Predictors of Major Adverse Cardiovascular Events in Stable Patients After ST Elevation Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
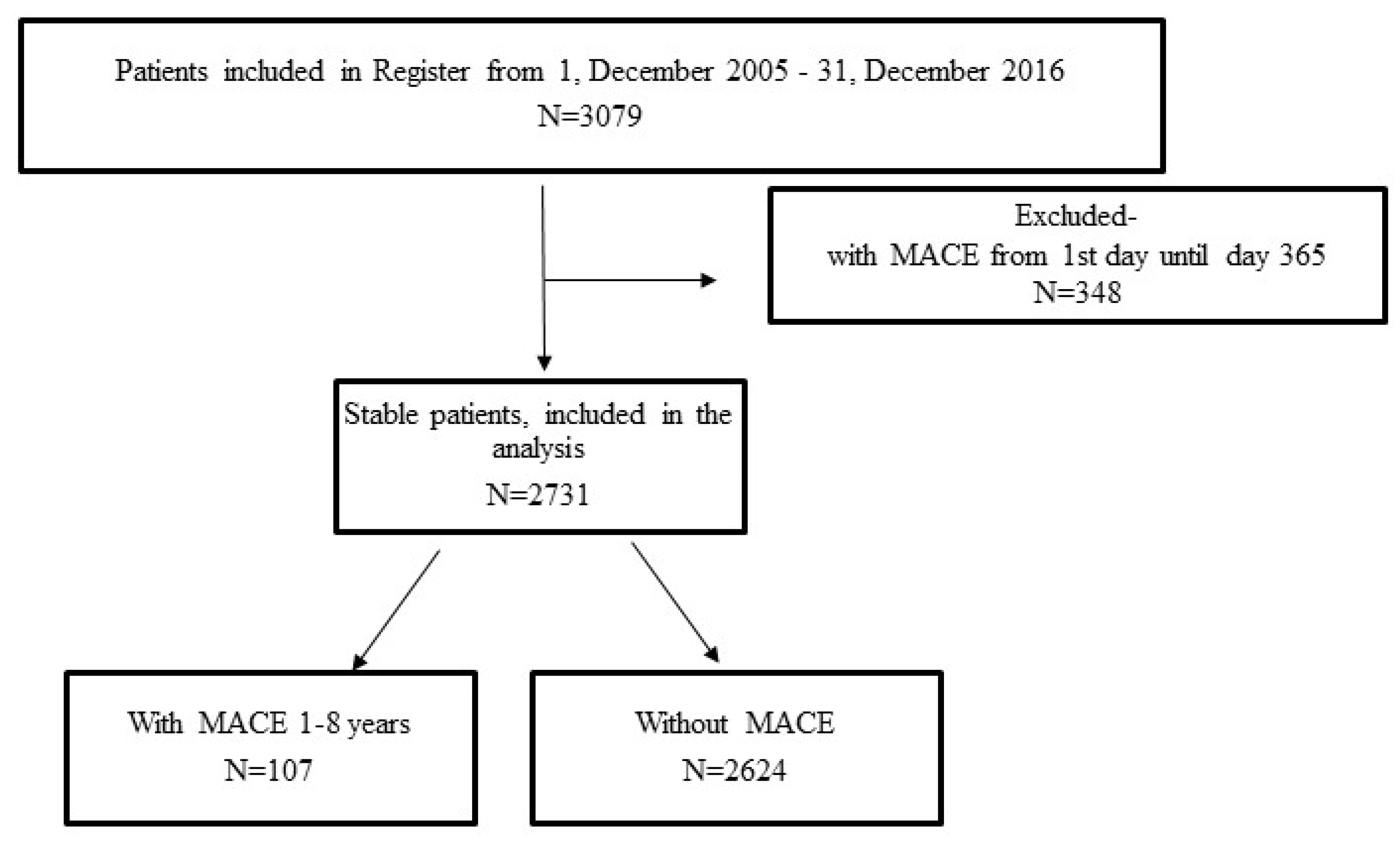
2.1. Study Population, Inclusion and Exclusion Criteria, Data Collection, and Definitions
2.2. Ethics
2.3. Statistical Analysis
3. Results
3.1. MACE Incidence in the First 12 Months
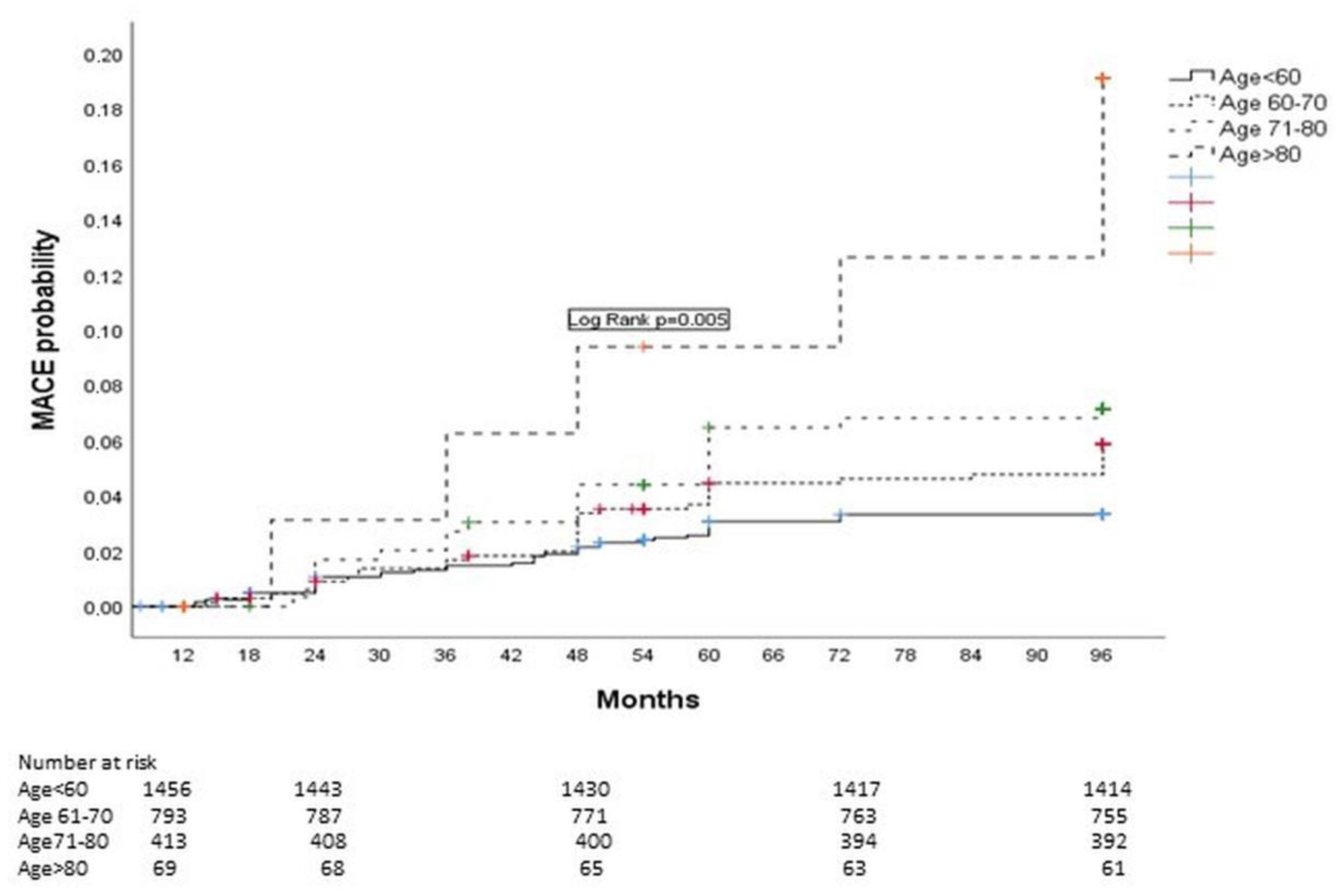
3.2. Patient Characteristics and MACE Incidence in Stable Post-STEMI Patients
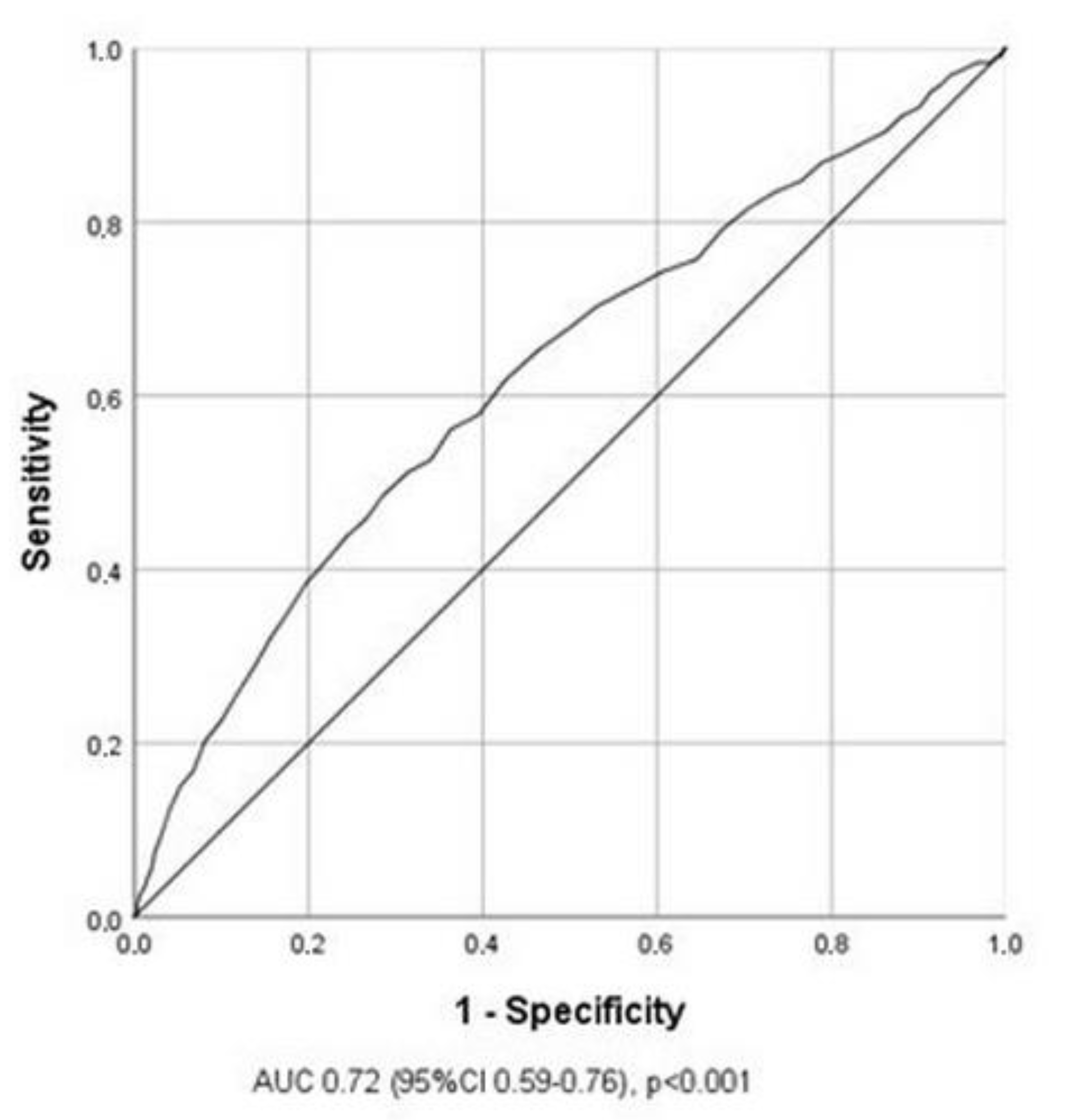
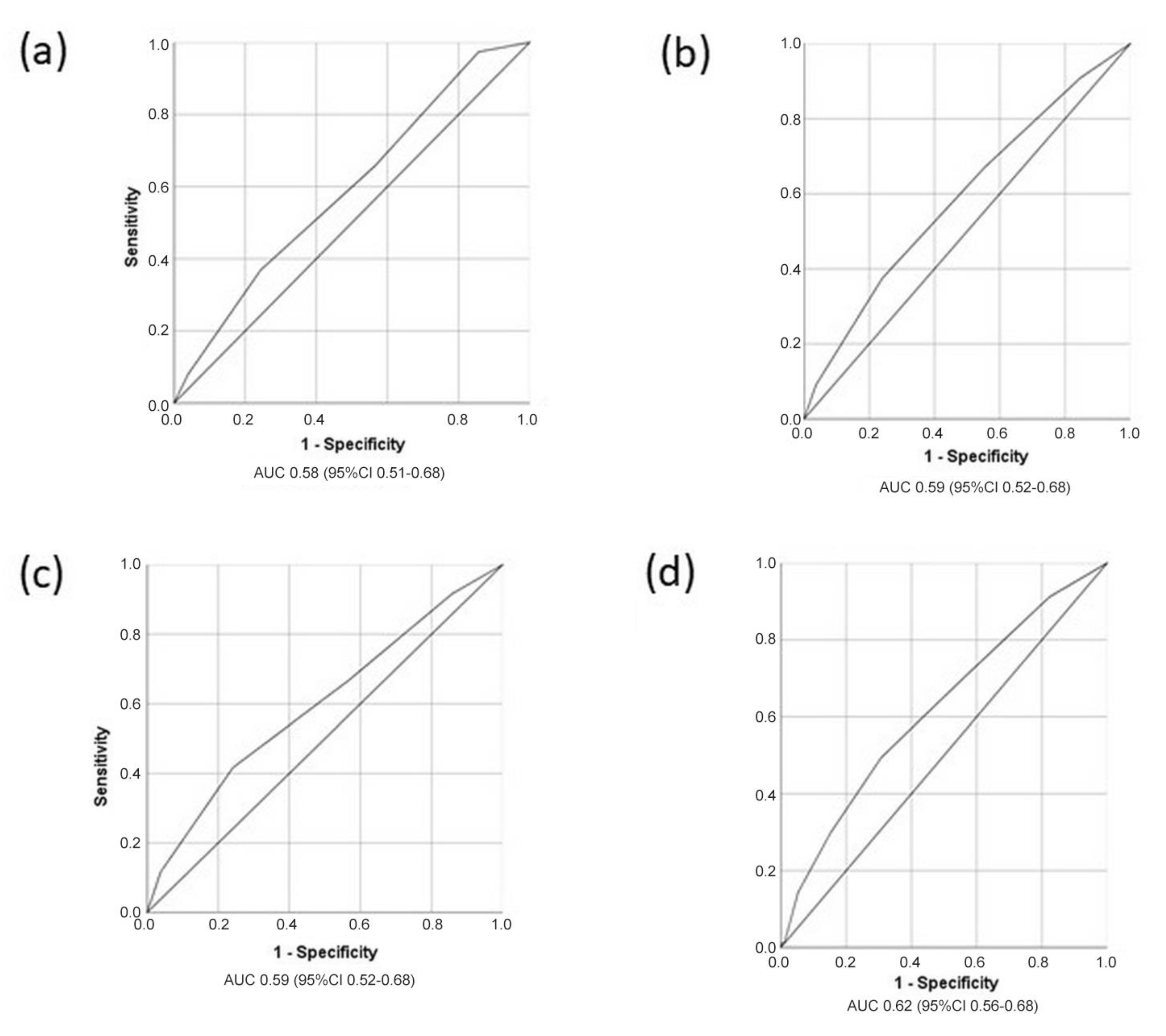
3.3. Predictors for the Occurrence of MACE in Stable Post-STEMI Patients
4. Discussion
4.1. Patient Characteristics, the Incidence of MACE, and MACE Predictors in Stable Post-STEMI Patients
4.2. Clinical and Future Implications
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brogan, R.A.; Alabas, O.; Almudarra, S.; Hall, M.; Dondo, T.B.; Mamas, M.A.; Baxter, P.D.; Batin, P.D.; Curzen, N.; de Belder, M.; et al. Relative survival and excess mortality following primary percutaneous coronary intervention for ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 68–77. [Google Scholar] [CrossRef]
- Rossello, X.; Bueno, H.; Pocock, S.J.; Van de Werf, F.; Danchin, N.; Annemans, L.; Medina, J.; Zeymer, U. Predictors of all-cause mortality and ischemic events within and beyond 1 year after an acute coronary syndrome: Results from the EPICOR registry. Clin. Cardiol. 2019, 42, 111–119. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Keeble, C.; Burton-Wood, N.; Somers, K.; Anderson, M.; Harland, C.; Baxter, P.D.; McLenachan, J.M.; Blaxill, J.M.; Blackman, D.J.; et al. Clinical outcomes following primary percutaneous coronary intervention for ST-elevation myocardial infarction according to sex and race. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Li, C.-Y.; Hsieh, M.-J.; Chen, C.-C.; Hsieh, I.-C.; Chen, T.-H.; Chen, S.-W.; Wang, C.-Y.; Chang, S.-H.; Lee, C.-H.; et al. Predictors of subsequent myocardial infarction, stroke, and death in stable post-myocardial infarction patients: A nationwide cohort study. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 634–642. [Google Scholar] [CrossRef]
