Trends and Hot Spots in Research Related to Rivaroxaban: Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Data Extraction Process
2.3. Visualization and Map Building
3. Results
3.1. Overall Number of Publications Related to Rivaroxaban
3.2. Number of Publications per Year and RRI
3.3. Countries of Publications
3.4. Most Productive Journals
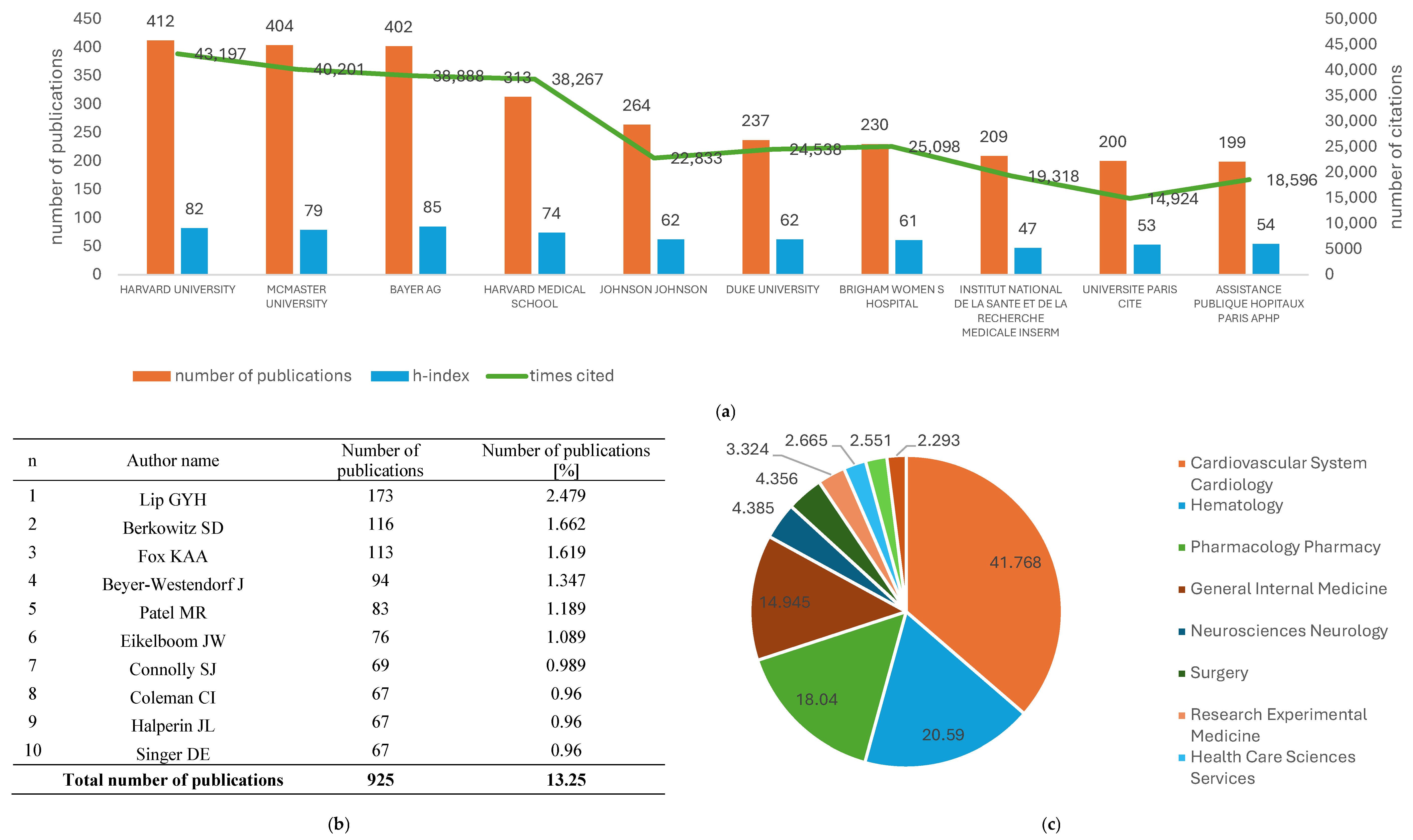
3.5. Most Productive Organizations
3.6. Most Productive Authors
3.7. Most Prominent Funders
3.8. Subjects of Area, Meso-Topics, and Micro-Topics Analysis

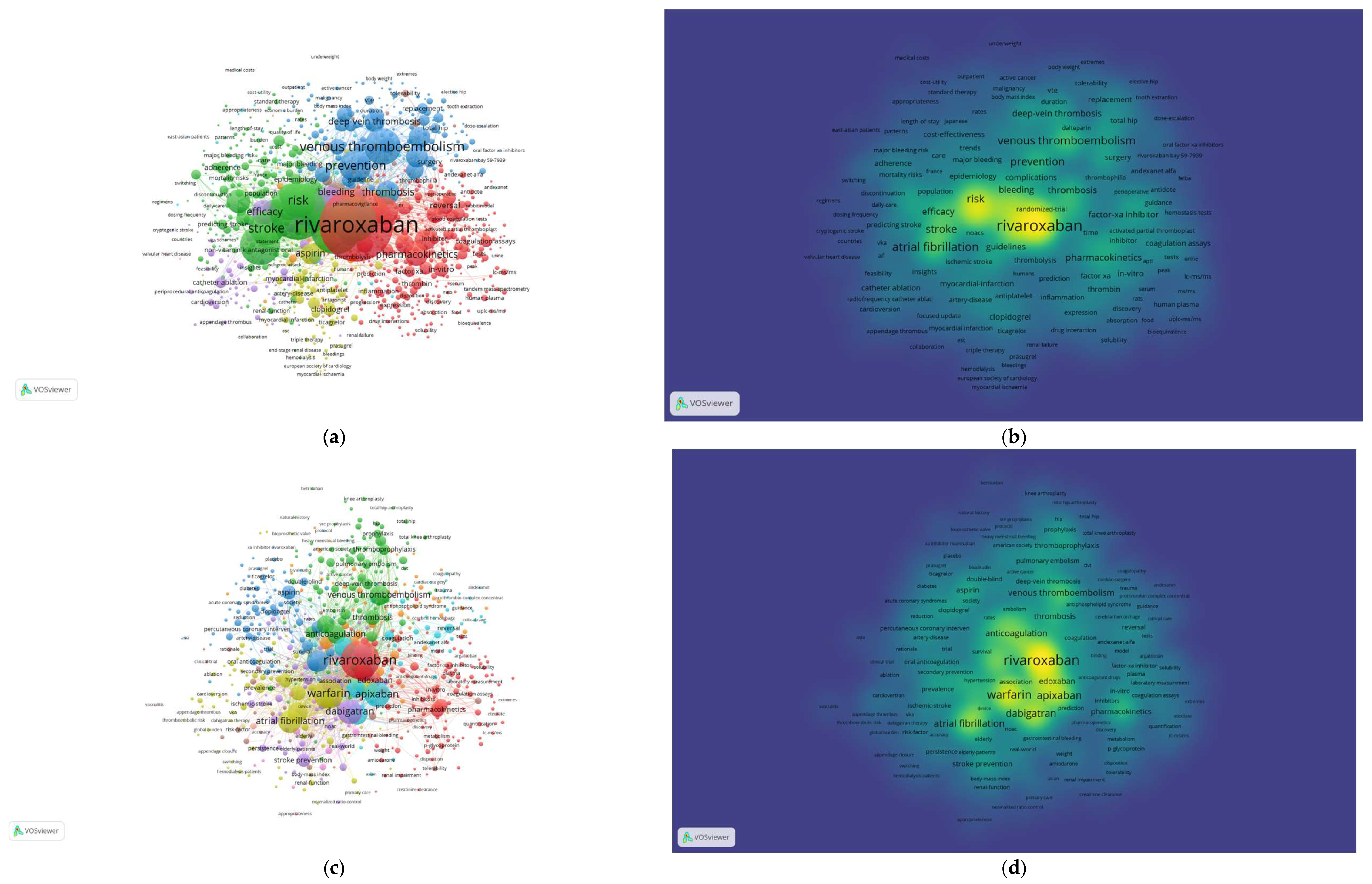
3.9. Co-Citations References
3.10. Co-Occurrence of Keywords

4. Discussion
4.1. New Trends in Research Related to Rivaroxaban
4.2. Hot Spots in Research Related to Rivaroxaban
4.3. The Analysis of Co-Cited References
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NOAC | novel oral anticoagulants |
| VKA | vitamin K antagonists |
| DVT | deep vein thrombosis |
| WOS | Web of Science |
References
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Alkhezi, O.S.; Buckley, L.F.; Fanikos, J. Trends in Oral Anticoagulant Use and Individual Expenditures Across the United States from 2014 to 2020. Am. J. Cardiovasc. Drugs 2024, 24, 433–444. [Google Scholar] [CrossRef]
- Gómez-Outes, A.; Terleira-Fernández, A.I.; Calvo-Rojas, G.; Suárez-Gea, M.L.; Vargas-Castrillón, E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis 2013, 2013, 640723. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Al-Jaghbeer, M.J. Primary Thromboprophylaxis for the Prevention of Venous Thromboembolism in Cancer Patients with Central Venous Catheters: A Literature Review. J. Clin. Med. 2024, 13, 1660. [Google Scholar] [CrossRef]
- Ruigómez, A.; Schink, T.; Voss, A.; Herings, R.M.C.; Smits, E.; Swart-Polinder, K.; Balabanova, Y.; Brobert, G.; Suzart-Woischnik, K.; Rodríguez, L.A.G. Safety profile of rivaroxaban in first-time users treated for venous thromboembolism in four European countries. PLoS ONE 2024, 19, e0298596. [Google Scholar] [CrossRef]
