Alpha-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Meta-Analysis
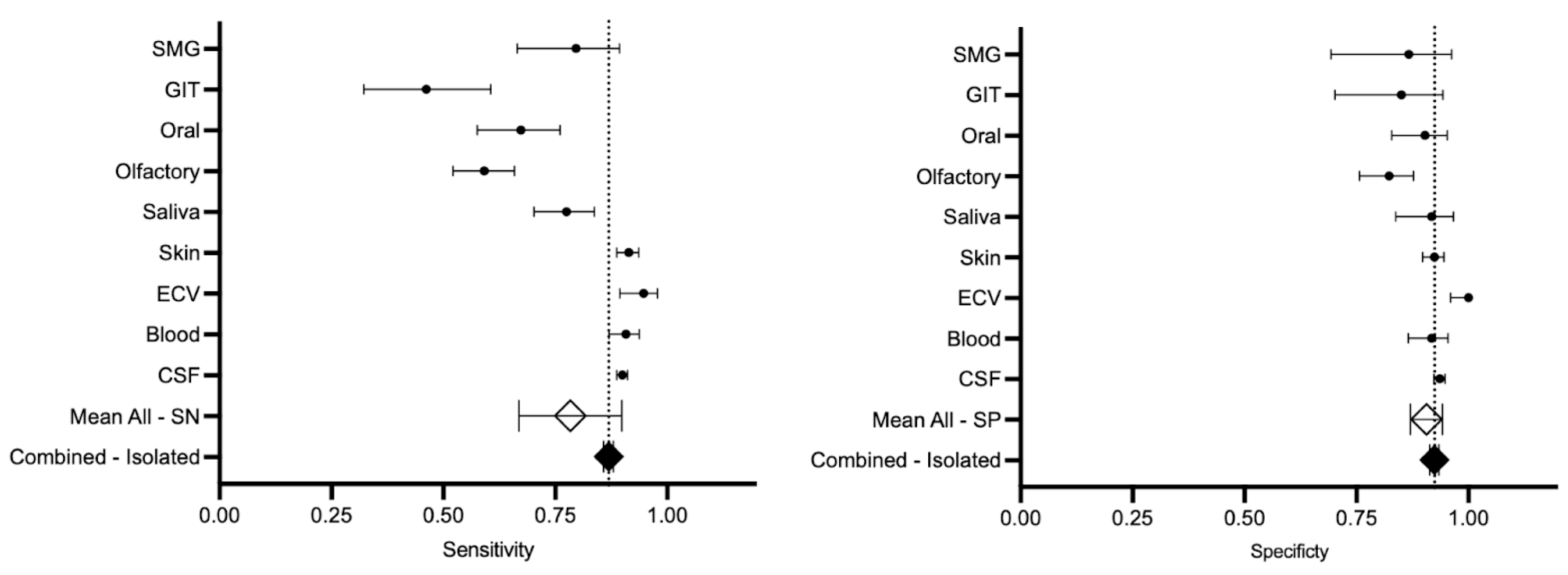
3.4.1. Sensitivity and Specificity
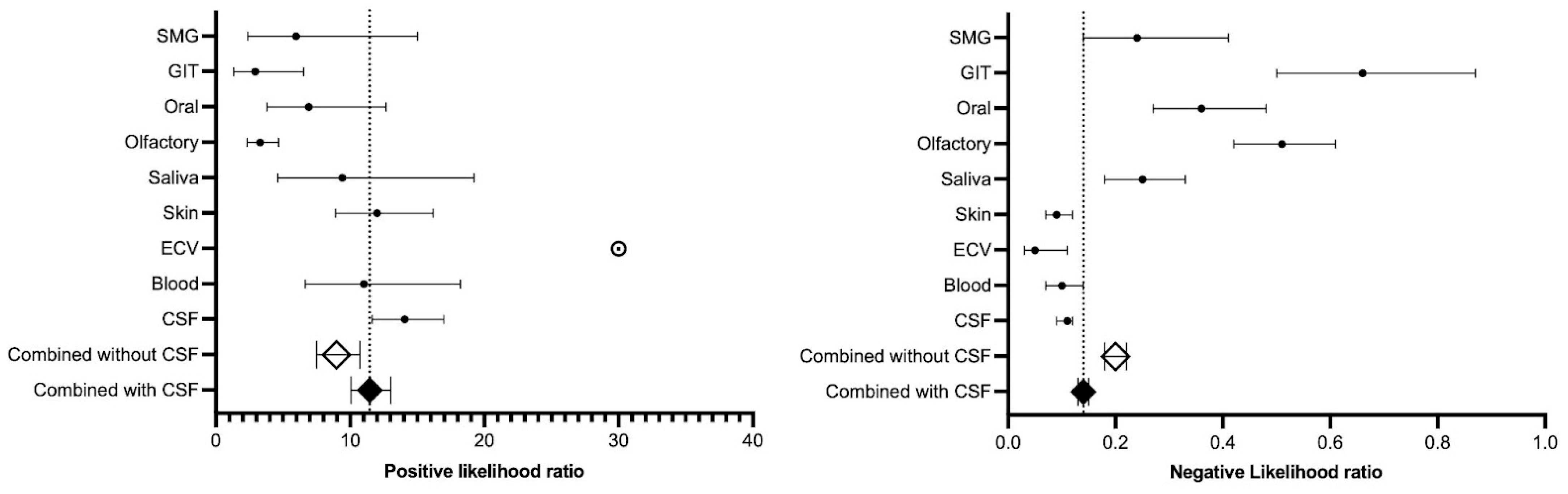
3.4.2. Positive and Negative Likelihood Ratio
3.4.3. ANOVA Results
3.4.4. Comparison Between RT-QuIC and PMCA
4. Discussion
4.1. General
4.2. Cerebrospinal Fluid
4.3. Blood
4.4. Neuronal Exosomes/Extracellular Vesicles
4.5. Skin
4.6. Saliva
4.7. Olfactory Mucosa
4.8. Oral Mucosa
4.9. Gastrointestinal Tract (Rectum/Sigmoid/Antrum)
4.10. Submandibular Gland
4.11. RT-QuIC Versus PMCA
5. Limitations
6. Future Directions
Limitations of αSyn-SAA
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| αSyn | alpha-synuclein |
| αSyn-SAA | alpha-synuclein seed amplification assay |
| CI | confidence interval |
| CNS | central nervous system |
| DOR | diagnostic odds ratio |
| ECV | extracellular vesicle |
| GIT | gastrointestinal tract |
| MSA | multiple system atrophy |
| OM | olfactory mucosa |
| p-αSyn | phosphorylated alpha-synuclein |
| PD | Parkinson’s disease |
| PMCA | protein misfolding cyclic amplification |
| RT-QuIC | real-time quaking-induced conversion |
| SMG | submandibular gland |
References
- Zupancic, M.; Mahajan, A.; Handa, K. Dementia with Lewy Bodies: Diagnosis and Management for Primary Care Providers. Prim. Care Companion CNS Disord. 2011, 13, 26212. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Cullen, V.; Kahn, I.; Krastins, B.; Outeiro, T.F.; Pepivani, I.; Ng, J.; Schulz-Schaeffer, W.; Kretzschmar, H.A.; McLean, P.J.; et al. Direct Quantification of CSF Alpha-Synuclein by ELISA and First Cross-Sectional Study in Patients with Neurodegeneration. Exp. Neurol. 2008, 213, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.-T.-T.; Satoh, K. The Latest Research on RT-QuIC Assays-A Literature Review. Pathogens 2021, 10, 305. [Google Scholar] [CrossRef]
- Pons, M.-L.; Loftus, N.; Vialaret, J.; Moreau, S.; Lehmann, S.; Hirtz, C. Proteomics Challenges for the Assessment of Synuclein Proteoforms as Clinical Biomarkers in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 818606. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Schoonjans, F.; Zalata, A.; Depuydt, C.E.; Comhaire, F.H. MedCalc: A New Computer Program for Medical Statistics. Comput. Methods Programs Biomed. 1995, 48, 257–262. [Google Scholar] [CrossRef]
- Swift, M.L. GraphPad Prism, Data Analysis, and Scientific Graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M. Metadta: A Stata Command for Meta-Analysis and Meta-Regression of Diagnostic Test Accuracy Data—A Tutorial. Arch. Public Health 2022, 80, 95. [Google Scholar] [CrossRef]
- Patel, A.; Cooper, N.; Freeman, S.; Sutton, A. Graphical Enhancements to Summary Receiver Operating Characteristic Plots to Facilitate the Analysis and Reporting of Meta-Analysis of Diagnostic Test Accuracy Data. Res. Synth. Methods 2021, 12, 34–44. [Google Scholar] [CrossRef]
- Blettner, M.; Sauerbrei, W.; Schlehofer, B.; Scheuchenpflug, T.; Friedenreich, C. Traditional Reviews, Meta-Analyses and Pooled Analyses in Epidemiology. Int. J. Epidemiol. 1999, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gumbs, A.A.; Hilal, M.A.; Croner, R.; Gayet, B.; Chouillard, E.; Gagner, M. The Initiation, Standardization and Proficiency (ISP) Phases of the Learning Curve for Minimally Invasive Liver Resection: Comparison of a Fellowship-Trained Surgeon with the Pioneers and Early Adopters. Surg. Endosc. 2021, 35, 5268–5278. [Google Scholar] [CrossRef]
- Kottas, M.; Kuss, O.; Zapf, A. A Modified Wald Interval for the Area under the ROC Curve (AUC) in Diagnostic Case-Control Studies. BMC Med. Res. Methodol. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Aerts, M.; Arbyn, M. ANOVA Model for Network Meta-Analysis of Diagnostic Test Accuracy Data. Stat. Methods Med. Res. 2018, 27, 1766–1784. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC Electron. J. Int. Fed. Clin. Chem. 2009, 19, 203–211. [Google Scholar]
- Zou, L.; Choi, Y.-H.; Guizzetti, L.; Shu, D.; Zou, J.; Zou, G. Extending the DeLong Algorithm for Comparing Areas under Correlated Receiver Operating Characteristic Curves with Missing Data. Stat. Med. 2024, 43, 4148–4162. [Google Scholar] [CrossRef]
- Faraggi, D.; Reiser, B. Estimation of the Area under the ROC Curve. Stat. Med. 2002, 21, 3093–3106. [Google Scholar] [CrossRef]
- Muschelli, J. ROC and AUC with a Binary Predictor: A Potentially Misleading Metric. J. Classif. 2020, 37, 696–708. [Google Scholar] [CrossRef]