- Pascual, I.; Avanzas, P.; Almendárez, M.; Lorca, R.; Vigil-Escalera, M.; Arboine, L.; Alperi, A.; Adeba, A.; Díaz, R.; Silva, J.; et al. STEMI, primary percutaneous coronary intervention and recovering of life expectancy: Insights from the SurviSTEMI study. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef]
- Özcan, C.; Deleskog, A.; Schjerning Olsen, A.M.; Nordahl Christensen, H.; Lock Hansen, M.; Hilmar Gislason, G. Coronary artery disease severity and long-term cardiovascular risk in patients with myocardial infarction: A Danish nationwide register-based cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; D’errigo, P.; Rosato, S.; Mureddu, G.F.; Badoni, G.; Seccareccia, F.; Baglio, G. Impact of myocardial revascularization on long-term outcomes in a nationwide cohort of first acute myocardial infarction survivors. Eur. Heart J. Suppl. 2022, 24 (Suppl. C), C225–C232. [Google Scholar] [CrossRef]
- Siniawski, D.; Masson, G.; Masson, W.; Barbagelata, L.; Destaville, J.; Lynch, S.; Vitagliano, L.; Parodi, J.B.; Berton, F.; Indavere, A.; et al. Residual cardiovascular risk, use of standard care treatments, and achievement of treatment goals in patients with cardiovascular disease. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 18, 200198. [Google Scholar] [CrossRef]
- Wita, K.; Wilkosz, K.; Wita, M.; Kułach, A.; Wybraniec, M.T.; Polak, M.; Matla, M.; Maciejewski, Ł.; Fluder, J.; Kalańska-Łukasik, B.; et al. Managed Care after Acute Myocardial Infarction (MC-AMI)—A Poland’s nationwide program of comprehensive post-MI care-improves prognosis in 12-month follow-up. Preliminary experience from a single high-volume center. Int. J. Cardiol. 2019, 296, 8–14. [Google Scholar] [CrossRef]
- Pocock, S.J.; Brieger, D.; Gregson, J.; Chen, J.Y.; Cohen, M.G.; Goodman, S.G.; Granger, C.B.; Grieve, R.; Nicolau, J.C.; Simon, T.; et al. Predicting risk of cardiovascular events 1 to 3 years post-myocardial infarction using a global registry. Clin. Cardiol. 