- Escobar, C.; Palacios, B.; Villarreal, M.; Gutiérrez, M.; Capel, M.; Hernández, I.; García, M.; Lledó, L.; Arenillas, J.F. Clinical Characteristics and Incidence of Hemorrhagic Complications in Patients Taking Factor Xa Inhibitors in Spain: A Long-Term Observational Study. J. Clin. Med. 2024, 13, 1677. [Google Scholar] [CrossRef]
- Chiasson, C.O.; Canneva, A.; Roy, F.O.; Doré, M. Rivaroxaban-Induced Hypersensitivity Syndrome. Can. J. Hosp. Pharm. 2017, 70, 301–304. [Google Scholar] [CrossRef]
- Al-Khafaji, R.A. Developing Deep Venous Thrombosis While on Rivaroxaban: A Review of Rivaroxaban. Available online: https://www.hilarispublisher.com/open-access/developing-deep-venous-thrombosis-while-on-rivaroxaban-a-review-of-rivaroxaban-45389.html (accessed on 25 June 2024).
- Dufrost, V.; Risse, J.; Kirchner, S.; Zuily, S.; Wahl, D. Failure of rivaroxaban to prevent thrombosis in four patients with anti-phospholipid syndrome. Rheumatology 2017, 56, 1433–1434. [Google Scholar] [CrossRef]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef]
- Mueck, W.; Kubitza, D.; Becka, M. Co-administration of rivaroxaban with drugs that share its elimination pathways: Pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef]
- Wu, T.; Wu, S.; Li, L.; Xiang, J.; Wang, N.; Chen, W.; Zhang, J. The impact of ABCB1, CYP3A4/5 and ABCG2 gene polymorphisms on rivaroxaban trough concentrations and bleeding events in patients with non-valvular atrial fibrillation. Hum. Genom. 2023, 17, 59. [Google Scholar] [CrossRef]
- Li, X.; Gu, Z.; Wang, Z.; Xu, Q.; Ma, C.; Lv, Q. Mutant CYP3A4/5 Correlated with Clinical Outcomes by Affecting Rivaroxaban Pharmacokinetics and Pharmacodynamics in Patients with Atrial Fibrillation. Cardiovasc. Drugs Ther. 2023, 38, 1315–1325. [Google Scholar] [CrossRef]
- Spiecker, M.; Darius, H.; Hankeln, T.; Soufi, M.; Sattler, A.M.; Schaefer, J.R.; Node, K.; Börgel, J.; Mügge, A.; Lindpaintner, K.; et al. Risk of Coronary Artery Disease Associated With Polymorphism of the Cytochrome P450 Epoxygenase CYP2J2. Circulation 2004, 110, 2132–2136. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the Breast Cancer Resistance Protein (BCRP/ABCG2) in Drug Transport—An Update. AAPS J. 2014, 17, 65–82. [Google Scholar] [CrossRef]