- De Luca, C.M.G.; Elia, A.E.; Portaleone, S.M.; Cazzaniga, F.A.; Rossi, M.; Bistaffa, E.; De Cecco, E.; Narkiewicz, J.; Salzano, G.; Carletta, O.; et al. Efficient RT-QuIC Seeding Activity for α-Synuclein in Olfactory Mucosa Samples of Patients with Parkinson’s Disease and Multiple System Atrophy. Transl. Neurodegener. 2019, 8, 24. [Google Scholar] [CrossRef]
- Shin, C.; Han, J.-Y.; Kim, S.-I.; Park, S.-H.; Yang, H.-K.; Lee, H.-J.; Kong, S.-H.; Suh, Y.-S.; Kim, H.-J.; Choi, Y.P.; et al. In Vivo and Autopsy Validation of Alpha-Synuclein Seeding Activity Using RT-QuIC Assay in the Gastrointestinal Tract of Patients with Parkinson’s Disease. Park. Relat. Disord. 2022, 103, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Tsunemi, T.; Ishiguro, Y.; Okuzumi, A.; Hatano, T.; Hattori, N. Comorbid Alpha Synucleinopathies in Idiopathic Normal Pressure Hydrocephalus. J. Neurol. 2022, 269, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic Criteria for Parkinson Disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.-E.; Farris, C.M.; Ma, Y.; Urenia, P.A.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of Heterogeneity among Participants in the Parkinson’s Progression Markers Initiative Cohort Using α-Synuclein Seed Amplification: A Cross-Sectional Study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-Synuclein RT-QuIC in the CSF of Patients with Alpha-Synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol. 2017, 74, 163–172. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and Ultra-Sensitive Quantitation of Disease-Associated α-Synuclein Seeds in Brain and Cerebrospinal Fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef]
- Candelise, N.; Schmitz, M.; Llorens, F.; Villar-Piqué, A.; Cramm, M.; Thom, T.; da Silva Correia, S.M.; da Cunha, J.E.G.; Möbius, W.; Outeiro, T.F.; et al. Seeding Variability of Different Alpha Synuclein Strains in Synucleinopathies. Ann. Neurol. 2019, 85, 691–703. [Google Scholar] [CrossRef]
- Garrido, A.; Fairfoul, G.; Tolosa, E.S.; Martí, M.J.; Green, A. α-Synuclein RT-QuIC in Cerebrospinal Fluid of LRRK2-Linked Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2019, 6, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.J.; Boehme, A.K.; Fairfoul, G.; Shahnawaz, M.; Ma, T.C.; Hutten, S.J.; Green, A.; Soto, C. Comparative Study of Cerebrospinal Fluid α-Synuclein Seeding Aggregation Assays for Diagnosis of Parkinson’s Disease. Mov. Disord. 2019, 34, 536–544. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Hepker, M.; Jin, H.; Anantharam, V.; Lewis, M.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. Ultrasensitive Detection of Aggregated α-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders. J. Neuroimmune Pharmacol. 2019, 14, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Wu, Q.; Han, D.; Yao, T.; Wang, J.; Lu, W.; Lv, S.; Jia, Q.; Li, X. Baseline Concentration of Misfolded α-Synuclein Aggregates in Cerebrospinal Fluid Predicts Risk of Cognitive Decline in Parkinson’s Disease. Neuropathol. Appl. Neurobiol. 2019, 45, 398–409. [Google Scholar] [CrossRef] [PubMed]
- van Rumund, A.; Green, A.J.E.; Fairfoul, G.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. α-Synuclein Real-Time Quaking-Induced Conversion in the Cerebrospinal Fluid of Uncertain Cases of Parkinsonism. Ann. Neurol. 2019, 85, 777–781. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC Assay with High Sensitivity and Specificity for Lewy Body-Associated Synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-Synuclein Strains in Parkinson’s Disease and Multiple System Atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Singer, W.; Schmeichel, A.M.; Shahnawaz, M.; Schmelzer, J.D.; Boeve, B.F.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Olson, A.D.; Savica, R.; et al. Alpha-Synuclein Oligomers and Neurofilament Light Chain in Spinal Fluid Differentiate Multiple System Atrophy from Lewy Body Synucleinopathies. Ann. Neurol. 2020, 88, 503–512. [Google Scholar] [CrossRef]
- Bargar, C.; Wang, W.; Gunzler, S.A.; LeFevre, A.; Wang, Z.; Lerner, A.J.; Singh, N.; Tatsuoka, C.; Appleby, B.; Zhu, X.; et al. Streamlined Alpha-Synuclein RT-QuIC Assay for Various Biospecimens in Parkinson’s Disease and Dementia with Lewy Bodies. Acta Neuropathol. Commun. 2021, 9, 62. [Google Scholar] [CrossRef]
- Brockmann, K.; Quadalti, C.; Lerche, S.; Rossi, M.; Wurster, I.; Baiardi, S.; Roeben, B.; Mammana, A.; Zimmermann, M.; Hauser, A.-K.; et al. Association between CSF Alpha-Synuclein Seeding Activity and Genetic Status in Parkinson’s Disease and Dementia with Lewy Bodies. Acta Neuropathol. Commun. 2021, 9, 175. [Google Scholar] [CrossRef]
- Donadio, V.; Wang, Z.; Incensi, A.; Rizzo, G.; Fileccia, E.; Vacchiano, V.; Capellari, S.; Magnani, M.; Scaglione, C.; Stanzani Maserati, M.; et al. In Vivo Diagnosis of Synucleinopathies: A Comparative Study of Skin Biopsy and RT-QuIC. Neurology 2021, 96, e2513–e2524. [Google Scholar] [CrossRef] [PubMed]
