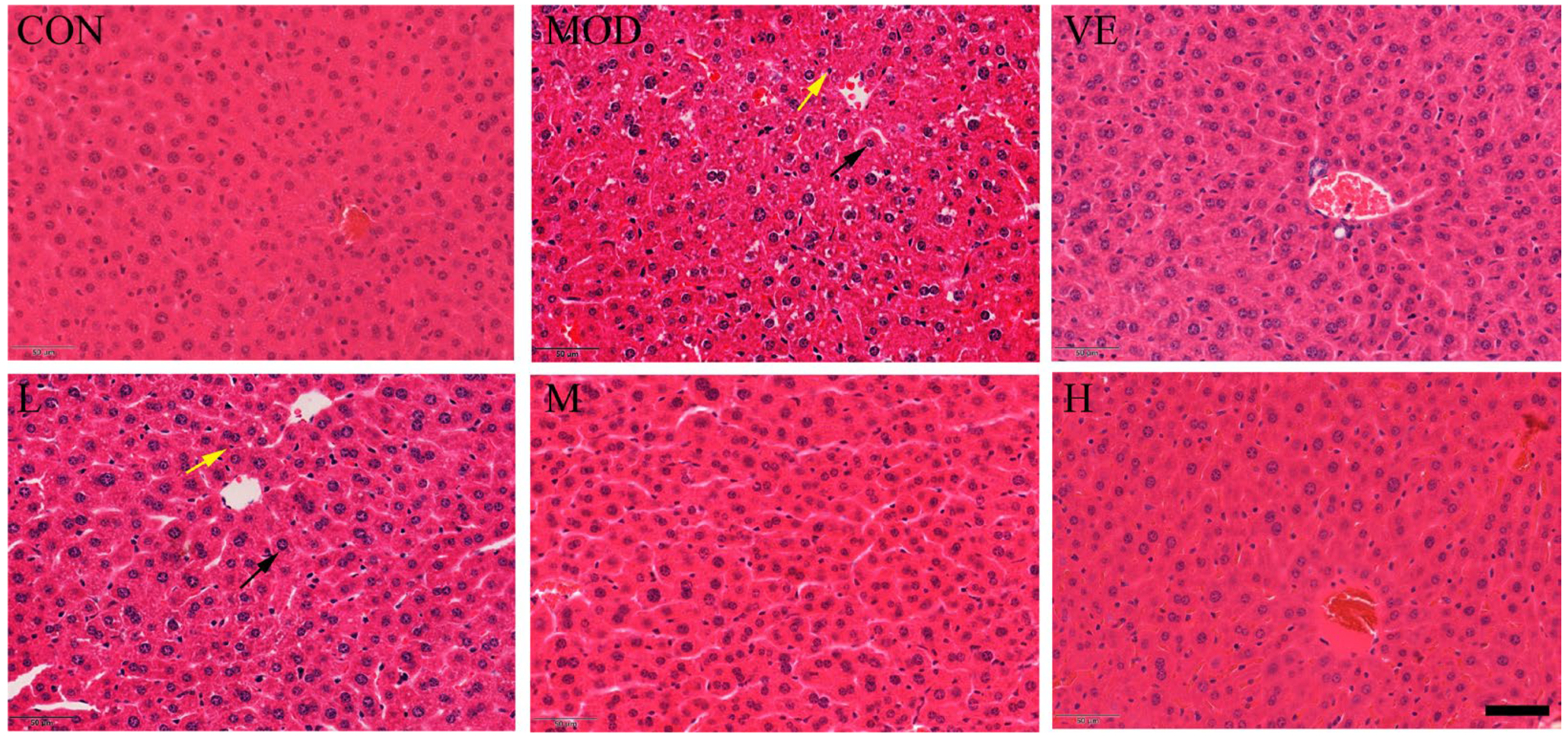
2.2. Effect of Osthole on Liver Pathological Changes
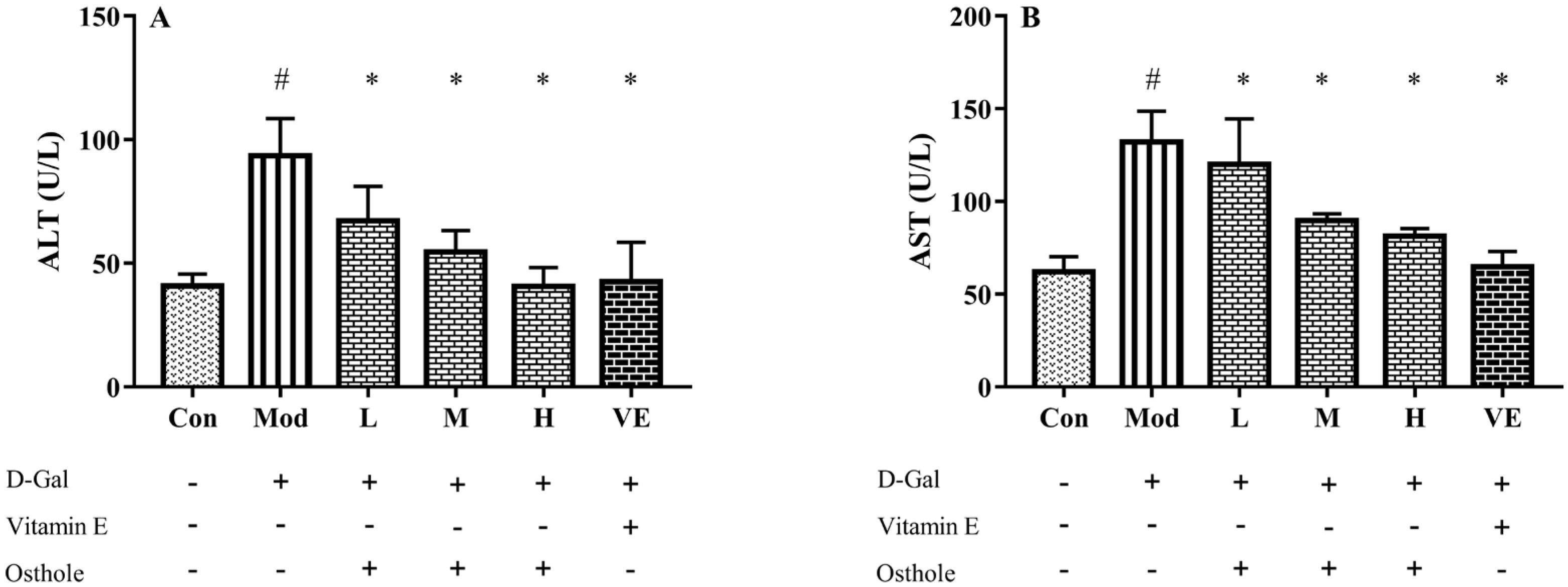
2.3. Effect of Osthole on Biochemical Indicators and Pro-Inflammatory Cytokines in Serum
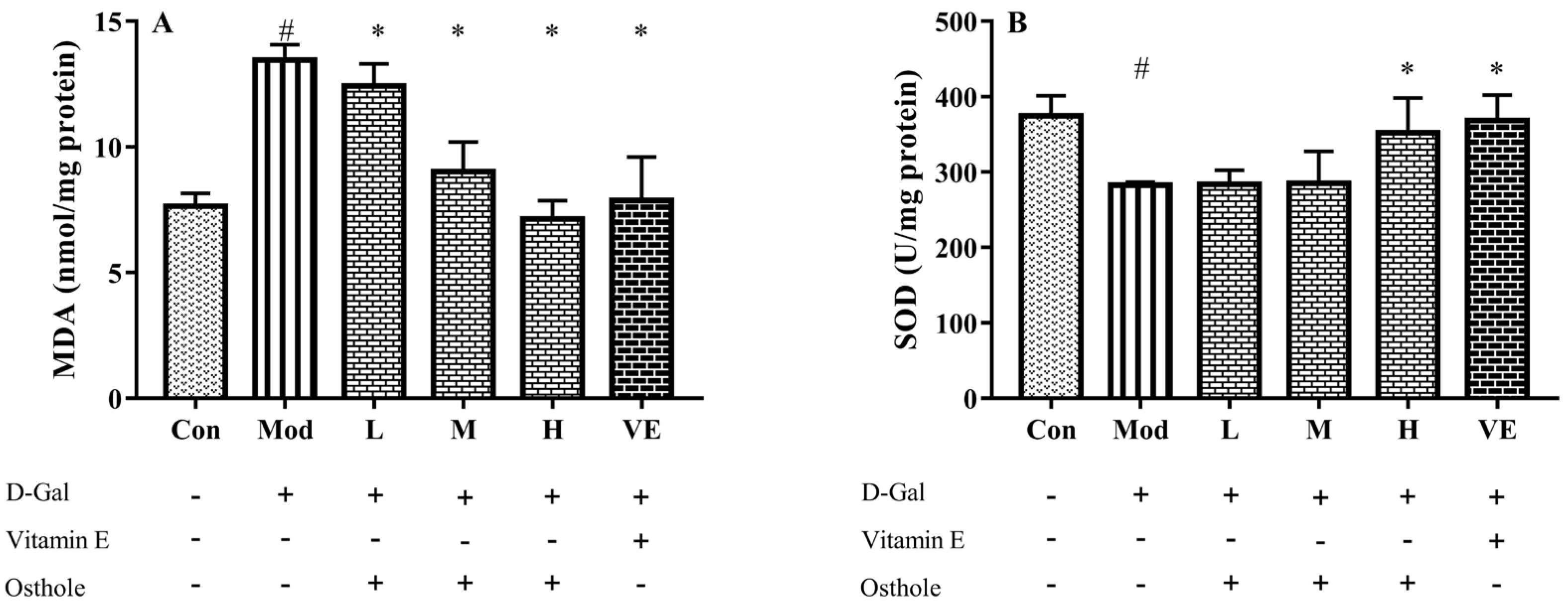
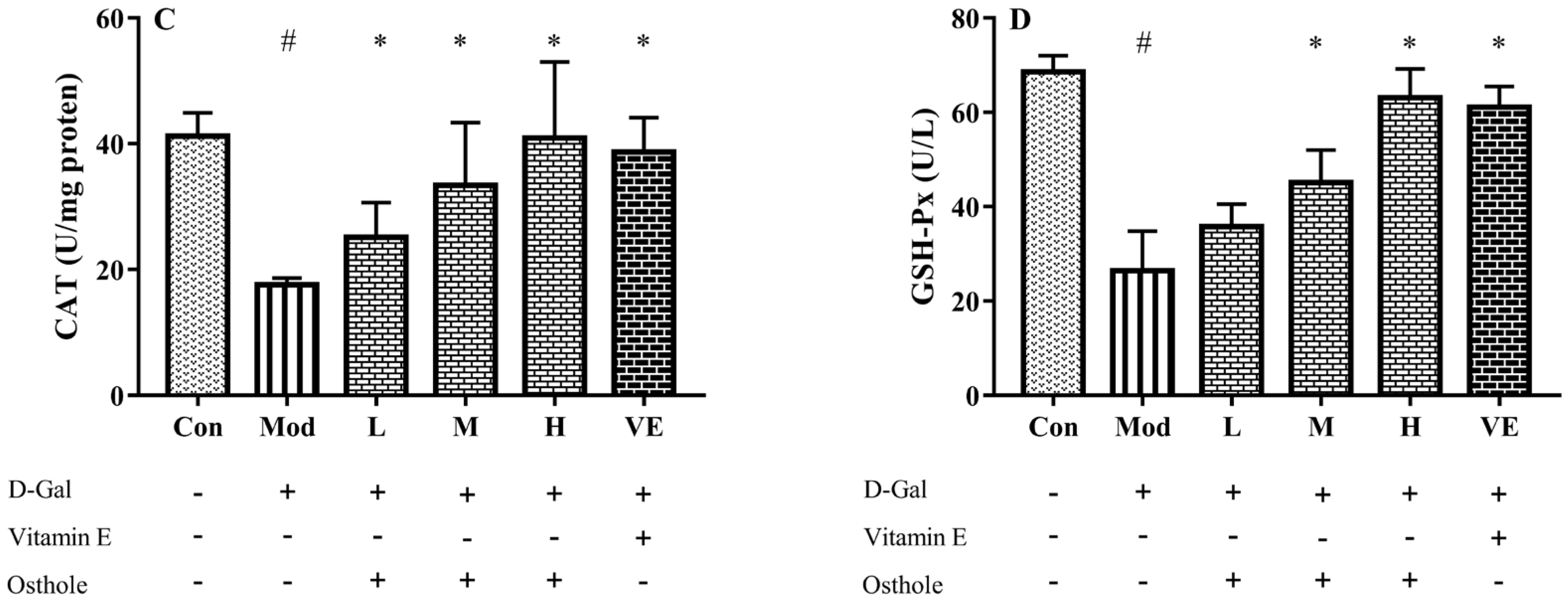
2.4. Effect of Osthole on Oxidative Stress in Liver Tissue
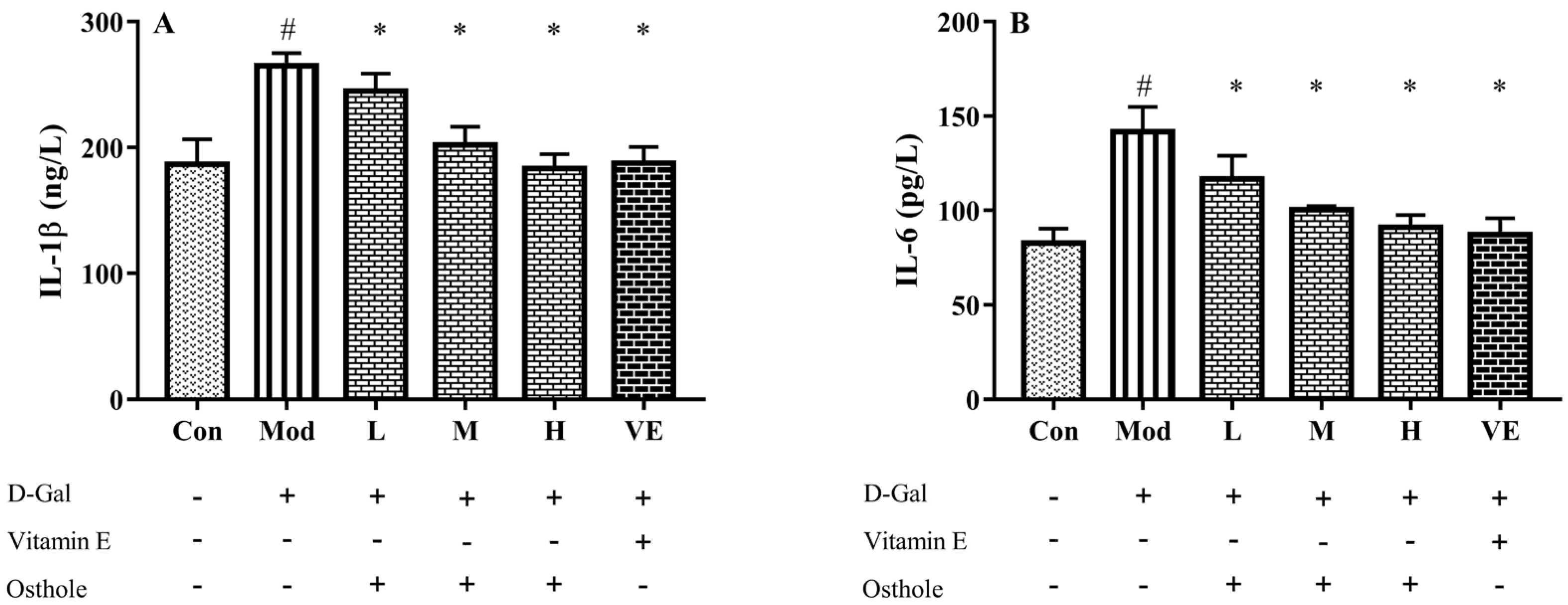
2.5. Effect of Osthole on Pro-Inflammatory Cytokines in Serum and Liver Tissue
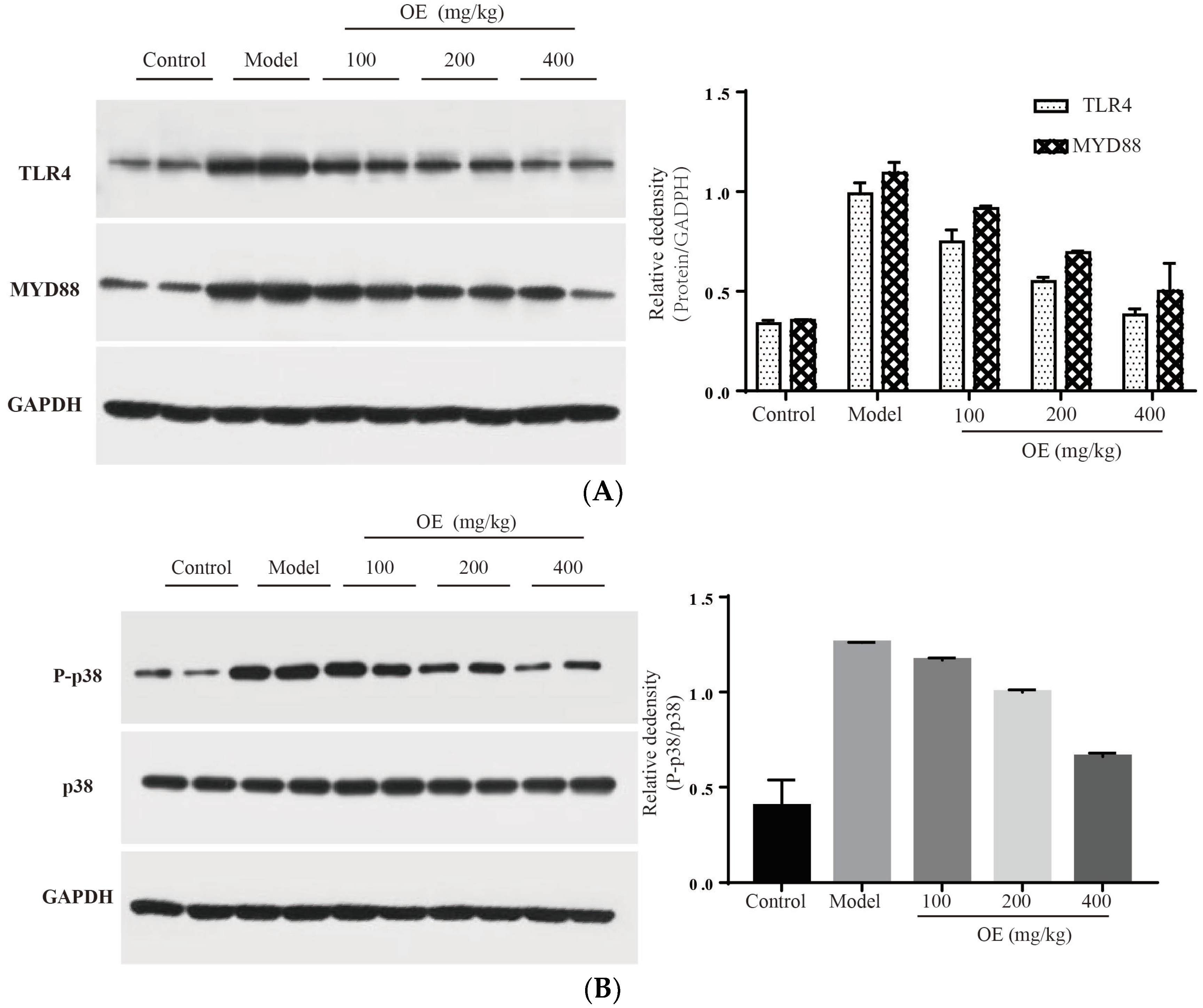
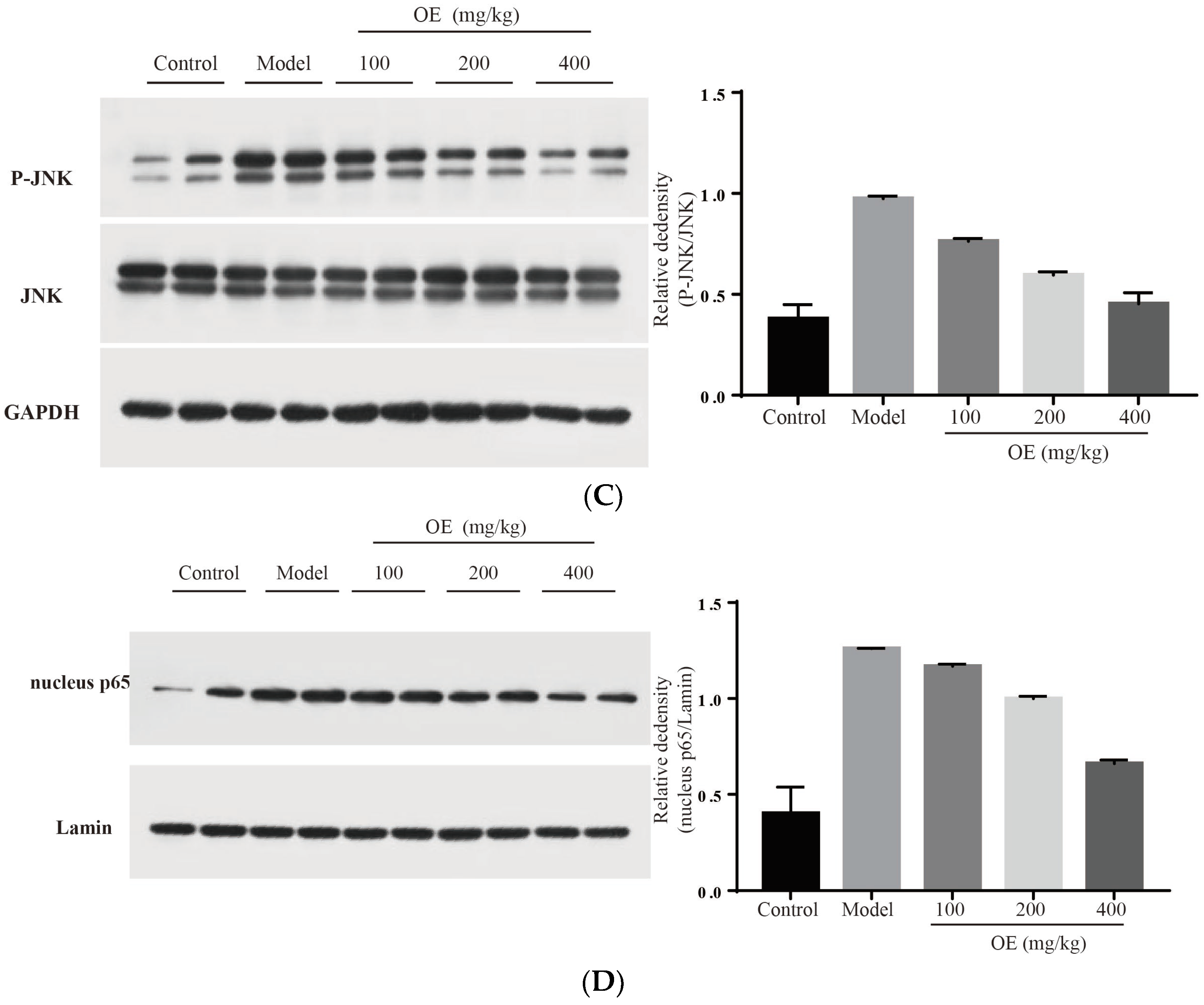
2.6. Effect of Oshtole on Signal Pathway-Mediated Inflammatory Response
3. Materials and Methods
3.1. Materials
3.2. Animal Experiment
3.3. Histological Assessment
3.4. Biochemical Indexes Assay
3.5. Western Blot Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sairam, K.V.; Gurupadayya, B.M.; Chandan, R.S.; Nagesha, D.K.; Vishwanathan, B. A Review on chemical profile of coumarins and their therapeutic role in the treatment of cancer. Curr. Drug Deliv. 