- Baturina, O.; Chashkina, M.; Andreev, D.; Mirzaev, K.; Bykova, A.; Suvorov, A.; Yeryshova, D.; Suchkova, S.; Sychev, D.; Syrkin, A. Pharmacokinetic and Pharmacogenetic Predictors of Major Bleeding Events in Patients with an Acute Coronary Syndrome and Atrial Fibrillation Receiving Combined Antithrombotic Therapy. J. Pers. Med. 2023, 13, 1371. [Google Scholar] [CrossRef] [PubMed]
- Wołowiec, Ł.; Kusiak, M.; Budzyński, J.; Wołowiec, A.; Jaśniak, A.; Wiciński, M.; Pedrycz-Wieczorska, A.; Rogowicz, D.; Grześk, G. Therapeutic Drug Monitoring of Direct Oral Anticoagulants in Patients with Extremely Low and High Body Weight—Pilot Study. J. Clin. Med. 2023, 12, 4969. [Google Scholar] [CrossRef] [PubMed]
- Foerster, K.I.; Huppertz, A.; Meid, A.D.; Müller, O.J.; Rizos, T.; Tilemann, L.; Haefeli, W.E.; Burhenne, J. Dried-Blood-Spot Technique to Monitor Direct Oral Anticoagulants: Clinical Validation of a UPLC-MS/MS-Based Assay. Anal. Chem. 2018, 90, 9395–9402. [Google Scholar] [CrossRef]
- Douxfils, J.; Pochet, L.; Lessire, S.; Vancraeynest, C.; Dogné, J.M.; Mullier, F. Mass spectrometry in the therapeutic drug monitoring of direct oral anticoagulants. Useful or useless? TrAC Trends Anal. Chem. 2016, 84, 41–50. [Google Scholar] [CrossRef]
- Margetić, S.; Ćelap, I.; Kes, V.B.; Lovrenčić-Huzjan, A.; Kobasić, I.; Goreta, S.Š.; Pavlović, N.; Brkljačić, D.D. Chromogenic anti-FXa assay calibrated with low molecular weight heparin in patients treated with rivaroxaban and apixaban: Possibilities and limitations. Biochem. Med. 2020, 30, 010702. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Kruszyna, Ł.; Miecznikowska, M.; Karaźniewicz-Łada, M. Application of a Novel UPLC-MS/MS Method for Analysis of Rivaroxaban Concentrations in Dried Blood Spot and Plasma Samples Collected from Patients with Venous Thrombosis. Molecules 2024, 29, 4140. [Google Scholar] [CrossRef] [PubMed]
