Estimation of Kidney Volumes in Autosomal Dominant Polycystic Kidney Disease: A Comparison Between Manual Segmentation and Ellipsoid Formula
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Technique
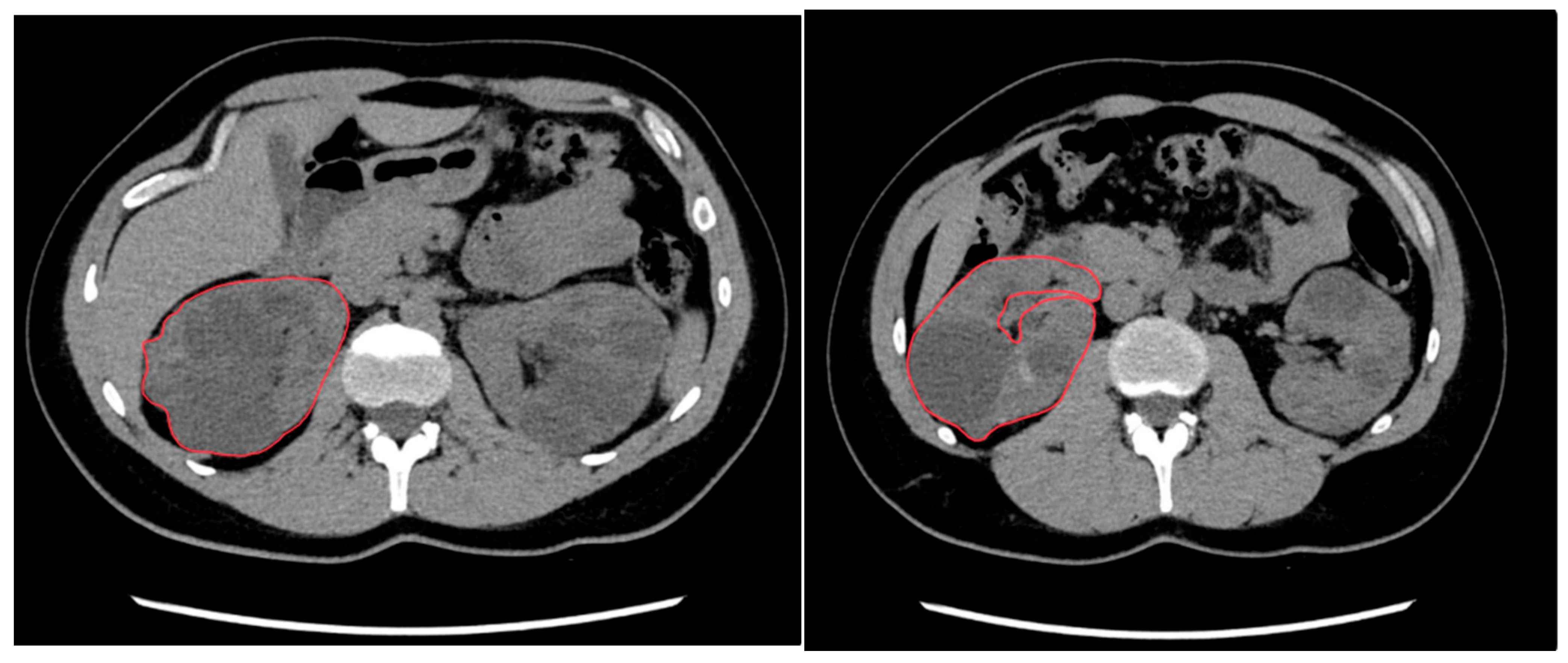
2.3. Manual Segmentation
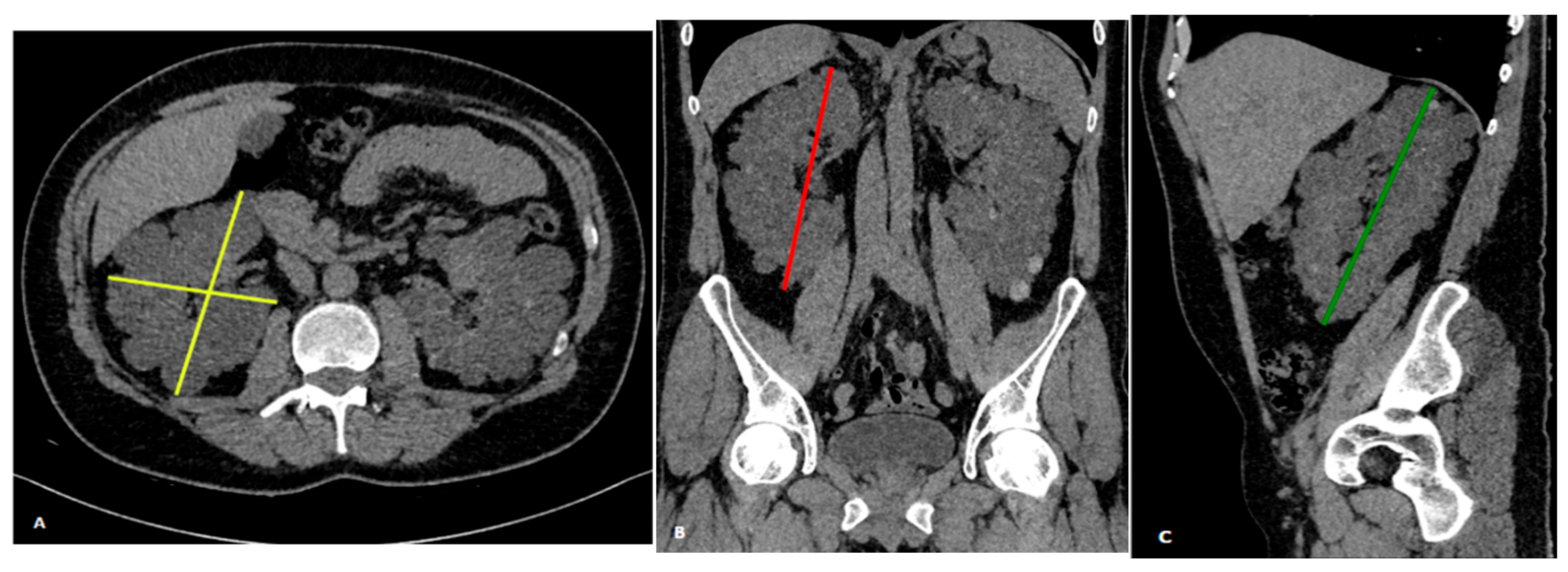
2.4. Ellipsoid Formula
2.5. Statical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal Dominant Polycystic Kidney Disease |
| PDK1 | Polycystic Kidney Disease 1 |
| PDK2 | Polycystic Kidney Disease 2 |
| ESRD | End-stage Renal Disease |
| eGFR | Estimated Glomerular Filtration Rate |
| CRISP | Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease |
| TKV | Total Kidney Volume |
| SKV | Single Kidney Volume |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| US | Ultrasound |
| MPR | Multi-planar Reconstructions |
| BMI | Body Mass Index |
| PROPKD | Predicting renal outcome in polycystic kidney disease score |
| htTKV | Height-adjusted Total Kidney Volume |
| MIC | Mayo Imaging Classification |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| ICC | Intraclass Correlation Coefficient |
| CI | Confidence Interval |
References
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Caplan, M.J. Polycystic kidney disease: Pathogenesis and potential therapies. Biochim. Biophys. Acta 2011, 1812, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gall, E.C.-L.; Olson, R.J.; Besse, W.; Heyer, C.M.; Gainullin, V.G.; Smith, J.M.; Audrézet, M.-P.; Hopp, K.; Porath, B.; Shi, B.; et al. Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am. J. Hum. Genet. 2018, 102, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Maxeiner, A.; Bichmann, A.; Oberländer, N.; El-Bandar, N.; Sugünes, N.; Ralla, B.; Biernath, N.; Liefeldt, L.; Budde, K.; Giessing, M.; et al. Native Nephrectomy before and after Renal Transplantation in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). J. Clin. Med. 2019, 8, 1622. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Wu, G.; Hayashi, T.; Xenophontos, S.L.; Veldhuisen, B.; Saris, J.J.; Reynolds, D.M.; Cai, Y.; Gabow, P.A.; Pierides, A.; et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996, 272, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Hateboer, N.; Dijk, M.A.V.; Bogdanova, N.; Coto, E.; Saggar-Malik, A.K.; Millan, J.L.S.; Torra, R.; Breuning, M.; Ravine, D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 1999, 353, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Patel, N. Autosomal dominant polycystic kidney disease. Am. Fam. Physician 2014, 90, 303–307. [Google Scholar] [PubMed]
- Grantham, J.J. Clinical Practice. Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2008, 359, 1477–1485. [Google Scholar] [CrossRef]
- Bennett, W.M. Autosomal dominant polycystic kidney disease: 2009 update for internists. Korean J. Intern. Med. 2009, 24, 165–168. [Google Scholar] [CrossRef]
- Boucher, C.; Sandford, R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2). Eur. J. Hum. Genet. 2004, 12, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Torres, V.E.; Chapman, A.B.; Guay-Woodford, L.M.; Bae, K.T.; King, B.F.J.; Wetzel, L.H.; Baumgarten, D.A.; Kenney, P.J.; Harris, P.C.; et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 2006, 354, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Rubinstein, D.; Hughes, R.; Stears, J.C.; Earnest, M.P.; Johnson, A.M.; Gabow, P.A.; Kaehny, W.D. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1992, 327, 916–920. [Google Scholar] [CrossRef]
- Grantham, J.J.; Mulamalla, S.; Swenson-Fields, K.I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2011, 7, 556–566. [Google Scholar] [CrossRef]
- Higashihara, E.; Nutahara, K.; Okegawa, T.; Shishido, T.; Tanbo, M.; Kobayasi, K.; Nitadori, T. Kidney volume and function in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 2014, 18, 157–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kline, T.L.; Korfiatis, P.; Edwards, M.E.; Bae, K.T.; Yu, A.; Chapman, A.B.; Mrug, M.; Grantham, J.J.; Landsittel, D.; Bennett, W.M.; et al. Image texture features predict renal function