Safety and Efficacy of Citrate Anticoagulation in Therapeutic Plasma Exchange: A Clinical Study
Abstract
1. Introduction
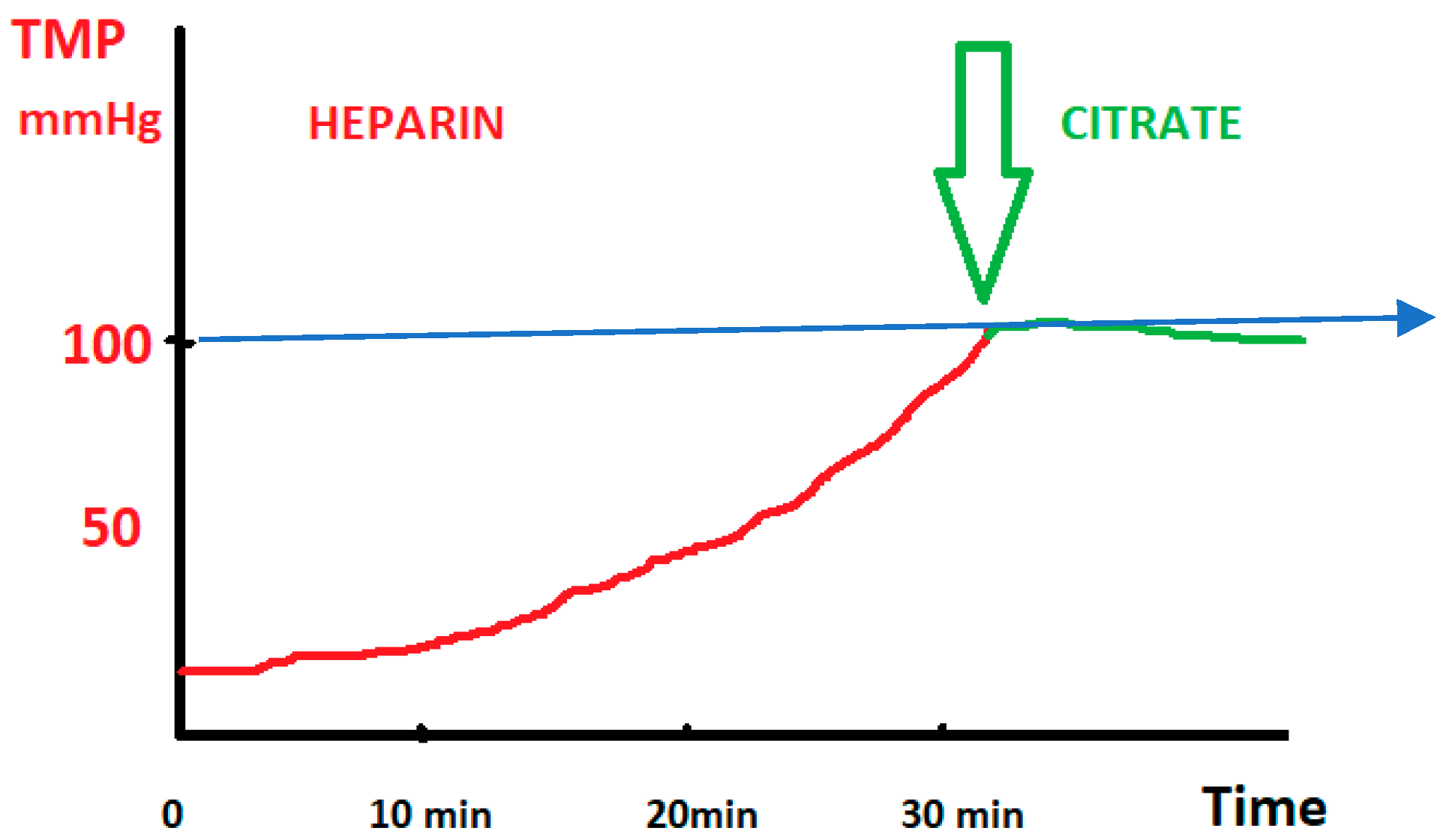
- The use of transmembrane pressure (TMP) monitoring as a guide to dynamically switch from heparin to citrate to prevent imminent circuit clotting;
- The evaluation of the safety and effectiveness of citrate anticoagulation in real-world patients across multiple clinical contexts.