- Jhang, R.-S.; Lin, S.-Y.; Peng, Y.-F.; Chao, H.-C.; Tsai, I.-L.; Lin, Y.-T.; Liao, H.-W.; Tang, S.-C.; Kuo, C.-H.; Jeng, J.-S. Using the PCI-IS Method to Simultaneously Estimate Blood Volume and Quantify Nonvitamin K Antagonist Oral Anticoagulant Concentrations in Dried Blood Spots. Anal Chem. 2020, 92, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G. Therapeutic monitoring of direct oral anticoagulants—An 8-year observational study. Acta Haematol. Pol. 2021, 52, 446–452. [Google Scholar] [CrossRef]
- Błoński, K. Analysis of Citations and Co-Citations of the Term ‘Word of Mouth’ Based on Publications in the Field of Social Sciences. Mark. Sci. Res. Organ. 2023, 48, 111–133. [Google Scholar] [CrossRef]
- Zupic, I.; Čater, T. Bibliometric Methods in Management and Organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- VOSviewer. VOSviewer—Visualizing Scientific Landscapes. Available online: https://www.vosviewer.com// (accessed on 23 September 2024).
- Abouzid, M.; Główka, A.K.; Karaźniewicz-Łada, M. Trend research of vitamin D receptor: Bibliometric analysis. Health Inform. J. 2021, 27, 14604582211043158. [Google Scholar] [CrossRef]
- Mian, M.K.; Sreedharan, S.; Limaye, N.S.; Hogan, C.; Darvall, J.N. Research Trends in Anticoagulation Therapy over the Last 25 Years. Semin. Thromb. Hemost. 2020, 46, 919–931. [Google Scholar] [CrossRef]
- Miao, L.; Shi, J.; Yu, H.; Song, L.; Zhu, C.; Shi, D.; Gao, J. Studies on Atrial Fibrillation and Venous Thromboembolism in the Past 20 Years: A Bibliometric Analysis Via CiteSpace and VOSviewer. J. Am. Heart Assoc. 2023, 12, e029810. [Google Scholar] [CrossRef]
- Song, W.; Ma, T.; Cheng, Q.; Wen, P.; Wu, J.; Hao, L.; Zhang, B.; Wang, Y.; Wang, Q.; Zhang, Y. Global Research Status and Trends in Venous Thromboembolism After Hip or Knee Arthroplasty From 1990 to 2021: A Bibliometric Analysis. Front. Med. 2022, 9, 837163. [Google Scholar] [CrossRef]
- Gómez García, T.; de Miguel Díez, J.; Baloira Villar, A. Pulmonary circulation: What has 2010 brought? Arch. Bronconeumol. 2011, 47 (Suppl. 1), 7–11. Available online: https://pubmed.ncbi.nlm.nih.gov/21300210/ (accessed on 14 October 2024).
