The Association of Glucose Control with Circulating Levels of Red Blood Cell-Derived Vesicles in Type 2 Diabetes Mellitus Patients with Atrial Fibrillation
Abstract
:1. Introduction
2. Results
2.1. General Patients’ Characteristics
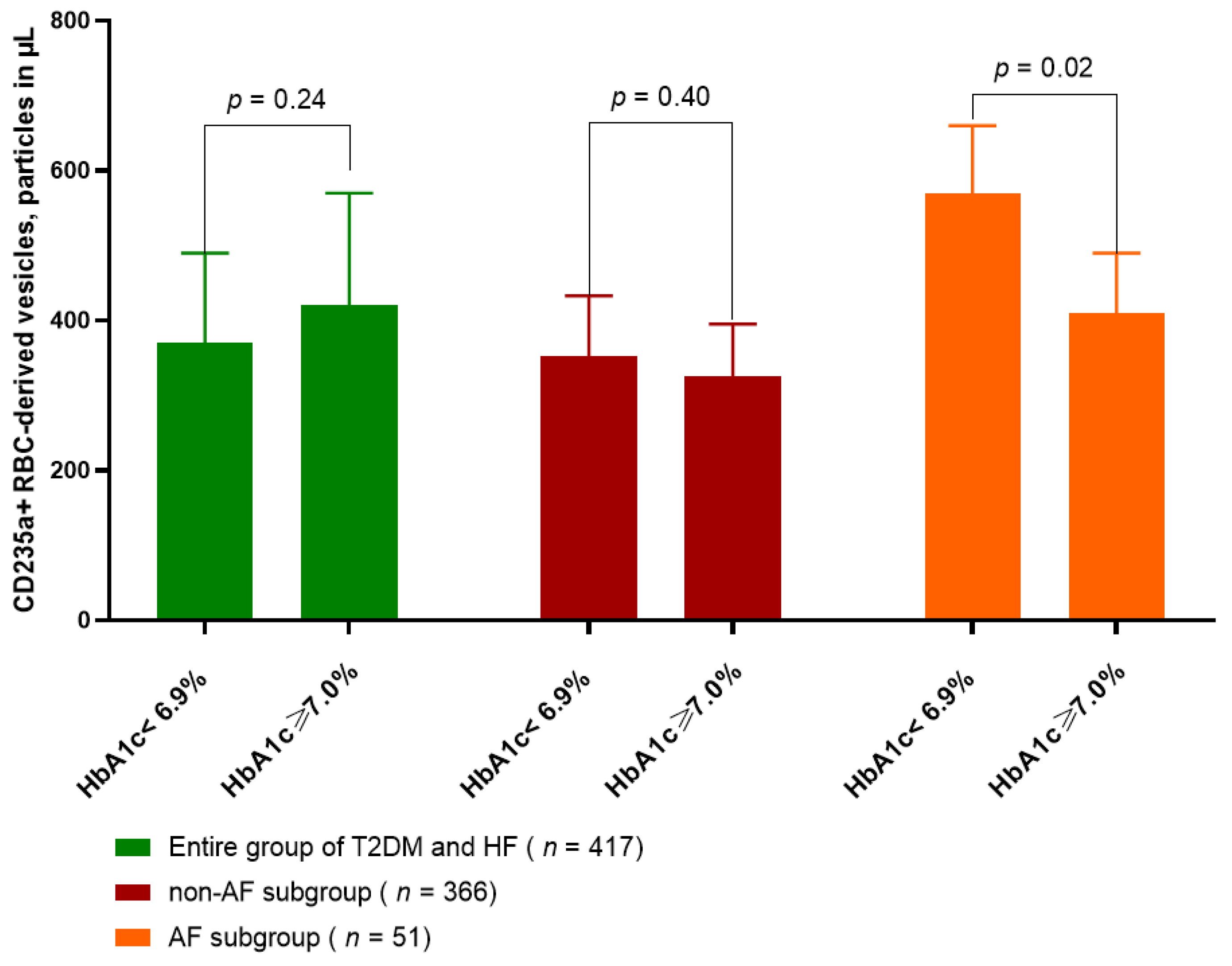
2.2. Amount of CD235a+ PS+ RBC-Derived Vesicles in T2DM Patients with HF Depending of Glycemia Control
2.3. Spearman’s Correlation between Quantity of CD235a+ PS+ RBC-Derived Vesicles and Other Patients Characteristics
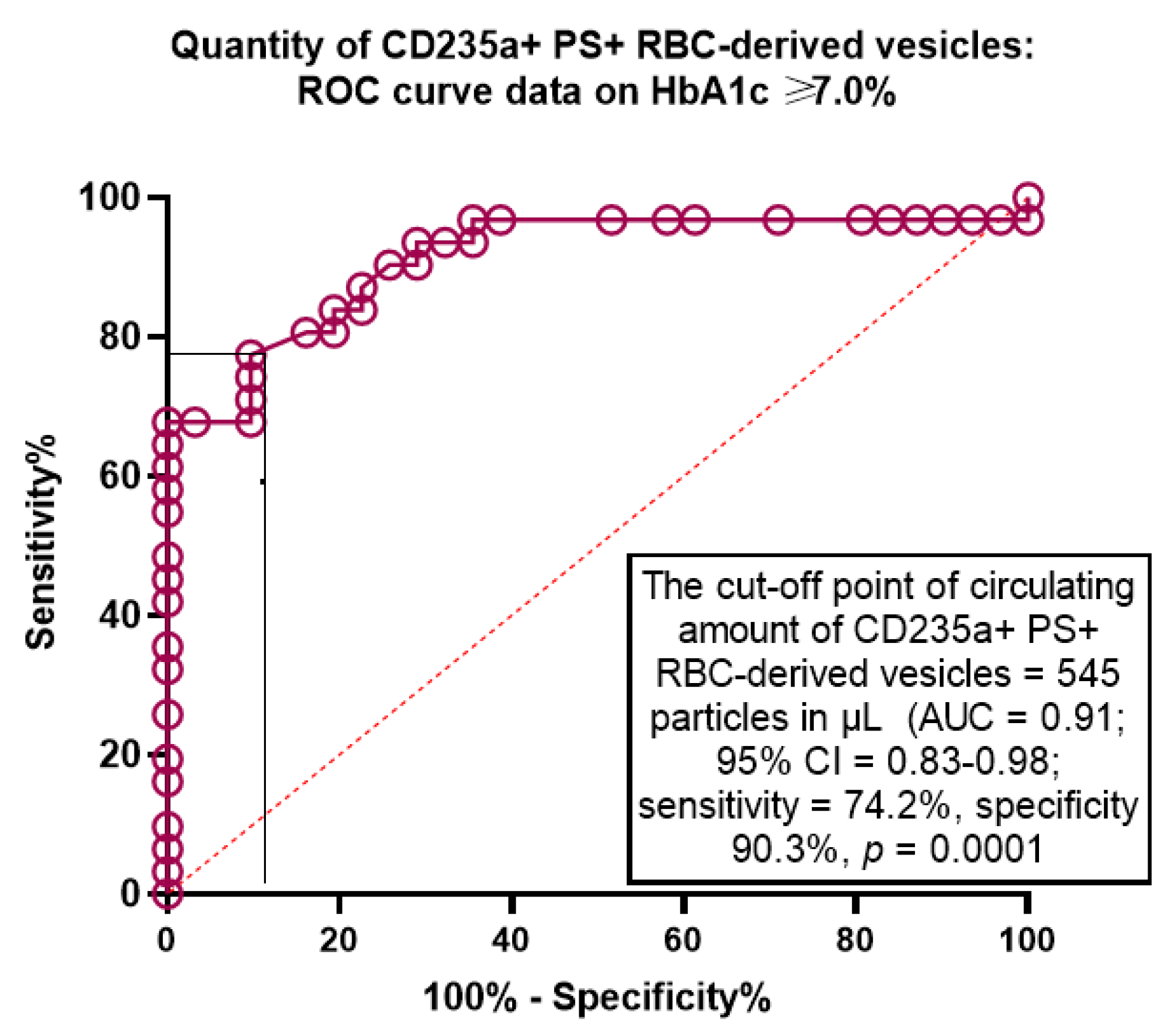
2.4. The Predictive Ability of Amount of CD235a+ PS+ RBC-Derived Vesicles for Poor Glycaemia Control: The Receive Operation Characteristics Curve Analysis
2.5. The Predictors of Poor Glycemic Control in T2DM Patients with HF: The Univariate and Multivariate Linear Regression
2.6. The Comparisons of Predictive Models for HbA1c ≥ 7.0%: The Results of Model Fit Statistics
3. Discussion
4. Materials and Methods
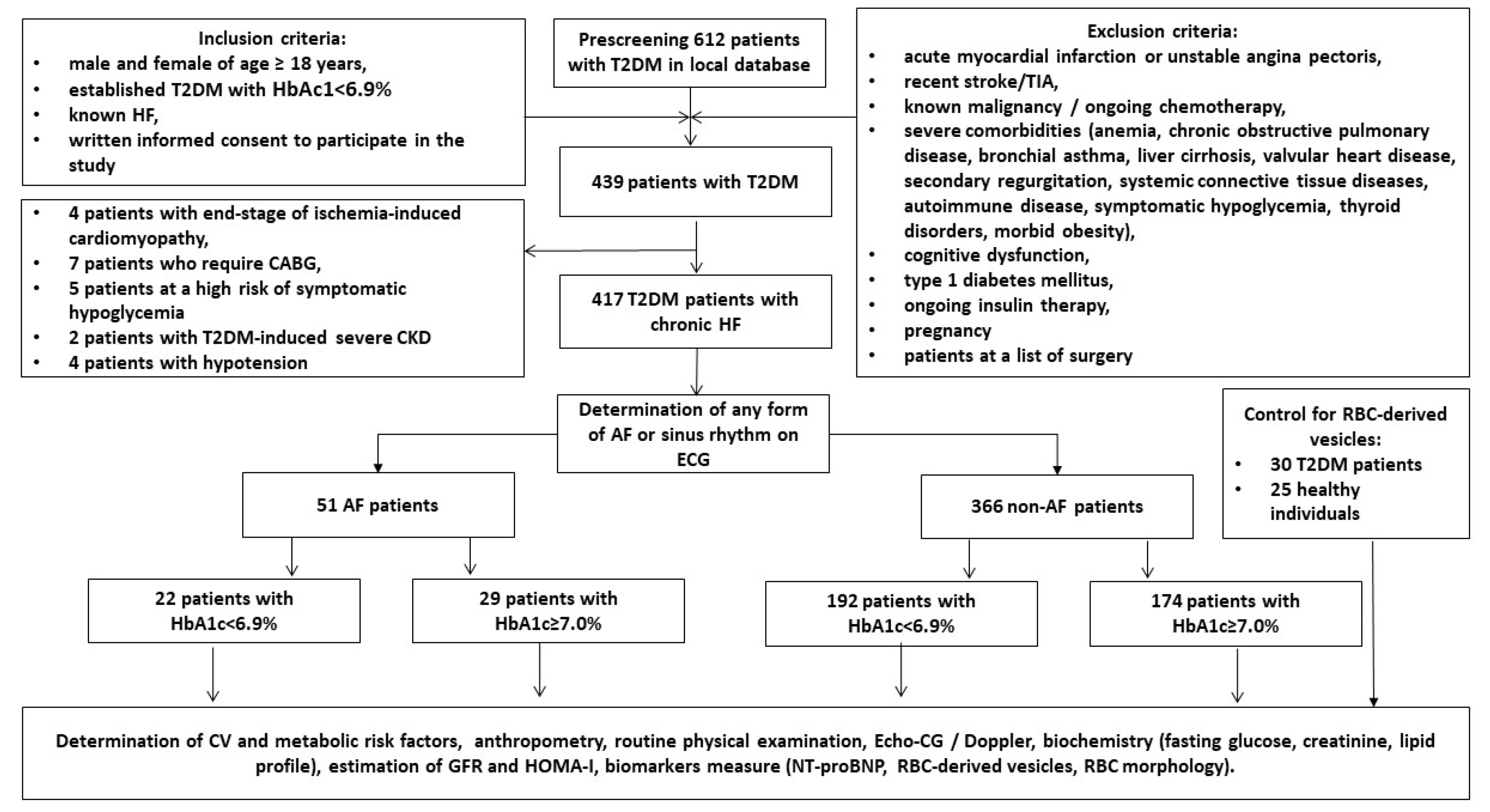
4.1. Study Design and Cohorts of Participants
4.2. Determination of Anthropometric Parameters, Co-Morbidities and Concomitant Diseases
4.3. Examination of Hemodynamics
4.4. Diet and Medications
4.5. Blood Sampling, Measurement of Circulating Biomarkers and Determination of Glomerular Filtration Rate and Insulin Resistance
4.6. Isolation, Detection and Quantitation of Circulating CD235a+ PS+ RBC-Derived EVs
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Skanes, A.C.; Tang, A.S. Atrial Fibrillation and Heart Failure: Untangling a Modern Gordian Knot. Can. J. Cardiol. 2018, 34, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, H.J.; Choe, J.C.; Park, J.S.; Lee, H.W.; Oh, J.-H.; Choi, J.H.; Lee, H.C.; Cha, K.S.; Hong, T.J.; et al. Treatment Strategies for Atrial Fibrillation With Left Ventricular Systolic Dysfunction―Meta-Analysis. Circ. J. 2018, 82, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- Stegmann, C.; Hindricks, G. Atrial Fibrillation in Heart Failure—Diagnostic, Therapeutic, and Prognostic Relevance. Curr. Heart Fail. Rep. 2019, 16, 108–115. [Google Scholar] [CrossRef]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: A report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Green, J.B.; Halperin, J.L.; Piccini, J.P. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1107–1115. [Google Scholar] [CrossRef]