2016, 13, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldivar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, A.W.H.; Lenon, G.B. Phytochemistry, ethnopharmacology, pharmacokinetics and toxicology of Cnidium monnieri (L.) Cusson. Int. J. Mol. Sci. 2020, 21, 1006. [Google Scholar] [CrossRef]
- Fan, H.; Gan, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li, X.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-kappa B and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef]
- Yang, J. Osthole alleviates inflammation by down-regulating NF-kappa B signaling pathway in traumatic brain injury. Immunopharmacol. Immunotoxicol. 2019, 41, 349. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Z.; Hou, J.; Huang, T.; Yang, M. Osthole improves collagen-induced arthritis in a rat model through inhibiting inflammation and cellular stress. Cell. Mol. Biol. Lett. 2018, 23, 19. [Google Scholar] [CrossRef]
- Bao, Y.; Meng, X.; Liu, F.; Wang, F.; Yang, J.; Wang, H.; Xie, G. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol. Med. Rep. 2018, 17, 4561–4566. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, X.J.; Juan, F.U. Protective effect of osthole on LPS-induced acute lung injury in mice and its mechanism. Pract. Pharm. Clin. Remedies 2015, 8, 893–897. (In Chinese) [Google Scholar] [CrossRef]
- Luo, L.-N.; Xie, D.Q.; Zhang, X.G.; Jiang, R. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp. Ther. Med. 2016, 12, 2009–2014. [Google Scholar] [CrossRef][Green Version]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Liu, F.; Gao, Z.; Kong, D.; Hu, X.; Shi, D.; Bao, Z.; Yu, Z. The anti-inflamm-aging and hepatoprotective effects of huperzine A in D-galactose-treated rats. Mech. Ageing Dev. 2013, 134, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Pi, F.W.; Guo, Y.H.; Cheng, Y.L.; Qian, H. Torularhodin ameliorates oxidative activity in vitro and D-Galactose-induced liver injury via the Nrf2/HO-1 signaling pathway in vivo. J. Agric. Food Chem. 2019, 67, 10059–10068. [Google Scholar] [CrossRef]
- Jiang, P.; Meng, W.; Shi, F.; Chen, C.; Sun, Y.; Jiao, L. Structural characteristics, antioxidant properties and antiaging activities of galactan produced by Mentha haplocalyx Briq. Carbohydr. Polym. 2020, 234, 115936. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Zhang, Y.; Tian, D.; Jiang, S.; Tang, Y. Hepatoprotective effect of pyrroloquinoline quinone against alcoholic liver injury through activating Nrf2-mediated antioxidant and inhibiting TLR4-mediated inflammation responses. Process Biochem. 2020, 92, 303–312. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Xie, Y.-L.; Gui, S.-H.; Zhang, X.; Mo, Z.-Z.; Sun, C.-Y.; Li, C.-L.; Luo, D.-D.; Zhang, Z.-B.; Su, Z.-R.; et al. Polydatin attenuates D-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct. 2016, 7, 4545–4555. [Google Scholar] [CrossRef]
- Dugum, M.; McCullough, A. Diagnosis and management of alcoholic liver disease. J. Clin. Transl. Hepatol. 2015, 3, 109–116. [Google Scholar] [CrossRef]
- Aydin, S.; Yanar, K.; Atukeren, P.; Dalo, E.; Sitar, M.E.; Uslu, E.; Caf, N.; Cakatay, U. Comparison of oxidative stress biomarkers in renal tissues of D-galactose induced, naturally aged and young rats. Biogerontology 2012, 13, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Huang, S.-S.; Lee, M.-M.; Deng, J.-S.; Huang, G.-J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-kappa B and MAPK signaling pathway in the carrageenan-induced paw edema. Int. Immunopharmacol. 