The Role of Vitamin D Supplementation in Type 1, Type 2, and Gestational Diabetes: A Comprehensive Updated Narrative Review
Abstract
1. Introduction
2. Methodology
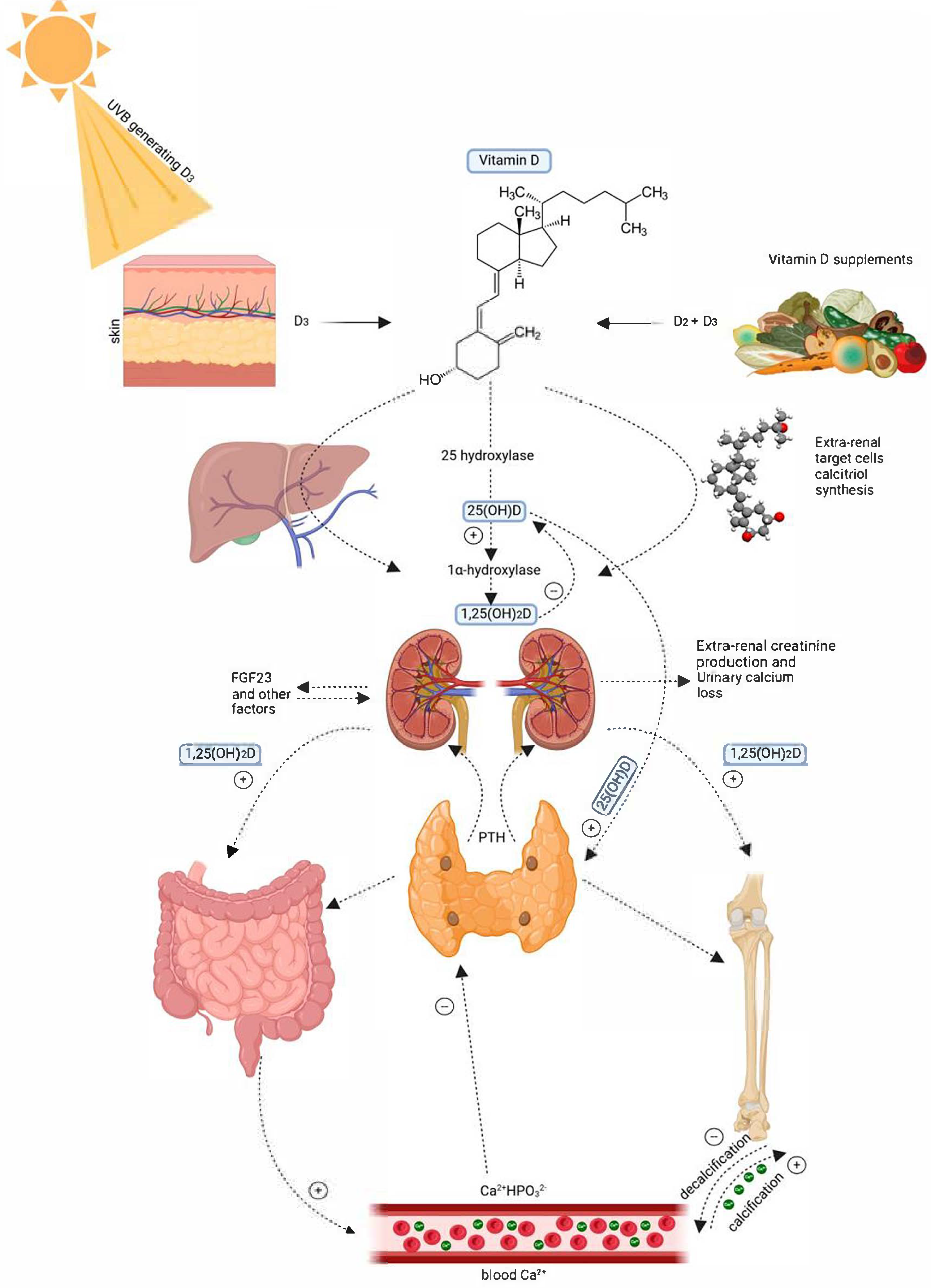
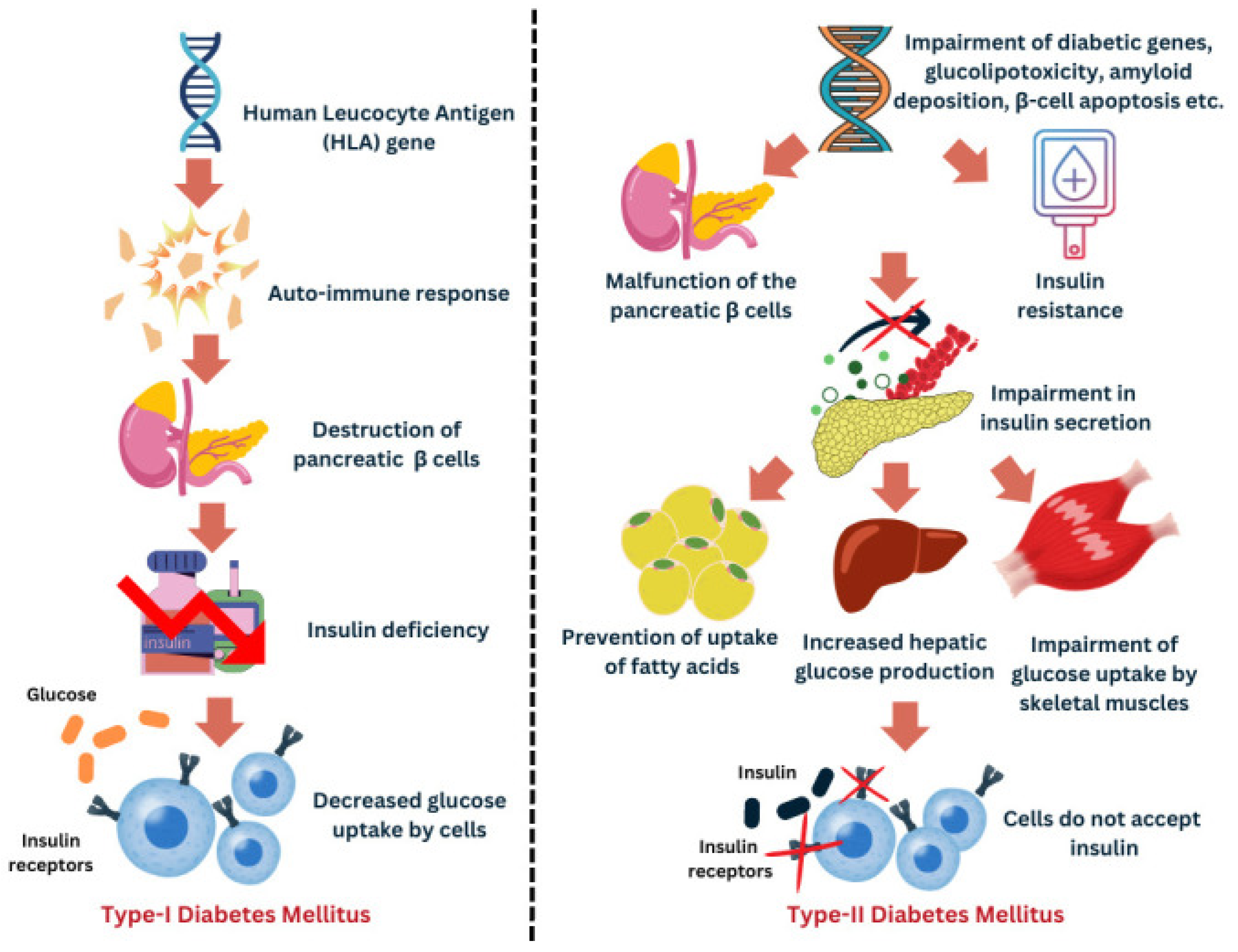
3. Mechanisms Linking Vitamin D with Diabetes Pathophysiology
4. Results
4.1. Vitamin D and Type 1 Diabetes Mellitus
4.2. Vitamin D and Type 2 Diabetes Mellitus
4.3. Vitamin D and Gestational Diabetes Mellitus
5. Discussion
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- D Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 27 June 2025).
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Voiculescu, V.M.; Nelson Twakor, A.; Jerpelea, N.; Pantea Stoian, A. Vitamin D: Beyond Traditional Roles—Insights into Its Biochemical Pathways and Physiological Impacts. Nutrients 2025, 17, 803. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Mohan, V.; Sandeep, S.; Deepa, R.; Shah, B.; Varghese, C. Epidemiology of type 2 diabetes: Indian scenario. Indian J. Med. Res. 2007, 125, 217–230. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 27 June 2025).