- Sobue, Y.; Watanabe, E.; Lip, G.Y.H.; Koshikawa, M.; Ichikawa, T.; Kawai, M.; Harada, M.; Inamasu, J.; Ozaki, Y. Thromboembolisms in atrial fibrillation and heart failure patients with a preserved ejection fraction (HFpEF) compared to those with a reduced ejection fraction (HFrEF). Hear. Vessel. 2017, 33, 403–412. [Google Scholar] [CrossRef]
- Achim, A.; Stanek, A.; Homorodean, C.; Spinu, M.; Onea, H.L.; Lazăr, L.; Marc, M.; Ruzsa, Z.; Olinic, D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Health 2022, 19, 9801. [Google Scholar] [CrossRef]
- Grant, P.J. Diabetes mellitus as a prothrombotic condition. J. Intern. Med. 2007, 262, 157–172. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Hung, J.; Hickling, S.; Nedkoff, L.; Murray, K.; Li, I.; Briffa, T.G. Incidence, predictors and mortality risk of new heart failure in patients hospitalised with atrial fibrillation. Heart 2021, 107, 1320–1326. [Google Scholar] [CrossRef]
- Patel, R.B.; Vaduganathan, M.; Shah, S.J.; Butler, J. Atrial fibrillation in heart failure with preserved ejection fraction: Insights into mechanisms and therapeutics. Pharmacol. Ther. 2017, 176, 32–39. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular Vesicles and Thrombogenicity in Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1774. [Google Scholar] [CrossRef]
- Gasecka, A.; Böing, A.N.; Filipiak, K.J.; Nieuwland, R. Platelet extracellular vesicles as biomarkers for arterial thrombosis. Platelets 2016, 28, 228–234. [Google Scholar] [CrossRef]
- Mørk, M.; Andreasen, J.J.; Rasmussen, L.H.; Lip, G.Y.; Pedersen, S.; Bæk, R.; Jørgensen, M.M.; Kristensen, S.R. Elevated blood plasma levels of tissue factor-bearing extracellular vesicles in patients with atrial fibrillation. Thromb. Res. 2018, 173, 141–150. [Google Scholar] [CrossRef]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef] [Green Version]
- Ruddox, V.; Sandven, I.; Munkhaugen, J.; Skattebu, J.; Edvardsen, T.; Otterstad, J.E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1555–1566. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.H.; Maan, A.M.; Heist, E.K.M. The Care of Patients With Atrial Fibrillation and Heart Failure. Crit. Pathw. Cardiol. A J. Evid.-Based Med. 2020, 20, 93–99. [Google Scholar] [CrossRef]
- Taniguchi, N.; Miyasaka, Y.; Suwa, Y.; Harada, S.; Nakai, E.; Shiojima, I. Heart Failure in Atrial Fibrillation ― An Update on Clinical and Echocardiographic Implications. Circ. J. 2020, 84, 1212–1217. [Google Scholar] [CrossRef]