2020, 43, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Amon, J.; Wong, G.C.; Lee, T.; Singer, J.; Cairns, J.; Shavadia, J.S.; Granger, C.; Gin, K.; Wang, T.Y.; van Diepen, S.; et al. Incidence and Predictors of Adverse Events Among Initially Stable ST-Elevation Myocardial Infarction Patients Following Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2022, 11, e025572. [Google Scholar] [CrossRef]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe: Epidemiological update. Eur. Heart J. 2013, 34, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Almendárez, M.; Álvarez-Velasco, R.; Avanzas, P.; Alperi, A.; Gutiérrez, L.; Ledesma, D.; Martínez, J.; Hernández-Vaquero, D.; Lorca, R.; Arboine, L.; et al. STEMI in women. Life expectancy recovery after primary percutaneous coronary intervention. Rev. Espanola Cardiol. 2023, 76, 1003–1012. [Google Scholar] [CrossRef]
- Mureddu, G.F.; D’Errigo, P.; Rosato, S.; Faggiano, P.; Badoni, G.; Ceravolo, R.; Altamura, V.; Di Martino, M.; Ambrosetti, M.; Oliva, F.; et al. The relative impact of components of high residual risk on the long-term prognosis after AMI. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 22, 200310. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kim, S.H.; Uhm, I.A.; Shin, J.-H.; Lim, Y.-H. Prognostic implications for patients after myocardial infarction: An integrative literature review and in-depth interviews with patients and experts. BMC Cardiovasc. Disord. 2022, 22, 348. [Google Scholar] [CrossRef]
- Rapsomaniki, E.; Thuresson, M.; Yang, E.; Blin, P.; Hunt, P.; Chung, S.C.; Stogiannis, D.; Pujades-Rodriguez, M.; Timmis, A.; Denaxas, S.C.; et al. Using big data from health records from four countries to evaluate chronic disease outcomes: A study in 114 364 survivors of myocardial infarction. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 172–183. [Google Scholar] [CrossRef]
- Widimsky, P.; Bilkova, D.; Penicka, M.; Novak, M.; Lanikova, M.; Porizka, V.; Groch, L.; Zelizko, M.; Budesinsky, T.; Aschermann, M.; et al. Long-term outcomes of patients with acute myocardial infarction presenting to hospitals without catheterization laboratory and randomized to immediate thrombolysis or interhospital transport for primary percutaneous coronary intervention. Five years’ follow-up of the PRAGUE-2 trial. Eur. Heart J. 2007, 28, 679–684. [Google Scholar] [CrossRef]