decline in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2017, 92, 1206–1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magistroni, R.; Corsi, C.; Martí, T.; Torra, R. A Review of the Imaging Techniques for Measuring Kidney and Cyst Volume in Establishing Autosomal Dominant Polycystic Kidney Disease Progression. Am. J. Nephrol. 2018, 48, 67–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cloutier, M.; Manceur, A.M.; Guerin, A.; Aigbogun, M.S.; Oberdhan, D.; Gauthier-Loiselle, M. The societal economic burden of autosomal dominant polycystic kidney disease in the United States. BMC Health Serv. Res. 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grantham, J.J.; Geiser, J.L.; Evan, A.P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987, 31, 1145–1152. [Google Scholar] [CrossRef]
- Grantham, J.J.; Torres, V.E. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat. Rev. Nephrol. 2016, 12, 667–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, E.W.C.; Chong, J.; Valluru, M.K.; Durkie, M.; Simms, R.J.; Harris, P.C.; Ong, A.C.M. Combining genotype with height-adjusted kidney length predicts rapid progression of ADPKD. Nephrol. Dial. Transplant. 2024, 39, 956–966. [Google Scholar] [CrossRef]
- Ghanem, A.; Borghol, A.H.; Debeh, F.G.M.; Paul, S.; AlKhatib, B.; Harris, P.C.; Garimella, P.S.; Hanna, C.; Kline, T.L.; Dahl, N.K.; et al. Biomarkers of Kidney Disease Progression in ADPKD. Kidney Int. Rep. 2024, 9, 2860–2882. [Google Scholar] [CrossRef]
- Gall, E.C.-L.; Audrézet, M.-P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.-P.; Moal, M.-C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 942–951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- US Food and Drug Administration. Qualification of Biomarker Total Kidney Volume in Studies for Treatment of Autosomal Dominant Polycystic Kidney Disease Draft Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qualification-biomarker-total-kidney-volume-studies-treatment-autosomal-dominant-polycystic-kidney (accessed on 6 June 2024).