- Simpson, J.; Castagno, D.; Doughty, R.N.; Poppe, K.K.; Earle, N.; Squire, I.; Richards, M.; Andersson, B.; Ezekowitz, J.A.; Komajda, M.; et al. Is heart rate a risk marker in patients with chronic heart failure and concomitant atrial fibrillation? Results from the MAGGIC meta-analysis. Eur. J. Heart Fail. 2015, 17, 1182–1191. [Google Scholar] [CrossRef] [Green Version]
- Farmakis, D.; Chrysohoou, C.; Giamouzis, G.; Giannakoulas, G.; Hamilos, M.; Naka, K.; Tzeis, S.; Xydonas, S.; Karavidas, A.; Parissis, J. The management of atrial fibrillation in heart failure: An expert panel consensus. Hear. Fail. Rev. 2020, 26, 1345–1358. [Google Scholar] [CrossRef]
- Choi, H.-I.; Lee, S.E.; Kim, M.-S.; Lee, H.-Y.; Cho, H.-J.; Choi, J.O.; Jeon, E.-S.; Hwang, K.-K.; Chae, S.C.; Baek, S.H.; et al. Prognostic Impact and Predictors of New-Onset Atrial Fibrillation in Heart Failure. Life 2022, 12, 579. [Google Scholar] [CrossRef]
- Mogensen, U.M.; Jhund, P.S.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Packer, M.; Rouleau, J.L.; Solomon, S.D.; Swedberg, K.; Zile, M.R.; et al. Type of Atrial Fibrillation and Outcomes in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2490–2500. [Google Scholar] [CrossRef]
- Hao, C.; Luo, J.; Liu, B.; Xu, W.; Li, Z.; Gong, M.; Qin, X.; Shi, B.; Wei, Y. Prognostic Significance of New-Onset Atrial Fibrillation in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction Following Acute Myocardial Infarction: Data from the NOAFCAMI-SH Registry. Clin. Interv. Aging 2022, 17, 479–493. [Google Scholar] [CrossRef]
- Cherian, T.S.; Shrader, P.; Fonarow, G.C.; Allen, L.A.; Piccini, J.P.; Peterson, E.D.; Thomas, L.; Kowey, P.R.; Gersh, B.J.; Mahaffey, K.W. Effect of Atrial Fibrillation on Mortality, Stroke Risk, and Quality-of-Life Scores in Patients With Heart Failure (from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation [ORBIT-AF]). Am. J. Cardiol. 2017, 119, 1763–1769. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Jhund, P.S.; Mogensen, U.M.; Rørth, R.; Abraham, W.T.; Desai, A.; Dickstein, K.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; et al. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide Levels in Heart Failure Patients With and Without Atrial Fibrillation. Circ. Heart Fail. 2017, 10, e004409. [Google Scholar] [CrossRef] [Green Version]