- Gao, Y.; Wang, Y.; Zhai, X.; He, Y.; Chen, R.; Zhou, J.; Li, M.; Wang, Q. Publication trends of research on diabetes mellitus and T cells (1997–2016): A 20-year bibliometric study. PLoS ONE 2017, 12, e0184869. [Google Scholar] [CrossRef]
- Perzborn, E.; Roehrig, S.; Straub, A.; Kubitza, D.; Misselwitz, F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat. Rev. Drug Discov. 2011, 10, 61–75. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Errickson, J.; Kong, X.; Ali, M.A.; Chipalkatti, N.; Haymart, B.; Kaatz, S.; Krol, G.D.; Sood, S.L.; Froehlich, J.B.; et al. A Comparison of Outcomes With Apixaban, Rivaroxaban, and Warfarin for Atrial Fibrillation and/or Venous Thromboembolism. JACC Adv. 2025, 4, 101714. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Spyropoulos, A.C.; Goodman, S.G.; Spinler, S.A.; Bonaca, M.P.; Redling, T.M.; Visveswaran, G.; Sohal, S. Rivaroxaban Versus Apixaban: A Comparison Without a Simple Solution. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ramacciotti, E.; Agati, L.B.; Calderaro, D.; Aguiar, V.C.R.; Spyropoulos, A.C.; de Oliveira, C.C.C.; dos Santos, J.L.; Volpiani, G.G.; Sobreira, M.L.; Joviliano, E.E.; et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): An open-label, multicentre, randomised, controlled trial. Lancet 2022, 399, 50–59. [Google Scholar] [CrossRef]
- Testa, S.; Prandoni, P.; Paoletti, O.; Morandini, R.; Tala, M.; Dellanoce, C.; Giorgi-Pierfranceschi, M.; Betti, M.; Danzi, G.B.; Pan, A.; et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J. Thromb. Haemost. 2020, 18, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Qiu, E.; Liu, Z.; Zhu, X.; Zeng, Y. Effectiveness and safety of rivaroxaban for anticoagulation therapy in COVID-19. Saudi Med. J. 2024, 45, 341–348. [Google Scholar] [CrossRef]
- Hyland, K. Enter the dragon: China and global academic publishing. Learn. Publ. 2023, 36, 394–403. [Google Scholar] [CrossRef]
- Rohde, G. Determination of rivaroxaban–A novel, oral, direct Factor Xa inhibitor—In human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 872, 43–50. [Google Scholar] [CrossRef]
- Kuhn, J.; Gripp, T.; Flieder, T.; Dittrich, M.; Hendig, D.; Busse, J.; Knabbe, C.; Birschmann, I. UPLC-MRM Mass Spectrometry Method for Measurement of the Coagulation Inhibitors Dabigatran and Rivaroxaban in Human Plasma and Its Comparison with Functional Assays. PLoS ONE 2015, 10, e0145478. [Google Scholar] [CrossRef]
- Schmitz, E.M.H.; Boonen, K.; Heuvel, D.v.D.; van Dongen, J.; Schellings, M.W.M.; Emmen, J.M.A.; van der Graaf, F.; Brunsveld, L.; van de Kerkhof, D. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography—Tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J. Thromb. Haemost. 2014, 12, 1636–1646. [Google Scholar] [CrossRef]
- Derogis, P.B.M.; Sanches, L.R.; de Aranda, V.F.; Colombini, M.P.; Mangueira, C.L.P.; Katz, M.; Faulhaber, A.C.L.; Mendes, C.E.A.; Ferreira, C.E.d.S.; França, C.N.; et al. Determination of rivaroxaban in patient’s plasma samples by anti-Xa chromogenic test associated to High Performance Liquid Chromatography tandem Mass Spectrometry (HPLC-MS/MS). PLoS ONE 2017, 12, e0171272. [Google Scholar] [CrossRef]