- Mammana, A.; Baiardi, S.; Quadalti, C.; Rossi, M.; Donadio, V.; Capellari, S.; Liguori, R.; Parchi, P. RT-QuIC Detection of Pathological α-Synuclein in Skin Punches of Patients with Lewy Body Disease. Mov. Disord. 2021, 36, 2173–2177. [Google Scholar] [CrossRef]
- Orrù, C.D.; Ma, T.C.; Hughson, A.G.; Groveman, B.R.; Srivastava, A.; Galasko, D.; Angers, R.; Downey, P.; Crawford, K.; Hutten, S.J.; et al. A Rapid α-Synuclein Seed Assay of Parkinson’s Disease CSF Panel Shows High Diagnostic Accuracy. Ann. Clin. Transl. Neurol. 2021, 8, 374–384. [Google Scholar] [CrossRef]
- Quadalti, C.; Calandra-Buonaura, G.; Baiardi, S.; Mastrangelo, A.; Rossi, M.; Zenesini, C.; Giannini, G.; Candelise, N.; Sambati, L.; Polischi, B.; et al. Neurofilament Light Chain and α-Synuclein RT-QuIC as Differential Diagnostic Biomarkers in Parkinsonisms and Related Syndromes. NPJ Park. Dis. 2021, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Orru, C.D.; Concha-Marambio, L.; Giaisi, S.; Groveman, B.R.; Farris, C.M.; Holguin, B.; Hughson, A.G.; LaFontant, D.-E.; Caspell-Garcia, C.; et al. High Diagnostic Performance of Independent Alpha-Synuclein Seed Amplification Assays for Detection of Early Parkinson’s Disease. Acta Neuropathol. Commun. 2021, 9, 179. [Google Scholar] [CrossRef]
- Bongianni, M.; Catalan, M.; Perra, D.; Fontana, E.; Janes, F.; Bertolotti, C.; Sacchetto, L.; Capaldi, S.; Tagliapietra, M.; Polverino, P.; et al. Olfactory Swab Sampling Optimization for α-Synuclein Aggregate Detection in Patients with Parkinson’s Disease. Transl. Neurodegener. 2022, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Painous, C.; Soto, M.; Pulido-Salgado, M.; Fernández, M.; Camara, A.; Sánchez, V.; Bargalló, N.; Caballol, N.; Pont-Sunyer, C.; et al. Combined CSF α-SYN RT-QuIC, CSF NFL and Midbrain-Pons Planimetry in Degenerative Parkinsonisms: From Bedside to Bench, and Back Again. Park. Relat. Disord. 2022, 99, 33–41. [Google Scholar] [CrossRef]
- Hall, S.; Orrù, C.D.; Serrano, G.E.; Galasko, D.; Hughson, A.G.; Groveman, B.R.; Adler, C.H.; Beach, T.G.; Caughey, B.; Hansson, O. Performance of αSynuclein RT-QuIC in Relation to Neuropathological Staging of Lewy Body Disease. Acta Neuropathol. Commun. 2022, 10, 90. [Google Scholar] [CrossRef]
- Majbour, N.; Aasly, J.; Abdi, I.; Ghanem, S.; Erskine, D.; van de Berg, W.; El-Agnaf, O. Disease-Associated α-Synuclein Aggregates as Biomarkers of Parkinson Disease Clinical Stage. Neurology 2022, 99, e2417–e2427. [Google Scholar] [CrossRef]
- Poggiolini, I.; Gupta, V.; Lawton, M.; Lee, S.; El-Turabi, A.; Querejeta-Coma, A.; Trenkwalder, C.; Sixel-Döring, F.; Foubert-Samier, A.; Pavy-Le Traon, A.; et al. Diagnostic Value of Cerebrospinal Fluid Alpha-Synuclein Seed Quantification in Synucleinopathies. Brain 2022, 145, 584–595. [Google Scholar] [CrossRef]
- Chahine, L.M.; Beach, T.G.; Adler, C.H.; Hepker, M.; Kanthasamy, A.; Appel, S.; Pritzkow, S.; Pinho, M.; Mosovsky, S.; Serrano, G.E.; et al. Central and Peripheral α-Synuclein in Parkinson Disease Detected by Seed Amplification Assay. Ann. Clin. Transl. Neurol. 2023, 10, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Weber, S.; Farris, C.M.; Dakna, M.; Lang, E.; Wicke, T.; Ma, Y.; Starke, M.; Ebentheuer, J.; Sixel-Döring, F.; et al. Accurate Detection of α-Synuclein Seeds in Cerebrospinal Fluid from Isolated Rapid Eye Movement Sleep Behavior Disorder and Patients with Parkinson’s Disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov. Disord. 2023, 38, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Gomes, B.; Farris, C.M.; Ma, Y.; Concha-Marambio, L.; Lebovitz, R.; Nellgård, B.; Dalla, K.; Constantinescu, J.; Constantinescu, R.; Gobom, J.; et al. α-Synuclein Seed Amplification Assay as a Diagnostic Tool for Parkinsonian Disorders. Park. Relat. Disord. 2023, 117, 105807. [Google Scholar] [CrossRef]
- Garrido, A.; Fairfoul, G.; Tolosa, E.; Marti, M.J.; Ezquerra, M.; Green, A.J.E. Brain and Cerebrospinal Fluid α-Synuclein Real-Time Quaking-Induced Conversion Identifies Lewy Body Pathology in LRRK2-PD. Mov. Disord. 2023, 38, 333–338. [Google Scholar] [CrossRef]
- Okuzumi, A.; Hatano, T.; Matsumoto, G.; Nojiri, S.; Ueno, S.-I.; Imamichi-Tatano, Y.; Kimura, H.; Kakuta, S.; Kondo, A.; Fukuhara, T.; et al. Propagative α-Synuclein Seeds as Serum Biomarkers for Synucleinopathies. Nat. Med. 2023, 29, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Verdurand, M.; Kaczorowski, F.; Dautricourt, S.; Desestret, V.; Formaglio, M.; Mollion, H.; Petitnicolas, G.; Afifi, A.; Fourier, A.; Garnier-Crussard, A.; et al. Toward Alpha-Synuclein Seed Amplification Assay in Clinical Practice. Alzheimer’s Dement. 2025, 17, e70066. [Google Scholar] [CrossRef]