- Brady, P.F.; Chua, W.; Nehaj, F.; Connolly, D.L.; Khashaba, A.; Purmah, Y.J.V.; Ul-Qamar, M.J.; Thomas, M.R.; Varma, C.; Schnabel, R.B.; et al. Interactions Between Atrial Fibrillation and Natriuretic Peptide in Predicting Heart Failure Hospitalization or Cardiovascular Death. J. Am. Heart Assoc. 2022, 11, e022833. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Mogensen, U.M.; Jhund, P.S.; Rørth, R.; Anand, I.S.; Carson, P.E.; Desai, A.S.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D.; et al. N-Terminal Pro-B-Type Natriuretic Peptide Levels for Risk Prediction in Patients With Heart Failure and Preserved Ejection Fraction According to Atrial Fibrillation Status. Circ. Heart Fail. 2019, 12, e005766. [Google Scholar] [CrossRef]
- Polovina, M.; Lund, L.H.; Đikić, D.; Petrović-Đorđević, I.; Krljanac, G.; Milinković, I.; Veljić, I.; Piepoli, M.F.; Rosano, G.M.; Ristić, A.D.; et al. Type 2 diabetes increases the long-term risk of heart failure and mortality in patients with atrial fibrillation. Eur. J. Heart Fail. 2019, 22, 113–125. [Google Scholar] [CrossRef]
- Westerman, M.; Porter, J.B. Red blood cell-derived microparticles: An overview. Blood Cells Mol. Dis. 2016, 59, 134–139. [Google Scholar] [CrossRef]
- Said, A.S.; Rogers, S.C.; Doctor, A. Physiologic Impact of Circulating RBC Microparticles upon Blood-Vascular Interactions. Front. Physiol. 2018, 8, 1120. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhao, W.; Christ, G.J.; Gladwin, M.T.; Kim-Shapiro, D.B. Nitric oxide scavenging by red cell microparticles. Free. Radic. Biol. Med. 2013, 65, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Helal, O.; Defoort, C.; Robert, S.; Marin, C.; Lesavre, N.; Lopez-Miranda, J.; Risérus, U.; Basu, S.; Lovegrove, J.; McMonagle, J.; et al. Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: Relationship with oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 665–671. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Suades, R.; Padró, T.; Vilahur, G.; Peña, E.; Ybarra, J.; Pou, J.M.; Badimon, L. Microparticle Shedding by Erythrocytes, Monocytes and Vascular Smooth Muscular Cells Is Reduced by Aspirin in Diabetic Patients. Rev. Esp. Cardiol. 2016, 69, 672–680. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef]
- Berezin, A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S176–S183. [Google Scholar] [CrossRef]
- Bheda, P. Metabolic transcriptional memory. Mol. Metab. 2020, 38, 100955. [Google Scholar] [CrossRef]
- Morrison, A.J. Chromatin-remodeling links metabolic signaling to gene expression. Mol. Metab. 2020, 38, 100973. [Google Scholar] [CrossRef]