2. Materials and Methods
2.1. Experimental Phase, Study Design, and Setting
2.2. Standardized Protocol for Regional Citrate Anticoagulation (RCA) in TPE
3. Statistical Analysis
4. Results
4.1. Results—Group 1
4.2. Results—Group 2
5. Discussion
6. Limitations and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellum, J.A.; Bellomo, R.; Ronco, C. (Eds.) Continuous Renal Replacement Therapy, 2nd ed.; Oxford University: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Connelly-Smith, L.; Alquist, C.R.; Aqui, N.A.; Hofmann, J.C.; Klingel, R.; Onwuemene, O.A.; Patriquin, C.J.; Pham, H.P.; Sanchez, A.P.; Schneiderman, J.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J. Clin. Apher. 2023, 38, 77–278. [Google Scholar] [CrossRef]
- Cervantes, C.E.; Bloch, E.M.; Sperati, C.J. Therapeutic Plasma Exchange: Core Curriculum 2023. Am. J. Kidney Dis. 2023, 81, 475–492. [Google Scholar] [CrossRef]
- Gertz, M.A. Acute hyperviscosity: Syndromes and management. Blood 2018, 132, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Cronin, R.E.; Reilly, R.F. Unfractionated Heparin for Hemodialysis: Still the Best Option. Semin. Dial. 2010, 23, 510–515. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mülling, N.; et al. Effect of Regional Citrate Anticoagulation vs. Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Raut, P.; Totoe, G.; Morgan, S.; Zantek, N.D. Management of systemic unfractionated heparin anticoagulation during therapeutic plasma exchange. J. Clin. Apher. 2016, 31, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.U.; Bharat, C.I.; Roberts, B.L.; Anstey, M.H. A cost comparison of regional citrate versus low-dose systemic heparin anticoagulation in continuous renal replacement therapy. Anaesth. Intensiv. Care 2019, 47, 281–287. [Google Scholar] [CrossRef]
- Betz, C.; Buettner, S.; Geiger, H.; Jung, O. Regional Citrate Anticoagulation in Therapeutic Plasma Exchange with Fresh Frozen Plasma—A Modified Protocol. Int. J. Artif. Organs 2013, 36, 803–811. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Xu, S.-Y.; Yin, L.; Yang, T.; Jin, K.; Zhang, Q.-B.; Sun, F.; Tan, D.-Y.; Xin, T.-Y.; Chen, Y.-G.; et al. Management of regional citrate anticoagulation for continuous renal replacement therapy: Guideline recommendations from Chinese emergency medical doctor consensus. Mil. Med. Res. 2023, 10, 23. [Google Scholar] [CrossRef]
- Saad, A.; Alsadi, J.; Al-Absi, D.T.; Almulla, M.; Simsekler, M.C.E.; Sadeq, A.A.; Omar, F.; Basha, M.; Khatab, I.; Abu Khater, N.; et al. An integrative risk assessment approach to enhancing patient safety in Continuous Renal Replacement Therapy (CRRT). J. Saf. Sci. Resil. 2024, 5, 344–354. [Google Scholar] [CrossRef]
- Baldwin, I.; Todd, S. Therapeutic plasma exchange in the intensive care unit and with the critically ill, a focus on clinical nursing considerations. J. Clin. Apher. 2022, 37, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, D.; Bai, K.; Xu, F.; Liu, C.; Dang, H. Novel blood product transfusion regimen to prevent clotting and citrate accumulation during continuous renal replacement therapy with regional citrate anticoagulation in children. Front. Pediatr. 2023, 11, 1086420. [Google Scholar] [CrossRef]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Panicali, L.; Facchini, M.G.; Mancini, E. Citrate Anticoagulation during Continuous Renal Replacement Therapy. Contrib. Nephrol. 2017, 190, 19–30. [Google Scholar] [CrossRef]