- Mrdovic, I.; Savic, L.; Lasica, R.; Krljanac, G.; Asanin, M.; Brdar, N.; Djuricic, N.; Marinkovic, J.; Perunicic, J. Efficacy and safety of tirofiban-supported primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Results of propensity analysis using the Clinical Center of Serbia STEMI Register. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 56–66. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular risk in post-myocardial infarction patients: Nationwide real world data demonstrate the importance of a long-term perspective. Eur. Heart J. 2015, 36, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Hvelplund, A.; Galatius, S.; Madsen, M.; Sørensen, R.; Madsen, J.K.; Iversen, A.Z.; Tilsted, H.-H.; Helqvist, S.; Mortensen, P.E.; Nielsen, P.H.; et al. Significance of the invasive strategy after acute myocardial infarction on prognosis and secondary preventive medication: A nationwide study of 6364 women and 11,915 men. J. Invasive Cardiol. 2012, 24, 19–24. [Google Scholar]
- Mureddu, G.F.; Greco, C.; Rosato, S.; D’Errigo, P.; De Luca, L.; Badoni, G.; Faggiano, P.; Seccareccia, F. High thrombotic risk increases adverse clinical events up to 5 years after acute myocardial infarction. A nationwide retrospective cohort study. Monaldi Arch. Chest Dis. 2019, 89, 3. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Huo, Y.; Van de Werf, F.; Newsome, S.; Chin, C.T.; Vega, A.M.; Medina, J.; Bueno, H. Predicting two-year mortality from discharge after acute coronary syndrome: An internationally-based risk score. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 727–737. [Google Scholar] [CrossRef]
- Abu-Assi, E.; López-López, A.; González-Salvado, V.; Redondo-Diéguez, A.; Peña-Gil, C.; Bouzas-Cruz, N.; Raposeiras-Roubín, S.; Abumuaileq, R.R.-Y.; García-Acuña, J.M.; González-Juanatey, J.R. The Risk of Cardiovascular Events After an Acute Coronary Event Remains High, Especially During the First Year, Despite Revascularization. Rev. Espanola Cardiol. 2016, 69, 11–18. [Google Scholar] [CrossRef]