- Berezin, A.E. Microparticles in Chronic Heart Failure. Adv. Clin. Chem. 2017, 81, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Abplanalp, W.A.; Jung, A.D.; Schuster, R.M.; Lentsch, A.B.; Gulbins, E.; Caldwell, C.C.; Pritts, T.A. Endocytosis of Red Blood Cell Microparticles by Pulmonary Endothelial Cells is Mediated By Rab5. Shock 2018, 49, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Goodman, M.D.; Jung, A.D.; Abplanalp, W.A.; Schuster, R.M.; Caldwell, C.C.; Lentsch, A.B.; Pritts, T.A. Microparticles from aged packed red blood cell units stimulate pulmonary microthrombus formation via P-selectin. Thromb. Res. 2019, 185, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 258, 138–143. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Cao, M.; Ma, R.; Wu, X.; Zhang, Y.; Ding, W.; Liu, Y.; Yu, Q.; Zhang, Y.; et al. Microparticles and blood cells induce procoagulant activity via phosphatidylserine exposure in NSTEMI patients following stent implantation. Int. J. Cardiol. 2016, 223, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Toto, R.D. Microalbuminuria: Definition, Detection, and Clinical Significance. J. Clin. Hypertens. 2004, 6, 2–7. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS): The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [Green Version]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Wakami, K.; Muto, K.; Kikuchi, S.; Goto, T.; Fukuta, H.; Seo, Y.; Ohte, N. Verification of Echocardiographic Assessment of Left Ventricular Diastolic Dysfunction in Patients With Preserved Left Ventricular Ejection Fraction Using the American Society of Echocardiography and European Association of Cardiovascular Imaging 2016 Recommendations. Circ. Rep. 2019, 1, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Van Der Pol, E.; Böing, A.N.; Gool, E.L.; Nieuwland, R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J. Thromb. Haemost. 2016, 14, 48–56. [Google Scholar] [CrossRef] [PubMed]

| Variables | Healthy Volunteers (n = 25) | T2DM Non-HF Patients (n = 30) | T2DM HF Patients | |||||
|---|---|---|---|---|---|---|---|---|
| p * Value | Entire Patient Cohort (n = 417) | p ** Value | AF Patients (n =51) | Non-AF Patients (n = 366) | p *** Value | |||
| Demographics and anthropomorphic parameters | ||||||||
| Age, year | 51 (47–55) | 52 (47–55) | 0.88 | 53 (41–64) | 0.88 | 55 (44–66) | 51 (40–64) | 0.06 |