- Wang, Z.; Gilliland, T.; Kim, H.J.; Gerasimenko, M.; Sajewski, K.; Camacho, M.V.; Bebek, G.; Chen, S.G.; Gunzler, S.A.; Kong, Q. A Minimally Invasive Biomarker for Sensitive and Accurate Diagnosis of Parkinson’s Disease. Acta Neuropathol. Commun. 2024, 12, 167. [Google Scholar] [CrossRef]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of Neuron-Derived Pathological α-Synuclein in Blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef]
- Kluge, A.; Borsche, M.; Streubel-Gallasch, L.; Gül, T.; Schaake, S.; Balck, A.; Prasuhn, J.; Campbell, P.; Morris, H.R.; Schapira, A.H.; et al. α-Synuclein Pathology in PRKN-Linked Parkinson’s Disease: New Insights from a Blood-Based Seed Amplification Assay. Ann. Neurol. 2024, 95, 1173–1177. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Schulte, C.; Deuschle, C.; Bunk, J.; Welzel, J.; Maetzler, W.; Berg, D. Association of Misfolded α-Synuclein Derived from Neuronal Exosomes in Blood with Parkinson’s Disease Diagnosis and Duration. J. Park. Dis. 2024, 14, 667–679. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Serrano, G.E.; Anantharam, V.; Kanthasamy, A.; Adler, C.H.; Beach, T.G.; Kanthasamy, A.G. Blinded RT-QuIC Analysis of α-Synuclein Biomarker in Skin Tissue from Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Becker, K.; Donadio, V.; Siedlak, S.; Yuan, J.; Rezaee, M.; Incensi, A.; Kuzkina, A.; Orrú, C.D.; Tatsuoka, C.; et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2020, 78, 30–40. [Google Scholar] [CrossRef]
- Kuzkina, A.; Bargar, C.; Schmitt, D.; Rößle, J.; Wang, W.; Schubert, A.-L.; Tatsuoka, C.; Gunzler, S.A.; Zou, W.-Q.; Volkmann, J.; et al. Diagnostic Value of Skin RT-QuIC in Parkinson’s Disease: A Two-Laboratory Study. NPJ Park. Dis. 2021, 7, 99. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Visanji, N.P.; Olszewska, D.A.; Sousa, M.; Bhakta, P.; Vasilevskaya, A.; Anastassiadis, C.; Tartaglia, M.C.; Kovacs, G.G.; Lang, A.E. Combining Skin α-Synuclein Real-Time Quaking-Induced Conversion and Circulating Neurofilament Light Chain to Distinguish Multiple System Atrophy and Parkinson’s Disease. Mov. Disord. 2022, 37, 648–650. [Google Scholar] [CrossRef]
- Kuzkina, A.; Panzer, C.; Seger, A.; Schmitt, D.; Rößle, J.; Schreglmann, S.R.; Knacke, H.; Salabasidou, E.; Kohl, A.; Sittig, E.; et al. Dermal Real-Time Quaking-Induced Conversion Is a Sensitive Marker to Confirm Isolated Rapid Eye Movement Sleep Behavior Disorder as an Early α-Synucleinopathy. Mov. Disord. 2023, 38, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Mao, H.; Gan, T.; Guo, W.; Dai, W.; Huang, W.; Wu, Z.; Li, H.; Huang, X.; Yang, X.; et al. A Skin-Specific α-Synuclein Seeding Amplification Assay for Diagnosing Parkinson’s Disease. NPJ Park. Dis. 2024, 10, 129. [Google Scholar] [CrossRef]
- Luan, M.; Sun, Y.; Chen, J.; Jiang, Y.; Li, F.; Wei, L.; Sun, W.; Ma, J.; Song, L.; Liu, J.; et al. Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. 2022, 37, 1059–1063. [Google Scholar] [CrossRef]
- Vivacqua, G.; Mason, M.; De Bartolo, M.I.; Węgrzynowicz, M.; Calò, L.; Belvisi, D.; Suppa, A.; Fabbrini, G.; Berardelli, A.; Spillantini, M. Salivary α-Synuclein RT-QuIC Correlates with Disease Severity in de Novo Parkinson’s Disease. Mov. Disord. 2023, 38, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-Synuclein Seeds in Olfactory Mucosa of Patients with Isolated REM Sleep Behaviour Disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef]
- Kuzkina, A.; Rößle, J.; Seger, A.; Panzer, C.; Kohl, A.; Maltese, V.; Musacchio, T.; Blaschke, S.J.; Tamgüney, G.; Kaulitz, S.; et al. Combining Skin and Olfactory α-Synuclein Seed Amplification Assays (SAA)-towards Biomarker-Driven Phenotyping in Synucleinopathies. NPJ Park. Dis. 2023, 9, 79. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Cai, H.; Kou, W.; Yang, C.; Li, S.; Zhang, N.; Feng, T. Detection of α-Synuclein in Oral Mucosa by Seed Amplification Assay in Synucleinopathies and Isolated REM Sleep Behavior Disorder. Mov. Disord. 2024, 39, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Fenyi, A.; Leclair-Visonneau, L.; Clairembault, T.; Coron, E.; Neunlist, M.; Melki, R.; Derkinderen, P.; Bousset, L. Detection of Alpha-Synuclein Aggregates in Gastrointestinal Biopsies by Protein Misfolding Cyclic Amplification. Neurobiol. Dis. 2019, 129, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Fenyi, A.; Duyckaerts, C.; Bousset, L.; Braak, H.; Del Tredici, K.; Melki, R.; Network, O.B.O.T.B.N.-C.N. Seeding Propensity and Characteristics of Pathogenic αSyn Assemblies in Formalin-Fixed Human Tissue from the Enteric Nervous System, Olfactory Bulb, and Brainstem in Cases Staged for Parkinson’s Disease. Cells 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Manne, S.; Kondru, N.; Jin, H.; Anantharam, V.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. α-Synuclein Real-Time Quaking-Induced Conversion in the Submandibular Glands of Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 268–278. [Google Scholar] [CrossRef]