- US Food and Drug Administration. Biomarker Qualification Program. FDA. Available online: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program (accessed on 9 May 2024).
- Kelsey, R. Tolvaptan in ADPKD—TEMPO 3:4 trial results. Nat. Rev. Nephrol. 2013, 9, 1. [Google Scholar] [CrossRef]
- Wyatt, C.M.; Le Meur, Y. REPRISE: Tolvaptan in advanced polycystic kidney disease. Kidney Int. 2018, 93, 292–295. [Google Scholar] [CrossRef]
- Perrone, R.D.; Abebe, K.Z.; Watnick, T.J.; Althouse, A.D.; Hallows, K.R.; Lalama, C.M.; Miskulin, D.C.; Seliger, S.L.; Tao, C.; Harris, P.C.; et al. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD). Kidney Int. 2021, 100, 684–696. [Google Scholar] [CrossRef]
- King, B.F.; Reed, J.E.; Bergstralh, E.J.; Sheedy, P.F.; Torres, V.E. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2000, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Commean, P.K.; Lee, J. Volumetric measurement of renal cysts and parenchyma using MRI: Phantoms and patients with polycystic kidney disease. J. Comput. Assist. Tomogr. 2000, 24, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, G.; Di Terlizzi, F.; Flor, N.; Morganti, A.; Sardanelli, F. Measurement of renal volume using respiratory-gated MRI in subjects without known kidney disease: Intraobserver, interobserver, and interstudy reproducibility. Eur. J. Radiol. 2011, 80, e212–e216. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, H.; Boykov, Y.; Kaur, M.; Wong, S.; Bokacheva, L.; Sajous, J.B.; Huang, A.J.; Heller, S.; Lee, V.S. Performance of an automated segmentation algorithm for 3D MR renography. Magn. Reson. Med. 2007, 57, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, S.W.; Wasser, M.N.; de Fijter, J.W.; Hoekstra, J.; van der Geest, R.J. Functional renal volume: Quantitative analysis at gadolinium-enhanced MR angiography–feasibility study in healthy potential kidney donors. Radiology 2005, 236, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.S.; Wang, T.H.; Parker, J.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies (OASIS): Cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J. Cogn. Neurosci. 2007, 19, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Akbari, P.; Pourafkari, M.; Iliuta, I.-A.; Guiard, E.; Quist, C.F.; Song, X.; Hillier, D.; Khalili, K.; Pei, Y. Prognostic Performance of Kidney Volume Measurement for Polycystic Kidney Disease: A Comparative Study of Ellipsoid vs. Manual Segmentation. Sci. Rep. 2019, 9, 10996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bevilacqua, M.U.; Hague, C.J.; Romann, A.; Sheitt, H.; Vasilescu, D.M.; Yi, T.W.; Levin, A. CT of Kidney Volume in Autosomal Dominant Polycystic Kidney Disease: Accuracy, Reproducibility, and Radiation Dose. Radiology 2019, 291, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Caroli, A.; Van Quach, L.; Petzold, K.; Bozzetto, M.; Serra, A.L.; Remuzzi, G.; Remuzzi, A. Kidney volume measurement methods for clinical studies on autosomal dominant polycystic kidney disease. PLoS ONE 2017, 12, e0178488. [Google Scholar] [CrossRef] [PubMed]