| Male/female n (%) | 14 (56.0)/11 (44.0) | 17 (57.0) 13 (43.0) | 0.80 | 231 (55.4)/186 (44.6) | 0.76 | 31 (60.7)/20 (39.3) | 200 (54.6)/166 (45.4) | 0.05 |
| BMI, kg/m2 | 23.9 ± 2.7 | 24.1 ± 2.3 | 0.82 | 25.8 ± 2.8 | 0.80 | 26.0 ± 2.7 | 25.5 ± 2.9 | 0.92 |
| Waist circumference, cm | 86.1 ± 3.6 | 88.2 ± 3.0 | 0.78 | 95.1 ± 3.2 | 0.04 | 96.4 ± 3.0 | 94.6 ± 2.7 | 0.78 |
| WHR, units | 0.73 ± 0.04 | 0.84 ± 0.05 | 0.04 | 0.85 ± 0.05 | 0.88 | 0.87 ± 0.04 | 0.85 ± 0.06 | 0.76 |
| Comorbidities and CV risk factors | ||||||||
| Dyslipidemia, n (%) | - | 24 (80.0) | 0.001 | 346 (83.0) | 0.86 | 48 (94.1) | 298 (81.4) | 0.02 |
| Hypertension, n (%) | - | 13 (43.3) | 0.001 | 352 (84.4) | 0.01 | 42 (82.3) | 310 (84.7) | 0.88 |
| Stable CAD, n (%) | - | - | - | 141 (33.8) | 0.001 | 26 (51.0) | 115 (31.4) | 0.01 |
| Smoking, n (%) | 4 (16) | 11 (36.7) | 0.001 | 168 (40.3) | 0.001 | 23 (45.1) | 145 (39.6) | 0.14 |
| Abdominal obesity, n (%) | - | 9 (30.0) | 0.001 | 179 (42.9) | 0.01 | 20 (39.2) | 159 (43.4) | 0.12 |
| LV hypertrophy, n (%) | - | 7 (23.3) | 0.001 | 334 (80.1) | 0.001 | 47 (92.2) | 287 (78.4) | 0.01 |
| CKD 1–3 grades, n (%) | - | 4 (13.3) | 0.001 | 112 (26.9) | 0.001 | 19 (37.2) | 93 (25.4) | 0.04 |
| Microalbuminuria, n (%) | - | 5 (16.7) | 0.001 | 75 (18.0) | 0.66 | 8 (15.7) | 67 (18.3) | 0.10 |
| HF phenotypes and functional classification | ||||||||
| HFpEF, n (%) | - | - | - | 132 (31.7) | 0.001 | 12 (23.5) | 120 (32.6) | 0.04 |
| HFmrEF, n (%) | - | - | - | 140 (33.6) | 0.001 | 16 (31.3) | 124 (33.9) | 0.80 |
| HFrEF, n (%) | - | - | - | 145 (34.8) | 0.001 | 23 (45.1) | 122 (33.3) | 0.01 |
| I/II HF NYHA class, n (%) | - | - | - | 282 (67.6) | 0.001 | 29 (59.9) | 253 (69.1) | 0.04 |
| III HF NYHA class, n (%) | - | - | - | 135 (32.4) | 0.001 | 22 (43.1) | 113 (30.9) | 0.01 |
| Hemodynamics parameters | ||||||||
| SBP, mm Hg | 124 ± 5 | 129 ± 7 | 0.82 | 132 ± 7 | 0.72 | 135 ± 5 | 129 ± 6 | 0.86 |
| DBP, mm Hg | 73 ± 4 | 76 ± 5 | 0.80 | 78 ± 5 | 0.80 | 79 ± 7 | 77 ± 5 | 0.86 |
| LVEDV, mL | 141 (122–155) | 144 (126–158) | 0.86 | 162 (154–170) | 0.01 | 169 (159–180) | 161 (152–169) | 0.04 |
| LVESV, mL | 52 (44–61) | 55 (47–64) | 0.87 | 86 (80–93) | 0.01 | 94 (86–99) | 84 (80–89) | 0.02 |
| LVEF, % | 63 (59–68) | 62 (56–67) | 0.88 | 46 (37–55) | 0.02 | 44 (35–52) | 48 (40–56) | 0.05 |
| LVMMI, g/m2 | 102 ± 4 | 108 ± 5 | 0.74 | 154 ± 5 | 0.001 | 170 ± 7 | 151 ± 5 | 0.04 |