- Yadav, S.; Deepika; Moar, K.; Kumar, A.; Khola, N.; Pant, A.; Kakde, G.S.; Maurya, P.K. Reconsidering Red Blood Cells as the Diagnostic Potential for Neurodegenerative Disorders. Biol. Cell 2024, 116, e2400019. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and Tau Concentrations in Cerebrospinal Fluid of Patients Presenting with Parkinsonism: A Cohort Study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma Exosomal α-Synuclein Is Likely CNS-Derived and Increased in Parkinson’s Disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Yang, C.; Yu, Z.; Jiang, Y.; Feng, T. Comparison of Biospecimens for α-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Eur. J. Neurol. 2023, 30, 3949–3967. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular Vesicles, from the Pathogenesis to the Therapy of Neurodegenerative Diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef]
- Shami-Shah, A.; Travis, B.G.; Walt, D.R. Advances in Extracellular Vesicle Isolation Methods: A Path towards Cell-Type Specific EV Isolation. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 447–460. [Google Scholar] [CrossRef]
- Manolopoulos, A.; Yao, P.J.; Kapogiannis, D. Extracellular Vesicles: Translational Research and Applications in Neurology. Nat. Rev. Neurol. 2025, 21, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Leshchyns’ka, I.; Sytnyk, V. Intracellular Transport and Cell Surface Delivery of the Neural Cell Adhesion Molecule (NCAM). Bioarchitecture 2015, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, D.; Wang, Y.; Xie, J.; Zhang, Z. Skin Alpha-Synuclein Deposit Patterns: A Predictor of Parkinson’s Disease Subtypes. EBioMedicine 2022, 80, 104076. [Google Scholar] [CrossRef]
- Kuzkina, A.; Schulmeyer, L.; Monoranu, C.-M.; Volkmann, J.; Sommer, C.; Doppler, K. The Aggregation State of α-Synuclein Deposits in Dermal Nerve Fibers of Patients with Parkinson’s Disease Resembles That in the Brain. Park. Relat. Disord. 2019, 64, 66–72. [Google Scholar] [CrossRef]
- Doppler, K.; Ebert, S.; Uçeyler, N.; Trenkwalder, C.; Ebentheuer, J.; Volkmann, J.; Sommer, C. Cutaneous Neuropathy in Parkinson’s Disease: A Window into Brain Pathology. Acta Neuropathol. 2014, 128, 99–109. [Google Scholar] [CrossRef]
- Brumberg, J.; Kuzkina, A.; Lapa, C.; Mammadova, S.; Buck, A.; Volkmann, J.; Sommer, C.; Isaias, I.U.; Doppler, K. Dermal and Cardiac Autonomic Fiber Involvement in Parkinson’s Disease and Multiple System Atrophy. Neurobiol. Dis. 2021, 153, 105332. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Tabikh, M.; Chahla, C.; Okdeh, N.; Kovacic, H.; Sabatier, J.-M.; Fajloun, Z. Parkinson Disease: Protective Role and Function of Neuropeptides. Peptides 2022, 151, 170713. [Google Scholar] [CrossRef]
- Jiao, L.-L.; Dong, H.-L.; Liu, M.-M.; Wu, P.-L.; Cao, Y.; Zhang, Y.; Gao, F.-G.; Zhu, H.-Y. The Potential Roles of Salivary Biomarkers in Neurodegenerative Diseases. Neurobiol. Dis. 2024, 193, 106442. [Google Scholar] [CrossRef]
- Bougea, A.; Koros, C.; Stefanis, L. Salivary Alpha-Synuclein as a Biomarker for Parkinson’s Disease: A Systematic Review. J. Neural. Transm. 2019, 126, 1373–1382. [Google Scholar] [CrossRef]
- Al-Nimer, M.S.M.; Mshatat, S.F.; Abdulla, H.I. Saliva α-Synuclein and A High Extinction Coefficient Protein: A Novel Approach in Assessment Biomarkers of Parkinson’s Disease. N. Am. J. Med. Sci. 2014, 6, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory Dysfunction in Parkinson Disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.I.; Bohl, J.; Del Tredici, K. Gastric Alpha-Synuclein Immunoreactive Inclusions in Meissner’s and Auerbach’s Plexuses in Cases Staged for Parkinson’s Disease-Related Brain Pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is Alpha-Synuclein in the Colon a Biomarker for Premotor Parkinson’s Disease? Evidence from 3 Cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, J.; Lee, H.J.; Ryu, H.-S.; Kim, K.; Lee, J.H.; Jung, K.W.; Kim, M.J.; Kim, M.-J.; Kim, Y.J.; et al. Alpha-Synuclein in Gastric and Colonic Mucosa in Parkinson’s Disease: Limited Role as a Biomarker. Mov. Disord. 2016, 31, 241–249. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, X.; Chen, L.; Hu, B.; Wang, H.; Wang, Y.; Huang, W. Pathological α-Synuclein Detected by Real-Time Quaking-Induced Conversion in Synucleinopathies. Exp. Gerontol. 2024, 187, 112366. [Google Scholar] [CrossRef]