- Aldama, G.; Rebollal, F.; Piñón, P. Prognosis after an acute myocardial infarction: Survivor life expectancy. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 820–822. [Google Scholar] [CrossRef]
- Ng, V.G.; Lansky, A.J.; Meller, S.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.; Shah, R.; Mehran, R.; Stone, G.W. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: The HORIZONS-AMI trial. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kyhl, K.; Ahtarovski, K.A.; Nepper-Christensen, L.; Ekström, K.; Ghotbi, A.A.; Schoos, M.; Göransson, C.; Bertelsen, L.; Helqvist, S.; Holmvang, L.; et al. Complete Revascularization Versus Culprit Lesion Only in Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Disease: A DANAMI-3-PRIMULTI Cardiac Magnetic Resonance Substudy. JACC Cardiovasc. Interv. 2019, 12, 721–730. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Stiermaier, T.; Eitel, C.; Metzler, B.; de Waha, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Relationship between diabetes and ischaemic injury among patients with revascularized ST-elevation myocardial infarction. Diabetes Obes. Metab. 2017, 19, 1706–1713. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. ESC 2023 guidelines for the treatment of acute coronary syndromes developed by the task force for the treatment of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 25, E1–E112. [Google Scholar] [CrossRef]
- Moukarbel, G.V.; Yu, Z.; Dickstein, K.; Hou, Y.; Wittes, J.T.; McMurray, J.J.V.; Pitt, B.; Zannad, F.; Pfeffer, M.A.; Solomon, S.D. The impact of kidney function on outcomes following high risk myocardial infarction: Findings from 27 610 patients. Eur. J. Heart Fail. 2014, 16, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with Heart failure with presserved or reduced left ventricular ejection fraction; an individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Stable Patients N = 2731 | With MACE N = 107 | Without MACE N = 2624 | p Value |
|---|---|---|---|---|
| Age, years med (IQR) | 60 (51, 67) | 62 (53, 71) | 59.5 (50, 67) | <0.001 |