2015, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Wen, J.L.; Wang, L.; Wang, X.P.; Chen, T.S. Intracellular catalase activity instead of glutathione level dominates the resistance of cells to reactive oxygen species. Cell Stress Chaperones 2019, 24, 609–619. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Feng, D.; Li, M.; Gao, Y.; Ramirez, T.; Cao, H.; Kim, S.-J.; Yang, Y.; Cai, Y.; Ju, C.; et al. Hepatic Mitochondrial DNA/Toll-Like Receptor 9/MicroRNA-223 Forms a Negative Feedback Loop to Limit Neutrophil Overactivation and Acetaminophen Hepatotoxicity in Mice. Hepatology 2017, 66, 220–234. [Google Scholar] [CrossRef]
- Negash, A.A.; Gale, M., Jr. Hepatitis regulation by the inflammasome signaling pathway. Immunol. Rev. 2015, 265, 143–155. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca Oleracea extract on acute alcoholic liver injury of rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef]
- Fouad, D.; Badr, A.; Attia, H.A. Hepatoprotective activity of raspberry ketone is mediated via inhibition of the NF-kappa B/TNF-alpha/caspase axis and mitochondrial apoptosis in chemically induced acute liver injury. Toxicol. Res. 2019, 8, 663–676. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Cha, H.; Lee, S.; Lee, J.H.; Park, J.W. Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2018, 121, 131–139. [Google Scholar] [CrossRef]
- Tashiro, S.; Tanaka, M.; Goya, T.; Aoyagi, T.; Kurokawa, M.; Imoto, K.; Kuwano, A.; Takahashi, M.; Suzuki, H.; Kohjima, M.; et al. Pirfenidone attenuates acetaminophen-induced liver injury via suppressing c-Jun N-terminal kinase phosphorylation. Toxicol. Appl. Pharmacol. 2022, 434, 115817. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Dey, S.; Pal, C.; Goyal, M.; Alam, A.; Iqbal, M.; Sarkar, S.; Siddiqui, A.A.; Banerjee, C.; et al. Nonsteroidal anti-inflammatory drug induces proinflammatory damage in gastric mucosa through NF-κB activation and neutrophil infiltration: Anti-inflammatory role of heme oxygenase-1 against nonsteroidal anti-inflammatory drug. Free Radic. Biol. Med. 2013, 65, 456–467. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Peng, L.; Chu, W.; Wang, P.; Fu, Y. Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways. Molecules 2023, 28, 443. https://doi.org/10.3390/molecules28010443
Ma Z, Peng L, Chu W, Wang P, Fu Y. Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways. Molecules. 2023; 28(1):443. https://doi.org/10.3390/molecules28010443
Chicago/Turabian StyleMa, Zhe, Lin Peng, Wenhui Chu, Pan Wang, and Yongqian Fu. 2023. "Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways" Molecules 28, no. 1: 443. https://doi.org/10.3390/molecules28010443
APA StyleMa, Z., Peng, L., Chu, W., Wang, P., & Fu, Y. (2023). Osthole Alleviates D-Galactose-Induced Liver Injury In Vivo via the TLR4/MAPK/NF-κB Pathways. Molecules, 28(1), 443. https://doi.org/10.3390/molecules28010443