- Concha-Marambio, L.; Pritzkow, S.; Shahnawaz, M.; Farris, C.M.; Soto, C. Seed Amplification Assay for the Detection of Pathologic Alpha-Synuclein Aggregates in Cerebrospinal Fluid. Nat. Protoc. 2023, 18, 1179–1196. [Google Scholar] [CrossRef]
- Bougea, A. Seeding Aggregation Assays in Lewy Bodies Disorders: A Narrative State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 10783. [Google Scholar] [CrossRef]
- Espay, A.J.; Lees, A.J.; Cardoso, F.; Frucht, S.J.; Erskine, D.; Sandoval, I.M.; Bernal-Conde, L.D.; Sturchio, A.; Imarisio, A.; Hoffmann, C.; et al. The α-Synuclein Seed Amplification Assay: Interpreting a Test of Parkinson’s Pathology. Park. Relat. Disord. 2025, 131, 107256. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Fraser, K.; Yang, M.; Fox, T.; Hirschhorn, E.; Njingti, E.; Scott, D.; Bedell, B.J.; Kistner, K.M.; Cedarbaum, J.M.; et al. Cinpanemab in Early Parkinson Disease: Evaluation of Biomarker Results from the Phase 2 SPARK Clinical Trial. Neurology 2024, 102, e209137. [Google Scholar] [CrossRef] [PubMed]






| Sample | Sensitivity | Specificity | N | Sample from PD | Processing of Sample | Assay | Cut-Off Value | Reference |
|---|---|---|---|---|---|---|---|---|
| Cerebrospinal fluid | 95.24% | 100.00% | 21 PD, 2 PSP, 35 HC | Biopsy | NA | RT-QuIC | Mean of negative controls +2SD | Fairfoul et al. (2016) [27] |
| 88.16% | 96.92% | 76 PD, 10 MSA, 65 NNC | Biopsy | NA | PMCA | ≥50 FU | Shahnawaz et al. (2017) [28] | |
| 91.67% | 100.00% | 12 PD, 2 PSP, 1 CBD, 12 HC | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Groveman et al. (2018) [29] | |
| 10.00% | 100.00% | 10 PD, 10 LBD, 10 HC | Autopsy | Frozen | RT-QuIC | 50% of maximum value | Candelise et al. (2019) [30] | |
| 90.00%; 40.00% | 80.00%; 80.00% | 10 PD LRRK2 negative, 10 HC; 15 PD LRRK2 positive, 10 HC | Biopsy | NA; NA | RT-QuIC | Mean of negative controls +2SD | Garrido et al. (2019) [31] | |
| 95.24%; 97.14% | 82.28%; 92.41% | 105 PD, 79 HC | Biopsy | NA | PMCA | ≥1000 FU | Kang et al. (2019) [32] | |
| 100.00% | 100.00% | 15 PD, 5 PSP, 16 HC | Biopsy | NA | RT-QuIC | Mean of all samples +10SD | Manne et al. (2019) [33] | |
| 85.25% | 91.37% | 278 PD, 278 NNC | Biopsy | NA | PMCA | ≥150 FU | Ning et al. (2019) [34] | |
| 84.31% | 98.04% | 51 PD, 17 MSA, 8 PSP, 51 HC | Biopsy | NA | RT-QuIC | Mean of negative controls +2SD | van Rumund et al. (2019) [35] | |
| 94.37% | 98.39% | 71 PD, 62 NNC, 33 MSA | Biopsy | NA | RT-QuIC | Mean of neuropathological controls +30SD | Rossi et al. (2020) [36] | |
| 93.62% | 100.00% | 94 PD, 56 NNC, 75 MSA | Biopsy | NA | PMCA | ≥50 FU | Shahnawaz et al. (2020) [37] | |
| 100.00% | 100.00% | 16 PD, 62 MSA, 29 HC | Biopsy | NA | PMCA | ≥150 AU | Singer et al. (2020) [38] | |
| 97.73% | 100.00% | 88 PD, 38 NNC, 9 PSP | Autopsy | NA | RT-QuIC | Mean background fluorescence +5SD | Bargar et al. (2021) [39] | |
| 85.05% | 92.31% | 107 PD, 26 HC | Biopsy | NA | RT-QuIC | Mean of negative controls +30SD | Brockmann et al. (2021) [40] | |
| 100.00% | 100.00% | 2 PD, 2 MSA, 1 PSP, 13 HC | Biopsy | NA | RT-QuIC | Mean of negative controls +3SD | Donadio et al. (2021) [41] | |
| 100.00% | 100.00% | 7 PD, 27 NNC | Biopsy | NA | RT-QuIC | 15% of maximum value | Mammana et al. (2021) [42] | |
| 97.22% | 87.06% | 108 PD, 85 HC | Biopsy | NA | RT-QuIC | 10% of maximum value | Orrù et al. (2021) [43] | |
| 91.50% | 97.14% | 153 PD/PDD, 68 MSA, 35 HC | Biopsy | NA | RT-QuIC | NA | Quadalti et al. (2021) [44] | |
| 86.67%; 96.67% | 96.43%; 100.00% | 30 PD, 1 MSA, 28 HC | Biopsy | NA | RT-QuIC | 10% of maximum value | Russo et al. (2021) [45] | |
| 91.30%; 100.00% | 87.50%; 100.00% | 23 PD, 8 NNC; 1 PD, 11 NNC | Biopsy | NA | RT-QuIC | 20% of maximum value | Bongianni et al. (2022) [46] | |
| 75.00%; 80.00% | 100.00%; 100.00% | 20 PD, 19 HC, 37 MSA, 23 PSP, 13 CBD | Biopsy | NA | RT-QuIC | Mean of negative controls +2SD | Compta et al. (2022) [47] | |
| 95.00% | 84.00% | 20 PD, 25 HC, 1 MSA, 4 PSP, 1 CBD | Autopsy | NA | RT-QuIC | 10% of maximum value | Hall et al. (2022) [48] | |
| 91.94% | 85.29% | 62 PD, 34 HC | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Majbour et al. (2022) [49] | |
| 84.62% | 63.16% | 13 NPH + PD/PDD, 19 NPH | Biopsy | NA | RT-QuIC | Mean of negative controls +2SD | Sakurai et al. (2022) [22] | |
| 89.19% | 96.36% | 74 PD, 55 HC | Biopsy | NA | RT-QuIC | Mean of initial fluorescence at 120 h + 5SD | Poggiolini et al. (2022) [50] | |
| 92.59% | 79.17% | 54 PD, 21 HC | Biopsy | NA | PMCA | ≥1000 FU | Chahine et al. (2023) [51] | |