| Age < 60 years, n (%) | 1456 (53.3) | 40 (38) | 1416 (53.9) | 0.005 |
| Age 61–70 years, n (%) | 793 (20.9) | 38 (36.2) | 755 (28.7) | 0.011 |
| Age 71–80 years, n (%) | 413 (15.1) | 21 (20) | 392 (14.9) | 0.010 |
| Age >80 years, n (%) | 69 (2.5) | 8 (7.5) | 61 (2.3) | 0.001 |
| Female, n (%) | 728 (26.7) | 24 (22.8) | 704 (26.8) | 0.369 |
| BMI, med (IQR) | 26.5 (24.5, 29.6) | 26.8 (24.9, 28.7) | 26.4 (24.8, 29.8) | 0.638 |
| Previous MI, n (%) | 252 (9.2) | 14 (13.3) | 238 (9.1) | 0.124 |
| Previous angina, n (%) | 185 (6.8) | 8 (7.6) | 177 (6.7) | 0.726 |
| Previous stroke, n (%) | 94 (3.4) | 9 (8.6) | 85 (3.3) | 0.003 |
| DM, n (%) | 499 (18.3) | 30 (28.6) | 469 (17.8) | 0.005 |
| Hypertension, n (%) | 1789 (65.5) | 77 (73.3) | 1712 (65.2) | 0.225 |
| HLP, n (%) | 1665 (60.1) | 69 (65.7) | 1596 (60.8) | 0.309 |
| Smoking, n (%) | 1530 (56) | 54 (51.4) | 1476 (56.2) | 0.606 |
| Family hystory, n (%) | 1939 (70.1) | 29 (27.6) | 910 (34.6) | 0.137 |
| Pain duration, hours med (IQR) | 2.5 (1.5, 4) | 3 (1.5, 4) | 2.5 (1.5, 4) | 0.807 |
| New or presumably new onset atrial fibrillation, n (%) * | 149 (5.5) | 11 (10.5) | 138 (5.2) | 0.021 |
| Complete AV block at admission, n (%) | 101 (3.4) | 6 (5.7) | 95 (3.6) | 0.526 |
| BBB at admisson ECG, n (%) ** | 128 (4.7) | 7 (6.1) | 121 (4.5) | 0.861 |
| Killip class >1, at admission, (%) | 260 (9.5) | 11 (10.5) | 249 (9.5) | 0.977 |
| Systolic BP at admission, med (IQR) | 140 (120, 150) | 140 (110, 155) | 135 (120, 150) | 0.610 |
| Heart rate at admission med (IQR) | 77 (66, 88) | 72 (69, 83) | 80 (70, 90) | 0.415 |
| Multivessel disease, n (%) | 1486 (54.4) | 67 (63.8) | 1419 (54) | 0.041 |
| 3-vessel disease, n (%) | 674 (24.7) | 36 (34.3) | 638 (24.9) | 0.020 |
| LM stenosis, n (%) | 158 (4.1) | 6 (5.9) | 152 (5.7) | 0.973 |
| Postprocedural flow TIMI <3, n (%) | 83 (3) | 2 (1.9) | 81 (3.1) | 0.498 |
| Stent implanted, n (%) | 2707 (99.1) | 102 (97.2) | 2605 (99.2) | 0.987 |
| Subacute stent thrombosis, n (%) | 12 (0.04) | 1 (0.9) | 11 (0.4) | 0.422 |
| Glicoprotein IIb/IIIa inhibitor, n (%) | 967 (35.4) | 30 (28.5) | 937 (35.7) | 0.151 |