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H. Gestational diabetes mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A.; Watanabe, R.M. What is Gestational Diabetes—Really? Diabetes 2025, 74, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Siam, N.H.; Snigdha, N.N.; Tabasumma, N.; Parvin, I. Diabetes Mellitus and Cardiovascular Disease: Exploring Epidemiology and Pathophysiology. Rev. Cardiovasc. Med. 2024, 25, 436. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Walker, G.E.; Vietti, R.; Cattaldo, S.; Mele, C.; Priano, L.; Mauro, A.; Bona, G.; Aimaretti, G.; Scacchi, M.; et al. Acute Vitamin D3 supplementation in severe obesity: Evaluation of multimeric adiponectin. Nutrients 2017, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, D.; Hamid, Z. The role of vitamin D in diabetes and cardiovascular disease: An updated review of the literature. Dis. Markers 2015, 2015, 580474. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, W.G.; Chen, J.J.; Zhang, Z.L.; Han, S.F.; Qin, L.Q. Vitamin D intake and risk of type 1 diabetes: A meta-analysis of observational studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: A systematic review and meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef]
- Zhang, M.X.; Pan, G.T.; Guo, J.F.; Li, B.Y.; Qin, L.Q.; Zhang, Z.L. Vitamin D deficiency increases the risk of gestational diabetes mellitus: A meta-analysis of observational studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef]
- Parlea, L.; Bromberg, I.L.; Feig, D.S.; Vieth, R.; Merman, E.; Lipscombe, L.L. Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet. Med. 2012, 29, e25–e32. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2022, 14, 2097. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M. Vitamin D and type 1 diabetes mellitus: State of the art. Trends Endocrinol. Metab. 2022, 33, 832–844. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in young adults. Am. J. Epidemiol. 2013, 177, 411–419. [Google Scholar] [CrossRef]
- Gabbay, M.A.; Sato, M.N.; Finazzo, C.; Duarte, A.J.; Dib, S.A. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β-cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 2012, 166, 601–607. [Google Scholar] [CrossRef]
- Manousaki, D.; Harroud, A.; Mitchell, R.E.; Ross, S.; Forgetta, V.; Timpson, N.J.; Smith, G.D.; Polychronakos, C.; Richards, J.B. Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLoS Med. 2021, 18, e1003536. [Google Scholar] [CrossRef]
- Miettinen, M.E.; Niinistö, S.; Erlund, I.; Cuthbertson, D.; Nucci, A.M.; Honkanen, J.; Vaarala, O.; Hyöty, H.; Krischer, J.P.; Knip, M.; et al. Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: The TRIGR nested case-control ancillary study. Diabetologia 2020, 63, 780–787. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Khan, I.A.; Khan, N.; Alharbi, F.K.; Alghamdi, J.; Alshahrani, M.Y.; Alharbi, K.F. Vitamin D deficiency and its association with glycemic control in children and adolescents with type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 2022, 107, e1077–e1085. [Google Scholar] [CrossRef]
- Barot, K.S.; Abbasi, Z.A.; Krishna Mohan, G.V.; Abid, S.A.; Hussain, S.A.; Wei, C.R.; Ali, N. Prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes mellitus: A systematic review and meta-analysis. Cureus 2025, 17, e83843. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Li, H.; Sun, Y.; Wu, L.; Chang, Y. Effect of vitamin D supplementation on glycemic control in type 1 diabetes mellitus: A meta-analysis. Clin. Nutr. 2024, 43, 10–18. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Littorin, B.; Blom, P.; Schölin, A.; Arnqvist, H.J.; Blohmé, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjörnsdottir, S.; Nyström, L.; et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Nascimento, B.F.; Moreira, C.F.F.; da Fonseca, E.R.; Fedeszen, P.M.K.; de Paula, T.P.; de Sena, A.S.S.; de Almeida, N.F.A.; Bandeira Filho, O.C.S.; Curval, D.R.; Padilha, P.C. Effects of vitamin D supplementation on glycemic control of children and adolescents with type 1 diabetes mellitus: A systematic review. J. Pediatr. Endocrinol. Metab. 2022, 35, 973–988. [Google Scholar] [CrossRef]