| 94.37% | 98.00% | 71 PD, 2 MSA, 50 HC | Biopsy | NA | PMCA | ≥50 AU | Concha-Marambio et al. (2023) [52] | |
| 100.00% | 70.83% | 55 PD, 27 MSA, 7 CBD, 16 PSP, 24 HC | Biopsy | NA | PMCA | ≥100 FU | Fernandes Gomes et al. (2023) [53] | |
| 87.50% | 100.00% | 8 PD, 3 HC | Autopsy | Frozen | RT-QuIC | Mean of negative controls +2SD | Garrido et al. (2023) [54] | |
| 100.00% | 100.00% | 6 PD, 3 MSA, 35 NNC | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Okuzumi et al. (2023) [55] | |
| 87.71% | 96.32% | 545 PD, 163 HC | Biopsy | NA | PMCA | ≥50 AU | Siderowf et al. (2023) [26] | |
| 100.00% | 100.00% | 41 LBD, 6 PD, 37 NNC, 42 AD | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Verdurand et al. (2025) [56] | |
| Blood | 94.57% | 92.19% | 221 PD, 39 MSA, 10 LBD, 9 RBD, 30 PSP. 25 AD, 128 HC | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Okuzumi et al. (2023) [55] |
| 80.49% | 90.48% | 82 PD, 42 HC | Biopsy | NA | RT-QuIC | Mean of all samples +4SD | Wang et al. (2024) [57] | |
| Neuronal exosomes/extracellular vesicles | 100.00% | 100.00% | 30 PD, 50 HC | Biopsy | NA | Specific technique | >100 ng α-synuclein monomers | Kluge et al. (2023) [58] |
| 61.54%; 88.89% | 100.00%; 100.00% | 13 PD Parkin positive, 10 HC; 9 idiopathic PD, 10 HC | Biopsy | NA; NA | Specific technique | >500 ng α-synuclein monomers | Kluge et al. (2024) [59] | |
| 98.75% | 100.00% | 80 PD, 20 HC | Biopsy | NA | RT-QuIC | Mean of all samples +5SD | Schaeffer et al. (2024) [60] | |
| Skin | 96.00%; 75.00% | 96.00%; 83.33% | 25 PD, 25 HC; 12 PD, 12 HC | Autopsy from scalp | Frozen tissues; FFPE tissues | RT-QuIC | Mean of all samples +10SD | Manne et al. (2020) [61] |
| 93.62%; 95.00% | 97.67%; 95.24% | 47 PD, 43 HC; 20 PD, 21 HC | Autopsy from abdomen and scalp; biopsy from C7 paravertebral and legs | Frozen tissues; fresh tissues | RT-QuIC; PMCA | Mean of all samples +3SD; NA | Wang et al. (2020) [62] | |
| 83.33% | 77.78% | 6 PD, 18 HC | Biopsy from C7 paravertebral, thigh, and leg | Frozen tissues | RT-QuIC | Mean of negative controls +3SD | Donadio et al. (2021) [41] | |
| 91.18% | 90.00% | 34 PD; 30 HC | Biopsy from neck, lower back, thigh, and lower leg | Frozen tissues | RT-QuIC | Mean of negative controls +5SD | Kuzkina et al. (2021) [63] | |
| 76.92% | 95.12% | 13 PD, 41 NNC | Biopsy from neck, leg, and thigh | NA | RT-QuIC | 15% of maximum value | Mammana et al. (2021) [42] | |
| 84.62% | 85.00% | 13 PD, 10 MSA, 7 PSP, 20 HC | Biopsy | NA | RT-QuIC | FU > 20,000 | Martinez-Valbuena et al. (2022) [64] | |
| 88.24% | 86.67% | 34 PD, 30 NNC | Biopsy from C7, Th10, and proximal leg. | Frozen tissues | RT-QuIC | Mean of negative controls +5SD | Kuzkina et al. (2023) [65] | |
| 92.47% | 93.33% | 332 PD, 285 HC | Biopsy from cervical region | Frozen tissues | Specific technique | Mean of all samples +10SD | Kuang et al. (2024) [66] | |
| Saliva | 76.00% | 94.44% | 75 PD, 36 HC | Biopsy | NA | RT-QuIC | Mean of all samples +2SD | Luan et al. (2022) [67] |
| 83.78% | 82.61% | 37 PD, 23 HC | Biopsy | NA | RT-QuIC | FU > 2990.5 | Vivacqua et al. (2023) [68] | |
| 75.00% | 96.15% | 48 PD, 26 HC | Biopsy | NA | RT-QuIC | Mean of all samples +4SD | Wang et al. (2024) [57] | |
| Olfactory mucosa | 55.55% | NA | 18 PD, 11 MSA, 6 CBD, 12 PSP | Biopsy | NA | RT-QuIC | AU > 500 | De Luca et al. (2019) [20] |
| 69.23%; 69.23% | 90.91% 100.00% | 13 PD, 10 MSA-C; 20 MSA-P; 11 HC | Biopsy | NA | RT-QuIC | AU > 30,000 | Bargar et al. (2021) [39] | |
| 46.34% | 89.83% | 41 PD, 59 HC | Biopsy | NA | RT-QuIC | Mean of all samples +3SD | Stefani et al. (2021) [69] | |
| 44.19%; 83.72% | 65.52%; 65.52% | 43 PD, 6 PSP, NNC 29 | Biopsy | NA | RT-QuIC | 20% of maximum value | Bongianni et al. (2022) [46] | |
| 48.15% | 90.00% | 27 PD, 3 MSA, 3 PSP, 30 NNC | Biopsy | NA | RT-QuIC | 10% of maximum value | Kuzkina et al. (2023) [70] | |
| Oral mucosa | 67.29% | 90.29% | 107 PD, 99 MSA, 33 RBD, 103 HC | Biopsy of oral mucosa | Frozen tissues | Specific technique | AU > 49,219 | Zheng et al. (2024) [71] |
| Gastrointestinal tract (rectum/sigmoid/antrum) | 55.56% | 90.91% | 18 PD, 11 HC | Biopsy from rectum, sigmoid, and antrum | Frozen tissues | PMCA | 30% of maximum value | Fenyi et al. (2019) [72] |
| 83.33% | 77.78% | 12 PD, 8 LBD, 9 HC | Autopsy gastric cardia | Formaldehyde-fixedtissues | PMCA | 30% of maximum value | Fenyi et al. (2021) [73] | |
| 100.00%; 10.00% | NA; 85.00% | 2 PD, 1, LBD, 1 MSA; 20 PD, 20 HC | Autopsy from stomach; biopsy from stomach, esophagus, colon, and rectum | FFPE tissues; FFPE tissues | RT-QuIC | Mean of all samples +10SD | Shin et al. (2022) [21] | |