| Citation Meso-Topics | Record Count | % of 6979 |
|---|---|---|
| Cardiac Arrhythmia | 3949 | 56.58 |
| Blood Clotting | 1592 | 22.81 |
| Cardiology Circulation | 170 | 2.44 |
| Vascular, Cardiac, and Thoracic Surgery | 132 | 1.89 |
| General Cardiology | 107 | 1.53 |
| Liver Diseases | 59 | 0.85 |
| Drug Delivery Chemistry | 58 | 0.83 |
| Soft Tissue, Bone, and Nerve Cancers | 56 | 0.80 |
| Virology-General | 51 | 0.73 |
| Pharmacology and Toxicology | 39 | 0.56 |
| Citation Micro-Topics | Record Count | % of 6979 |
|---|---|---|
| Atrial Fibrillation | 3916 | 56.11 |
| Pulmonary Embolism | 1098 | 15.73 |
| Antiphospholipid Syndrome | 237 | 3.40 |
| Acute Myocardial Infarction | 148 | 2.12 |
| Tissue Factor | 115 | 1.65 |
| Peripheral Arterial Disease | 98 | 1.40 |
| Coronavirus | 51 | 0.73 |
| Heparin-induced Thrombocytopenia | 51 | 0.73 |
| Portal Hypertension | 45 | 0.65 |
| Solid Dispersion | 42 | 0.60 |
| Time Period | Occurrences | Time Period | Occurrences | Time Period | Occurrences | Time Period | Occurrences |
|---|---|---|---|---|---|---|---|
| 2021–2024 | 2016–2020 | 2011–2015 | 2006–2010 | ||||
| rivaroxaban | 1624 | rivaroxaban | 2042 | rivaroxaban | 777 | venous thromboembolism | 83 |
| warfarin | 933 | warfarin | 1323 | warfarin | 466 | rivaroxaban | 81 |
| apixaban | 650 | dabigatran | 1108 | dabigatran | 442 | prevention | 66 |
| dabigatran | 599 | apixaban | 812 | apixaban | 319 | enoxaparin | 56 |
| atrial fibrillation | 495 | atrial fibrillation | 677 | venous thromboembolism | 287 | deep-vein thrombosis | 45 |
| risk | 437 | management | 514 | atrial fibrillation | 247 | double-blind | 45 |
| management | 387 | stroke | 507 | prevention | 211 | thromboprophylaxis | 43 |
| stroke | 379 | venous thromboembolism | 503 | enoxaparin | 195 | bay-59-7939 | 40 |
| safety | 372 | risk | 501 | atrial-fibrillation | 171 | dabigatran etexilate | 36 |
| venous thromboembolism | 356 | safety | 501 | thromboprophylaxis | 167 | factor-xa inhibitor | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak, K.; Kruszyna, Ł.; Wesołowska, A.; Karaźniewicz-Łada, M. Trends and Hot Spots in Research Related to Rivaroxaban: Bibliometric Analysis. Clin. Pract. 2025, 15, 190. https://doi.org/10.3390/clinpract15100190
Pawlak K, Kruszyna Ł, Wesołowska A, Karaźniewicz-Łada M. Trends and Hot Spots in Research Related to Rivaroxaban: Bibliometric Analysis. Clinics and Practice. 2025; 15(10):190. https://doi.org/10.3390/clinpract15100190
Chicago/Turabian StylePawlak, Kornel, Łukasz Kruszyna, Anna Wesołowska, and Marta Karaźniewicz-Łada. 2025. "Trends and Hot Spots in Research Related to Rivaroxaban: Bibliometric Analysis" Clinics and Practice 15, no. 10: 190. https://doi.org/10.3390/clinpract15100190
APA StylePawlak, K., Kruszyna, Ł., Wesołowska, A., & Karaźniewicz-Łada, M. (2025). Trends and Hot Spots in Research Related to Rivaroxaban: Bibliometric Analysis. Clinics and Practice, 15(10), 190. https://doi.org/10.3390/clinpract15100190