- Mathieu, C.; Gysemans, C.; Giulietti, A.; Bouillon, R. Vitamin D and diabetes. Diabetologia 2005, 48, 1247–1257. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.R.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Delahanty, L.M.; Barrett-Connor, E.; Crandall, J.P.; D2d Research Group. Vitamin D supplementation prevention of type 2 diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.A.; Daneshzad, E.; Fung, T.T.; Zahidi, F.; Muhammadi, M.; Bellissimo, N.; Azadbakht, L. What is the impact of vitamin D supplementation on glycemic control in people with type-2 diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2023, 23, 15. [Google Scholar] [CrossRef]
- Cheng, L.; Lv, C.; Xue, L.; Zhang, C.; Wang, L.; Wang, X.; Chen, S.; Li, X.; Feng, W.; Xie, H.; et al. The prevention and improvement effects of vitamin D on type 2 diabetes mellitus: Evidence from an umbrella review on meta-analyses of cohort studies and randomized controlled trials. Front. Nutr. 2024, 11, 1462535. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776, Erratum in JAMA Cardiol. 2020, 5, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vojdeman, F.J.; Heegaard, N.H.H.; Madsbad, S.; Jørgensen, M.E.; Linneberg, A.; Andersen, S.; Thuesen, B.H. Vitamin D deficiency and risk of type 2 diabetes: A cohort study. Diabetes Care 2022, 45, 1486–1493. [Google Scholar] [CrossRef]
- Papandreou, D.; Hamid Mehmood, Z.-T.-N. An Updated Mini Review of Vitamin D and Obesity: Adipogenesis and Inflammation State. Open Access Maced. J. Med. Sci. 2016, 4, 526–532. [Google Scholar]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Poel, Y.; Hummel, P.; Lips, P.; Stam, F.; Van Der Ploeg, T.; Simsek, S. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur J Intern Med. 2012, 23, 465–469. [Google Scholar] [CrossRef]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, M.K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Corcoy, R.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Adelantado, J.M.; Chico, A. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin. Nutr. 2020, 39, 976–984. [Google Scholar] [CrossRef]
- Soheilykhah, S.; Mojibian, M.; Moghadam, M.J.; Jannati Moghadam, M.; Shojaoddiny-Ardekani, A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol. Endocrinol. 2013, 29, 396–399. [Google Scholar] [CrossRef]
- Kron-Rodrigues, M.R.; de Souza, A.I.; Lima, M.C.; de Lima, J.R.; da Silva, E.P.; de Lima, J.G. Supplementation of vitamin D in the postdelivery period of women diagnosed with previous gestational diabetes mellitus: A systematic review. Rev. Bras. De Ginecol. Obstetrícia 2021, 43, 700–707. [Google Scholar]
- von Herrath, M.G.; Nepom, G.T.; Babu, S.R.; Atkinson, M.A.; Hummel, P.; Lips, P.; Simsek, S. Is vitamin D deficiency involved in the pathogenesis of type 1 diabetes? A systematic review. Eur. J. Clin. Nutr. 2012, 66, 566–571. [Google Scholar] [CrossRef]
- Ghosh, S.; Raj, P.; Mohapatra, P.R. Vitamin D in type 1 diabetes mellitus: Current status and future prospects. Indian. J. Endocrinol. Metab. 2013, 17, 311–317. [Google Scholar] [CrossRef]
- Karras, S.; Anagnostis, P.; Paschou, S.A.; Kandaraki, E.; Goulis, D.G. Vitamin D status during pregnancy: Time for a more unified approach beyond borders? Eur. J. Clin. Nutr. 2015, 69, 874–877. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D supplementation: A review of the evidence arguing for a daily dose of 2000 International Units (50 µg) of vitamin D for adults in the general population. Nutrients 2024, 16, 391. [Google Scholar] [CrossRef]
| Study | Design | Population | Vitamin D Dose | Duration | Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Gabbay et al. [27] | Randomized Controlled Trial | T1DM patients with vitamin D deficiency | 2000 IU/day cholecalciferol | 1 year | Improved HbA1c, reduced insulin requirements | Vitamin D supplementation improved metabolic control in deficient patients |