| LAVI, mL/m2 | 31 (29–34) | 34 (30–36) | 0.80 | 43 (37–52) | 0.001 | 48 (41–56) | 40 (36–45) | 0.01 |
| E/e′, unit | 6.45 ± 0.3 | 6.51 ± 0.4 | 0.82 | 13.5 ± 0.3 | 0.001 | 14.9 ± 0.3 | 12.8 ± 0.2 | 0.01 |
| Biomarkers | ||||||||
| eGFR, mL/min/1.73 m2 | 109 ± 5.5 | 102 ± 4.5 | 0.90 | 75 ± 4.0 | 0.001 | 72 ± 3.6 | 76 ± 6.0 | 0.80 |
| HOMA-IR | 4.32 ± 0.7 | 5.95 ± 0.9 | 0.01 | 7.95 ± 2.3 | 0.44 | 8.02 ± 2.2 | 7.90 ± 2.5 | 0.88 |
| Fasting glucose, mmol/L | 5.1 ± 0.7 | 6.08 ± 0.8 | 0.01 | 6.12 ± 1.3 | 0.72 | 6.50 ± 1.4 | 5.80 ± 1.5 | 0.84 |
| HbA1c, % | 5.20 ± 0.04 | 6.40 ± 0.05 | 0.01 | 6.59 ± 0.02 | 0.76 | 7.12 ± 0.10 | 6.42 ± 0.09 | 0.72 |
| Creatinine, µmol/L | 69.5 ± 7.0 | 77.4 ± 8.0 | 0.78 | 108.6 ± 8.5 | 0.01 | 110.4 ± 9.4 | 104.8 ± 8.9 | 0.84 |
| TC, mmol/L | 4.93 ± 0.50 | 5.48 ± 0.40 | 0.02 | 6.43 ± 0.60 | 0.04 | 6.50 ± 0.72 | 6.26 ± 0.50 | 0.88 |
| HDL-C, mmol/L | 1.04 ± 0.12 | 1.01 ± 0.15 | 0.88 | 0.97 ± 0.17 | 0.74 | 0.92 ± 0.20 | 0.99 ± 0.13 | 0.89 |
| LDL-C, mmol/L | 2.88 ± 0.13 | 3.10 ± 0.14 | 0.01 | 4.38 ± 0.10 | 0.02 | 4.60 ± 0.11 | 4.20 ± 0.12 | 0.76 |
| TG, mmol/L | 1.70 ± 0.10 | 1.80 ± 0.12 | 0.86 | 2.21 ± 0.17 | 0.01 | 2.28 ± 0.10 | 2.15 ± 0.15 | 0.68 |
| NT-proBNP, pmol/mL | 48 (10–95) | 56 (0–102) | 0.88 | 2615 (1380–3750) | 0.001 | 3620 (1240–4250) | 2190 (1170–3250) | 0.02 |
| Concomitant medications | ||||||||
| ACEI, n (%) | - | 13 (43.3) | 0.001 | 198 (47.5) | 0.78 | 23 (45.1) | 175 (47.8) | 0.86 |
| ARB, n (%) | - | - | - | 67 (16.1) | 0.001 | 6 (11.7) | 61 (16.7) | 0.16 |
| ARNI, n (%) | - | - | - | 165 (39.6) | 0.001 | 20 (39.2) | 145 (39.6) | 0.92 |
| Beta-blocker, n (%) | - | - | - | 372 (89.2) | 0.001 | 51 (100.0) | 321 (87.7) | 0.05 |
| Ivabradine, n (%) | - | - | - | 59 (14.1) | 0.001 | 0 | 59 (14.1) | 0.001 |
| Calcium channel blocker, n (%) | - | 5 (16.7) | 0.001 | 75 (18.0) | 0.42 | 8 (15.7) | 67 (18.3) | 0.05 |
| MRA, n (%) | - | - | - | 283 (67.8) | 0.001 | 39 (76.5) | 244 (66.7) | 0.04 |
| Loop diuretic, n (%) | - | - | - | 358 (85.9) | 0.001 | 43 (84.3) | 315 (86.1) | 0.90 |
| Antiplatelet, n (%) | - | - | - | 367 (88.0) | 0.001 | 38 (74.5) | 329 (89.9) | 0.04 |
| Anticoagulant, n (%) | - | - | - | 51 (12.2) | 0.001 | 51 (12.2) | 0 | 0.001 |
| Metformin, n (%) | - | 30 (100) | 0.001 | 387 (92.8) | 0.80 | 45 (88.2) | 342 (93.4) | 0.02 |
| Statins, n (%) | - | 24 (80.0) | 0.001 | 408 (97.8) | 0.01 | 49 (96.1) | 359 (98.1) | 0.92 |
| Variables | Dependent Variable: HbA1c ≥ 7.0% | |||
|---|---|---|---|---|