| CK MB max, med (IQR) | 1826 (980, 3308) | 2276 (1113, 3549) | 1816 (906, 3315) | 0.067 |
| eGFR, med (IQR) | ||||
| Troponin max, med (IQR) | 34 (10, 97.1) | 48 (16, 150) | 31 (10, 93) | 0.009 |
| Anemia at admission, n (%) | 198 (7.2) | 12 (11.4) | 186 (7.1) | 0.082 |
| WBC count at admission, med (IQR) | 11.3 (9.2, 13.6) | 10.5 (8.8, 13.5) | 11.1 (9.2, 13.6) | 0.950 |
| Glycemia at admission, mmol/L med (IQR) | 7.2 (5.9, 9) | 7.6 (6.1, 11) | 7.1 (5.9, 8.9) | 0.010 |
| CKD, n (%) | 1831 (67) | 74 (70.6) | 1801 (68.6) | 0.672 |
| EF, med (IQR) | 50 (40, 55) | 45 (40, 55) | 50 (45, 55) | <0.001 |
| EF < 40%, n (%) | 278 (10.3) | 15 (14.3) | 263 (10) | <0.001 |
| EF 40–49%, n (%) | 977 (35.7) | 59 (56.2) | 918 (34.9) | <0.001 |
| EF ≥ 50%, n (%) | 1476 (54) | 31 (29.5) | 1445 (55) | <0.001 |
| Ongoing medication at day 366 after index event *** | ||||
| Aspirin, n (%) | 2697 (98.7) | 99 (94.2) | 2598 (98.9) | 0.567 |
| Beta blockers, n (%) | 2587 (94.7) | 93 (88.5) | 2494 (94.7) | 0.268 |
| ACE inhibitors/ARB, n (%) | 2413 (88.3) | 88 (83.8) | 2325 (88.5) | 0.813 |
| Statin, n (%) | 2577 (94.4) | 95 (90.4) | 2482 (94.5) | 0.990 |
| Diuretic, n (%) | 499 (18.3) | 72 (68.6) | 427 (16.2) | 0.002 |
| Calcium antagonist, n (%) | 103 (3.8) | 4 (3.8) | 99 (3.8) | 0.909 |
| OAC, n (%) | 30 (1.1) | 3 (2.8) | 27 (1.1) | 0.622 |
| Amiodarone, n (%) | 81 (2.9) | 8 (7.6) | 73 (2.8) | 0.003 |
| At 2 Years | At 4 Years | At 6 Years | At 8 Years | |
|---|---|---|---|---|
| MACE, n (%) | 36(1.3) | 66(2.4) | 102(3.7) | 107(3.9) |
| Death, n (%) | 18(0.6) | 36(1.3) | 52(1.9) | 57(2.1) |
| Non-fatal recurrent infarction, n (%) | 12(0.4) | 27(1.0) | 45(1.7) | 45(1.6) |
| TVR, n (%) | 15(0.5) | 28(1.1) | 45(1.7) | 47(1.8) |
| Non-fatal stroke, n (%) | 3(0.01) | 11(0.4) | 12(0.4) | 14(0.5) |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age > 60 years | 2.10 (1.09–3.05) | 0.001 | 2.07 (1.20–2.51) | 0.010 |
| Age 60–69 vs. <60 years | 1.68 (1.03–2.64) | 0.031 | 1.65 (1.06–2.37) | 0.026 |
| Age 70–79 vs. <60 years | 1.85 (1.06–3.25) | 0.033 | 1.82 (1.05–3.20) | 0.032 |
| Age ≥80 vs. <60 years | 3.51 (1.28–6.99) | 0.010 | 3.16 (1.11–9.65) | 0.032 |