| Dong et al. [20] | Meta-analysis of RCTs | T1DM patients | Varied | Varied | Modest reductions in HbA1c and fasting glucose | More effective in individuals with low baseline vitamin D |
| Chen et al. [32] | Meta-analysis of RCTs | T1DM patients | Varied | Varied | Improved glycemic control | Supports adjunctive role of vitamin D in T1DM management |
| Ferrari and Locatelli [25] | Systematic Review | Individuals with or at risk for T1DM | Varied | Varied | Reviewed immune modulation and metabolic effects of vitamin D | Vitamin D may preserve β-cell function and improve glycemic outcomes |
| Hyppönen et al. [34] | Birth Cohort Study (Epidemiological) | Finnish infants followed into adulthood | Regular supplementation (infancy) | Long-term follow-up | ~80% reduced T1DM risk in those supplemented with vitamin D | Strong inverse association between early vitamin D and T1DM risk |
| Munger et al. [26] | Epidemiological Cohort Study | Adults with varied serum 25(OH)D levels | N/A (observational serum levels) | Not specified | Higher serum vitamin D linked with 44% lower T1DM risk | Supports protective effect of higher vitamin D status |
| Littorin et al. [35] | Case-control nested in population study | Newly diagnosed T1D patients (15–34 yrs, n=459) vs. matched controls (n=208), Sweden | No supplementation; 25OHD measured | At diagnosis + 8-year follow-up | T1D group had significantly lower 25OHD at diagnosis; further decline over 8 year | Low vitamin D levels at diagnosis may be linked to T1D development, especially in males. |
| Zipitis and Akobeng [33] | Systematic Review of Observational Studies | Children receiving vitamin D supplementation | Varied | Childhood | 29% reduced risk of T1DM with vitamin D supplementation | Observational evidence supports protective role of vitamin D |
| Nascimento et al. [36] | Systematic review | Children and adolescents with type 1 diabetes (8 studies included) | Various doses across studies | 2–12 months | Mixed results; some studies showed improved HbA1c, others no significant effect | Vitamin D may help improve glycemic control, but evidence remains inconsistent. |
| Manousaki et al., 2021 [28] | Mendelian Randomization Study | General population (T1DM genetics) | N/A | N/A | Low genetically predicted vitamin D ↑ T1DM risk | Genetic evidence for causal relationship |
| Miettinen et al.(TRIGR), 2020 [29] | Nested Case-Control (TRIGR) | Children at risk of T1DM | Serum 25(OH)D levels | childhood | No significant association | Vitamin D status in childhood not strongly linked to T1DM |
| Study | Design | Population | Vitamin D Dose | Duration | Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Pittas et al. [38] | Randomized Controlled Trial (D2d study) | Adults with prediabetes | 4000 IU/day | 2.5 years | No significant reduction in diabetes incidence overall; benefit seen in vitamin-D-deficient subgroup | Efficacy may depend on baseline vitamin D status |
| Dong et al. [20] | Meta-analysis of RCTs | T2DM patients | Varied | Varied | Modest improvements in FPG, HOMA-IR, HbA1c (mainly in deficient individuals) | Supplementation improves metabolic indices in vitamin-D-deficient patients |
| Chen et al. [32] | Meta-analysis of RCTs | T2DM patients | Varied | Varied | Reductions in HbA1c and fasting glucose | Supports beneficial effect of vitamin D on glycemic control |
| Barbarawi et al. [41] | Meta-analysis of RCTs | T2DM patients | Varied | Varied | Improved lipid profile, reduced BP, reduced inflammatory markers | Vitamin D may improve cardiometabolic outcomes |
| Mai et al. [17] | Interventional study | Adults with severe obesity (n = 48) | Single high dose (600,000 IU cholecalciferol) | 1 month | Increased total and HMW adiponectin after supplementation | High-dose vitamin D3 improved adiponectin profile in obese patients. |
| Vojdeman et al. [42] | Observational Cohort Study | Danish adults | N/A | Not specified | Vitamin D deficiency associated with increased T2DM risk, especially in younger adults | Supports a link between low vitamin D and increased diabetes incidence |