| Univariate Linear Regression | Multivariate Linear Regression | |||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| III NYHA class vs I/II NYHA class | 1.03 (1.00–1.07) | 0.050 | - | |
| AF versus sinus rhythm | 1.08 (0.93–1.17) | 0.82 | - | |
| HFrEF vs HFpEF/HFmrEF | 1.04 (1.02–1.07) | 0.042 | 1.05 (1.01–1.09) | 0.050 |
| LAVI | 1.05 (1.03–1.09) | 0.044 | 1.03 (1.00–1.07) | 0.050 |
| NT-proBNP | 1.07 (1.03–1.12) | 0.010 | 1.07 (1.02–1.10) | 0.040 |
| CD235a+ PS+ RBC-derived vesicles ≥ 545 particles in µL vs. <545 particles in µL | 1.05 (1.02–1.09) | 0.040 | 1.06 (1.01–1.11) | 0.044 |
| Models | AUC | NRI | IDI | |||
|---|---|---|---|---|---|---|
| M (95% CI) | p-Value | M (95% CI) | p-Value | M (95% CI) | p-Value | |
| NT-proBNP | 0.66 (0.60–0.74) | - | Reference | - | Reference | - |
| CD235a+ PS+ RBC-derived vesicles | 0.91 (0.83–0.98) | 0.001 | 0.45 (0.39–0.52) | 0.001 | 0.49 (0.44–0.55) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, A.A.; Obradovic, Z.; Kopp, K.; Berezina, T.A.; Lichtenauer, M.; Wernly, B.; Berezin, A.E. The Association of Glucose Control with Circulating Levels of Red Blood Cell-Derived Vesicles in Type 2 Diabetes Mellitus Patients with Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 729. https://doi.org/10.3390/ijms24010729
Berezin AA, Obradovic Z, Kopp K, Berezina TA, Lichtenauer M, Wernly B, Berezin AE. The Association of Glucose Control with Circulating Levels of Red Blood Cell-Derived Vesicles in Type 2 Diabetes Mellitus Patients with Atrial Fibrillation. International Journal of Molecular Sciences. 2023; 24(1):729. https://doi.org/10.3390/ijms24010729
Chicago/Turabian StyleBerezin, Alexander A., Zeljko Obradovic, Kristen Kopp, Tetiana A. Berezina, Michael Lichtenauer, Bernhard Wernly, and Alexander E. Berezin. 2023. "The Association of Glucose Control with Circulating Levels of Red Blood Cell-Derived Vesicles in Type 2 Diabetes Mellitus Patients with Atrial Fibrillation" International Journal of Molecular Sciences 24, no. 1: 729. https://doi.org/10.3390/ijms24010729
APA StyleBerezin, A. A., Obradovic, Z., Kopp, K., Berezina, T. A., Lichtenauer, M., Wernly, B., & Berezin, A. E. (2023). The Association of Glucose Control with Circulating Levels of Red Blood Cell-Derived Vesicles in Type 2 Diabetes Mellitus Patients with Atrial Fibrillation. International Journal of Molecular Sciences, 24(1), 729. https://doi.org/10.3390/ijms24010729