- Turco, D.; Busutti, M.; Mignani, R.; Magistroni, R.; Corsi, C. Comparison of Total Kidney Volume Quantification Methods in Autosomal Dominant Polycystic Disease for a Comprehensive Disease Assessment. Am. J. Nephrol. 2017, 45, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, F.G.; Svarstad, E.; Munthe-Kaas, A.Z.; Schad, L.R.; Lundervold, A.; Rørvik, J. Assessment of kidney volumes from MRI: Acquisition and segmentation techniques. Am. J. Roentgenol. 2012, 199, 1060–1069. [Google Scholar] [CrossRef]
- Christensen, R.H.; Lundgren, T.; Stenvinkel, P.; Brismar, T.B. Renal volumetry with magnetic resonance imaging. Acta Radiol. Open 2017, 6, 2058460117731120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Pietro, S.; Torcitto, A.G.; Marcantoni, C.; Giordano, G.; Campisi, C.; Failla, G.; Saporito, L.; Giunta, R.; Veroux, M.; Foti, P.V.; et al. Calculation of Kidney Volumes with Magnetic Resonance in Patients with Autosomal Dominant Polycystic Kidney Disease: Comparison between Methods. Diagnostics 2023, 13, 3573. [Google Scholar] [CrossRef]
- Seuss, H.; Janka, R.; Prümmer, M.; Cavallaro, A.; Hammon, R.; Theis, R.; Sandmair, M.; Amann, K.; Bäuerle, T.; Uder, M.; et al. Development and Evaluation of a Semi-automated Segmentation Tool and a Modified Ellipsoid Formula for Volumetric Analysis of the Kidney in Non-contrast T2-Weighted MR Images. J. Digit. Imaging 2016, 30, 244–254. [Google Scholar] [CrossRef]
- Bhutani, H.; Smith, V.; Rahbari-Oskoui, F.; Mittal, A.; Grantham, J.J.; Torres, V.E.; Mrug, M.; Bae, K.T.; Wu, Z.; Ge, Y.; et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease: CRISP Investigators. Kidney Int. 2015, 88, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ge, Y.; Tao, C.; Zhu, J.; Chapman, A.B.; Torres, V.E.; Yu, A.S.; Mrug, M.; Bennett, W.M.; Flessner, M.F.; et al. Automated Segmentation of Kidneys from MR Images in Patients with Autosomal Dominant Polycystic Kidney Disease: Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Clin. J. Am. Soc. Nephrol. 2016, 11, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Racimora, D.; Vivier, P.-H.; Chandarana, H.; Rusinek, H. Segmentation of polycystic kidneys from MR images. In Proceedings of the Medical Imaging 2010: Computer-Aided Diagnosis, San Diego, CA, USA, 13–18 February 2010. [Google Scholar]
- Daum, V.; Helbig, H.; Janka, R.; Eckardt, K.; Zeltner, R. Quantitative Measurement of Kidney and Cyst Sizes in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). In Proceedings of the 3rd Russian-Bavarian Conference on Biomedical Engineering, Erlangen, Germany, 2–3 July 2007. [Google Scholar]
- Mignani, R.; Corsi, C.; De Marco, M.; Caiani, E.G.; Santucci, G.; Severi, S.; Cagnoli, L. Assessment of kidney volume in polycystic kidney disease using magnetic resonance imaging without contrast medium. Am. J. Nephrol. 2011, 33, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.L.; Korfiatis, P.; Edwards, M.E.; Warner, J.D.; Irazabal, M.V.; King, B.F.; Torres, V.E.; Erickson, B.J. Automatic total kidney volume measurement on follow-up magnetic resonance images to facilitate monitoring of autosomal dominant polycystic kidney disease progression. Nephrol. Dial. Transplant. 2015, 31, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-L.; Singaravelan, A.; Lai, C.