| Submandibular gland | 100.00% | 93.75% | 13 PD, 16 HC | Autopsy | FFPE | RT-QuIC | Mean of all samples +10SD | Manne et al. (2020) [74] |
| 73.17% | 78.57% | 41 PD, 14 HC | Biopsy | FFPE tissues | RT-QuIC | Mean of all samples +10SD | Chahine et al. (2023) [51] |
| Biomatrices | n | Sensitivity (95% CI) | Specificity (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Pooled a | Single-Population b | Z-Test | Pooled a | Single-Population b | Z-Test | ||
| CSF | 2382 | 0.88 (95% CI, 0.83–0.94) | 0.89 (95% CI, 0.88–0.91) | −1.08 | 0.93 (95% CI, 0.90–0.96) | 0.93 (95% CI, 0.92–0.94) | 0 |
| Blood | 303 | 0.88 (95% CI, 0.70–1.06) | 0.90 (95% CI, 0.86–0.93) | −0.78 | 0.91 (95% CI, 0.89–0.93) | 0.91 (95% CI, 0.86–0.95) | 0 |
| Extracellular vesicles | 132 | 0.88 (95% CI, 0.69–1.08) | 0.94 (95% CI, 0.89–0.97) | −1.70 | 1.00 (95% CI, 1.00–1.00) | 1.00 (95% CI, 0.95–1.00) | 0 |
| Skin | 536 | 0.88 (95% CI, 0.83–0.92) | 0.91 (95% CI, 0.88–0.93) | −1.60 | 0.90 (95% CI, 0.86–0.94) | 0.92 (95% CI, 0.89–0.94) | −1.14 |
| Saliva | 160 | 0.78 (95% CI, 0.71–0.84) | 0.77 (95% CI, 0.70–0.83) | 0.21 | 0.91 (95% CI, 0.81–1.01) | 0.91 (95% CI, 0.83–0.96) | 0 |
| Olfactory | 198 | 0.59 (95% CI, 0.47–0.71) | 0.59 (95% CI, 0.52–0.65) | 0 | 0.83 (95% CI, 0.71–0.95) | 0.82 (95% CI, 0.75–0.87) | 0.26 |
| Oral | 107 | 0.67 (95% CI, 0.57–0.76) | 0.67 (95% CI, 0.57–0.76) | 0 | 0.90 (95% CI, 0.82–0.95) | 0.90 (95% CI, 0.82–0.95) | 0 |
| Gastrointestinal tract | 52 | 0.59 (95% CI, 0.15–1.02) | 0.46 (95% CI, 0.32–0.60) | 1.32 | 0.84 (95% CI, 0.76–0.93) | 0.85 (95% CI, 0.70–0.94) | −0.14 |
| Submandibular gland | 54 | 0.84 (95% CI, 0.49–1.191) | 0.79 (95% CI, 0.66–0.89) | 0.66 | 0.86 (95% CI, 0.67–1.05) | 0.86 (95% CI, 0.69–0.96) | 0 |
| All c except CSF | 1542 | 0.77 (95% CI, 0.70–0.84) | 0.82 (95% CI, 0.80–0.84) | −3.43 * | 0.89 (95% CI, 0.86–0.92) | 0.90 (95% CI, 0.89–0.92) | −0.90 |
| All c including CSF | 3924 | 0.78 (95% CI, 0.66–0.89) | 0.86 (95% CI, 0.85–0.87) | −9.22 * | 0.90 (95% CI, 0.87–0.94) | 0.92 (95% CI, 0.91–0.93) | −3.09 * |
| Rank | Biomatrix | Relative Sensitivity (95% CI) | Relative Specificity (95% CI) | DOR (SE) |
|---|---|---|---|---|
| 1 | Extracellular vesicles | 0.94 (0.90–0.98) | 1.00 (1.00–1.00) | ∞ (Perfect specificity) |
| 2 | Cerebrospinal fluid | 0.89 (0.88–0.91) | 0.93 (0.92–0.94) | 131.25 (16.14) |
| 3 | Skin | 0.91 (0.89–0.93) | 0.92 (0.90–0.94) | 129.16 (29.12) |
| 4 | Blood | 0.90 (0.87–0.94) | 0.91 (0.87–0.95) | 109.44 (37.46) |
| 5 | Saliva | 0.77 (0.71–0.83) | 0.91 (0.85–0.97) | 38.38 (16.80) |
| 6 | Submandibular gland | 0.79 (0.68–0.90) | 0.86 (0.74–0.98) | 25.41 (16.12) |
| 7 | Oral mucosa | 0.67 (0.58–0.76) | 0.90 (0.84–0.96) | 19.13 (7.49) |
| 8 | Olfactory mucosa | 0.58 (0.51–0.65) | 0.90 (0.84–0.96) | 6.49 (1.63) |
| 9 | Gastrointestinal tract | 0.44 (0.30–0.57) | 0.85 (0.73–0.96) | 4.45 (2.34) |
| Feature | RT-QuIC (Real-Time Quaking-Induced Conversion) | PMCA (Protein Misfolding Cyclic Amplification) |
|---|---|---|
| Principle | Misfolded proteins seed the conversion of recombinant proteins, detected via fluorescence | Cyclic amplification of misfolded proteins using brain homogenates and sonication |
| Amplification Mechanism | Shaking-induced conversion | Sonication-induced conversion |
| Readout | Fluorescence detection in real time | End-point detection (Western blot, Thioflavin T) |
| Assay Duration | 12–48 h | Several days |
| Sensitivity | High | Very high (more sensitive than RT-QuIC) |
| Specificity | High | High, but depends on conditions |
| Substrate Used | Recombinant proteins | Brain homogenates (more complex) |
| Risk of Contamination | Lower | Higher due to exponential amplification |
| Equipment Required | Fluorescence plate reader | Sonicator and specialized incubation equipment |
| Quantification Capability | Yes, real-time fluorescence monitoring | No, usually an endpoint assay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Fornari Caprara, A.L. Alpha-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Clin. Pract. 2025, 15, 107. https://doi.org/10.3390/clinpract15060107
Rissardo JP, Fornari Caprara AL. Alpha-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Clinics and Practice. 2025; 15(6):107. https://doi.org/10.3390/clinpract15060107
Chicago/Turabian StyleRissardo, Jamir Pitton, and Ana Leticia Fornari Caprara. 2025. "Alpha-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis" Clinics and Practice 15, no. 6: 107. https://doi.org/10.3390/clinpract15060107
APA StyleRissardo, J. P., & Fornari Caprara, A. L. (2025). Alpha-Synuclein Seed Amplification Assays in Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Clinics and Practice, 15(6), 107. https://doi.org/10.3390/clinpract15060107