| EF < 50% | 2.49 (1.55–3.82) | <0.001 | 2.47 (1.52–4.10) | <0.001 |
| EF < 40% vs. EF ≥ 50% | 2.56 (1.54–4.90) | <0.001 | 2.38 (1.36–3.69) | 0.002 |
| EF 40–49% vs. EF ≥ 50% | 2.42 (1.67–4.15) | <0.001 | 2.32 (1.31–4.13) | 0.004 |
| Amiodarone (ongoing therapy) | 1.72 (1.05–1.97) | 0.044 | ||
| Diabetes | 1.71 (1.14–2.67) | 0.010 | 1.49 (1.09–2.31) | 0.049 |
| Diuretics (ongoing therapy) | 1.69 (1.24–2.13) | 0.040 | ||
| 3-vessel disease | 1.68 (1.12–2.52) | 0.011 | 1.44 (1.06–2.43) | 0.048 |
| Glycemia at admission | 1.02 (1.01–1.03) | 0.010 | ||
| Previous stroke | 1.58 (1.01–2.76) | 0.044 | ||
| New-onset AF | 1.50 (0.86–1.67) | 0.117 | ||
| Troponin | 1.01 (0.98–1.02) | 0.354 | ||
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Age > 60 years | 64.8% | 64.5% | 14.4% | 97.3% |
| EF < 50% | 62.8% | 69.5% | 25.6% | 98.1% |
| 3-vessel disease | 51.2% | 75.5% | 14.3% | 96.9% |
| Diabetes | 50.9% | 82% | 15.5% | 97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, L.; Simic, D.; Lasica, R.; Krljanac, G.; Stankovic, S.; Mrdovic, I.; Asanin, M. Predictors of Major Adverse Cardiovascular Events in Stable Patients After ST Elevation Myocardial Infarction. Clin. Pract. 2025, 15, 106. https://doi.org/10.3390/clinpract15060106
Savic L, Simic D, Lasica R, Krljanac G, Stankovic S, Mrdovic I, Asanin M. Predictors of Major Adverse Cardiovascular Events in Stable Patients After ST Elevation Myocardial Infarction. Clinics and Practice. 2025; 15(6):106. https://doi.org/10.3390/clinpract15060106
Chicago/Turabian StyleSavic, Lidija, Damjan Simic, Ratko Lasica, Gordana Krljanac, Sanja Stankovic, Igor Mrdovic, and Milika Asanin. 2025. "Predictors of Major Adverse Cardiovascular Events in Stable Patients After ST Elevation Myocardial Infarction" Clinics and Practice 15, no. 6: 106. https://doi.org/10.3390/clinpract15060106
APA StyleSavic, L., Simic, D., Lasica, R., Krljanac, G., Stankovic, S., Mrdovic, I., & Asanin, M. (2025). Predictors of Major Adverse Cardiovascular Events in Stable Patients After ST Elevation Myocardial Infarction. Clinics and Practice, 15(6), 106. https://doi.org/10.3390/clinpract15060106