-Y.; Li, Z.-L.; Lin, C.-N.; Wu, W.-S.; Kao, T.-W.; Chu, P.-L. Applying a Deep Learning Model for Total Kidney Volume Measurement in Autosomal Dominant Polycystic Kidney Disease. Bioengineering 2024, 11, 963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, B.A.; Barash, I.; Kim, D.C.; Sanger, M.D.; Babb, J.S.; Chandarana, H. Intraobserver and interobserver variability of renal volume measurements in polycystic kidney disease using a semiautomated MR segmentation algorithm. Am. J. Roentgenol. 2012, 199, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Shi, T.; Tao, C.; Yu, A.S.L.; Torres, V.E.; Perrone, R.D.; Chapman, A.B.; Brosnahan, G.; Steinman, T.I.; Braun, W.E.; et al. Expanded Imaging Classification of Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 1640–1651. [Google Scholar] [CrossRef]
- Gregory, A.V.; Chebib, F.T.; Poudyal, B.; Holmes, H.L.; Yu, A.S.; Landsittel, D.P.; Bae, K.T.; Chapman, A.B.; Frederic, R.-O.; Mrug, M.; et al. Utility of New Image-Derived Biomarkers for Autosomal Dominant Polycystic Kidney Disease Prognosis Using Automated Instance Cyst Segmentation. Kidney Int. 2023, 104, 334–342. [Google Scholar] [CrossRef]
- Riyahi, S.; Dev, H.; Blumenfeld, J.D.; Rennert, H.; Yin, X.; Attari, H.; Barash, I.; Chicos, I.; Bobb, W.; Donahue, S.; et al. Hemorrhagic Cysts and Other Biomarkers for Predicting Renal Dysfunction Progression in Autosomal Dominant Polycystic Kidney Disease. J. Magn. Reson. Imaging 2020, 53, 564–576. [Google Scholar] [CrossRef]
- Caroli, A.; Kline, T.L. Abdominal Imaging in ADPKD: Beyond Total Kidney Volume. J. Clin. Med. 2023, 12, 5133. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggialetti, N.; Dipalma, C.; Colucci, E.; Villanova, I.; Lorusso, G.; Arcidiacono, M.G.; Piscopo, G.; Stabile Ianora, A.A. Estimation of Kidney Volumes in Autosomal Dominant Polycystic Kidney Disease: A Comparison Between Manual Segmentation and Ellipsoid Formula. Clin. Pract. 2025, 15, 191. https://doi.org/10.3390/clinpract15110191
Maggialetti N, Dipalma C, Colucci E, Villanova I, Lorusso G, Arcidiacono MG, Piscopo G, Stabile Ianora AA. Estimation of Kidney Volumes in Autosomal Dominant Polycystic Kidney Disease: A Comparison Between Manual Segmentation and Ellipsoid Formula. Clinics and Practice. 2025; 15(11):191. https://doi.org/10.3390/clinpract15110191
Chicago/Turabian StyleMaggialetti, Nicola, Claudia Dipalma, Eva Colucci, Ilaria Villanova, Giovanni Lorusso, Maria Grazia Arcidiacono, Giovanni Piscopo, and Amato Antonio Stabile Ianora. 2025. "Estimation of Kidney Volumes in Autosomal Dominant Polycystic Kidney Disease: A Comparison Between Manual Segmentation and Ellipsoid Formula" Clinics and Practice 15, no. 11: 191. https://doi.org/10.3390/clinpract15110191
APA StyleMaggialetti, N., Dipalma, C., Colucci, E., Villanova, I., Lorusso, G., Arcidiacono, M. G., Piscopo, G., & Stabile Ianora, A. A. (2025). Estimation of Kidney Volumes in Autosomal Dominant Polycystic Kidney Disease: A Comparison Between Manual Segmentation and Ellipsoid Formula. Clinics and Practice, 15(11), 191. https://doi.org/10.3390/clinpract15110191