| Pittas et al., 2019 [38] | RCT | Adults at high risk of T2DM | 4000 IU/day cholecalciferol | Median 2.5 years | No significant ↓ in diabetes incidence overall | Subgroup benefit in those with low vitamin D |
| Farahmand et al., 2023 [39] | Systematic Review and Meta-analysis | People with T2DM | Varied | Varied | ↓ HbA1c, ↓ fasting glucose, ↓ insulin resistance | Supports glycemic control in T2DM |
| Cheng et al., 2024 [40] | Umbrella Review | Mixed (T2DM, cohort + RCTs) | Varied | Varied | Improved control and reduced T2DM risk | Strong evidence for benefit in T2DM |
| Study | Design | Population | Vitamin D Dose | Timing | Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Soheilykhah et al. [48] | RCT | Pregnant women | 50,000 IU every two weeks | During pregnancy | Improved insulin sensitivity, reduced fasting glucose levels | Higher dose vitamin D may improve glycemic control |
| Corcoy et al. [47] | RCT | Pregnant Women | 1600 IU daily | During Pregnancy | Improved Vitamin D sufficiency | Small statistically reduction in FBG-No effect on insulin |
| Poel et al. [45] | Systematic Review and Meta-analysis of RCTs | Pregnant women across multiple studies | Varied | Varied | Modest improvement in insulin resistance | Vitamin D may improve insulin sensitivity; results variable |
| Harvey et al. [46] | Systematic Review (includes RCTs) | Pregnant women | Varied across studies | Varied | Possible improvements in metabolic markers | Inconsistencies in trial findings noted |
| Littorin et al. [35] | Case-control nested in population study | Newly diagnosed T1D patients (15–34 yrs, n = 459) vs. matched controls (n = 208), Sweden | No supplementation; 25OHD measured | At diagnosis + 8-year follow-up | T1D group had significantly lower 25OHD at diagnosis; further decline over 8 year | Low vitamin D levels at diagnosis may be linked to T1D development, especially in males. |
| Kron-Rodrigues et al. [49] | Systematic Review and meta-analysis of 11 studies | Pregnant women | Varied | Varied | No differences in the frequency of cesarean deliveries | No high-quality evidence to support vitamin D supplementation |
| Bi et al. [21] | Prospective Observational Study | Pregnant women | N/A | Early pregnancy | Insufficient vitamin D linked to impaired glucose tolerance and GDM | Early deficiency may increase GDM risk |
| Parlea et al. [23] | Observational Cohort | Pregnant women | N/A | Early pregnancy | Low 25(OH)D associated with higher GDM incidence later in pregnancy | Low vitamin D status may contribute to GDM |
| Buchanan and Xiang, 2005 [10] | Review Study | Women with Gestational Diabetes | N/A | N/A | Explores pathogenesis and consequences | Summarizes GDM as predictor of future diabetes risk |
| Buchanan et al. [11] | Narrative review | Pregnant women with/at risk of gestational diabetes | N/A | N/A | Explores complex pathophysiology beyond glucose intolerance | GDM is a heterogeneous condition with diverse underlying mechanisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, A.; Papandreou, D.; Papadopoulou, S.K.; Cheikh Ismail, L. The Role of Vitamin D Supplementation in Type 1, Type 2, and Gestational Diabetes: A Comprehensive Updated Narrative Review. Clin. Pract. 2025, 15, 148. https://doi.org/10.3390/clinpract15080148
Nasser A, Papandreou D, Papadopoulou SK, Cheikh Ismail L. The Role of Vitamin D Supplementation in Type 1, Type 2, and Gestational Diabetes: A Comprehensive Updated Narrative Review. Clinics and Practice. 2025; 15(8):148. https://doi.org/10.3390/clinpract15080148
Chicago/Turabian StyleNasser, Asala, Dimitrios Papandreou, Sousana K. Papadopoulou, and Leila Cheikh Ismail. 2025. "The Role of Vitamin D Supplementation in Type 1, Type 2, and Gestational Diabetes: A Comprehensive Updated Narrative Review" Clinics and Practice 15, no. 8: 148. https://doi.org/10.3390/clinpract15080148
APA StyleNasser, A., Papandreou, D., Papadopoulou, S. K., & Cheikh Ismail, L. (2025). The Role of Vitamin D Supplementation in Type 1, Type 2, and Gestational Diabetes: A Comprehensive Updated Narrative Review. Clinics and Practice, 15(8), 148. https://doi.org/10.3390/clinpract15080148