- Teh, S.P.; Ho, Q.Y.; Kee, Y.S.T.; Thangaraju, S.; Tan, R.Y.; Teo, S.H.; Tan, H.K.; Tan, C.S.; Choong, H.L.L.; Ng, L.C.; et al. Regional citrate anticoagulation vs systemic heparin anticoagulation for double-filtration plasmapheresis. J. Clin. Apher. 2023, 38, 16–23. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Wang, F. Segmental citrate anticoagulation for double-filtration plasmapheresis: A case report and literature review. Med. Int. 2020, 2, 18. [Google Scholar] [CrossRef]
- Barac, S.; Onofrei, R.R.; Neagoe, P.V.; Popescu, A.I.; Pantea, S.; Rață, A.L. An Observational Study on Patients with Acute Limb Ischemia and SARS-CoV-2 Infection: Early and Late Results in Limb Salvage Rate. J. Clin. Med. 2021, 10, 5083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khamis, F.; Al-Zakwani, I.; Al Hashmi, S.; Al Dowaiki, S.; Al Bahrani, M.; Pandak, N.; Al Khalili, H.; Memish, Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 2020, 99, 214–218. [Google Scholar] [CrossRef]
- Rosca, C.I.; Branea, H.S.; Sharma, A.; Nicoras, V.A.; Borza, C.; Lighezan, D.F.; Morariu, S.I.; Kundnani, N.R. Rhythm Disturbances in Post-Acute COVID-19 Syndrome in Young Men without Pre-Existing Known Cardiovascular Disease—A Case Series. Biomedicines 2023, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Kissling, S.; Legallais, C.; Pruijm, M.; Teta, D.; Vogt, B.; Burnier, M.; Rondeau, E.; Ridel, C. A new prescription model for regional citrate anticoagulation in therapeutic plasma exchanges. BMC Nephrol. 2017, 18, 81. [Google Scholar] [CrossRef]
- Jiao, J.; Yu, Y.; Wei, S.; Tian, X.; Yang, X.; Feng, S.; Li, Y.; Sun, S.; Zhang, P.; Bai, M. Heparin anticoagulation versus regional citrate anticoagulation for membrane therapeutic plasma exchange in patients with increased bleeding risk. Ren. Fail. 2023, 45, 2210691. [Google Scholar] [CrossRef] [PubMed]
- Schriner, J.B.; Van Gent, J.M.D.; Meledeo, M.A.; Olson, S.D.; Cotton, B.A.; Cox, C.S.J.; Gill, B.S. Impact of Transfused Citrate on Pathophysiology in Massive Transfusion. Crit. Care Explor. 2023, 5, e0925. [Google Scholar] [CrossRef] [PubMed]
- Szamosfalvi, B.; Puri, V.; Sohaney, R.; Wagner, B.; Riddle, A.; Dickinson, S.; Napolitano, L.; Heung, M.; Humes, D.; Yessayan, L. Regional Citrate Anticoagulation Protocol for Patients with Presumed Absent Citrate Metabolism. Kidney360 2021, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, M.Z.; Laaribi, I.; Benaini, I.; Yeznasni, A.; Berrajaa, S.; Oujidi, Y.; Bkiyar, H.; Abda, N. Therapeutic plasma exchange in the treatment of COVID-19 induced cytokine storm: The first Moroccan experience. BMC Infect Dis. 2023, 23, 829. [Google Scholar]

| Age-mean | 49.39 (±12.63) |
| Weight | 86.8 (±16.92) |
| Gender | |
| Male | 14 |
| Female | 9 |
| Comorbidities | |
| COVID-19 | 19 |
| Myasthenia Gravis | 1 |
| Polyradiculonevritis | 2 |
| Hypertriglyceridemia | 1 |
| Prismaflex with TPE 2000 plasma filter | 15 |
| HF 440 with Granopen 60 plasma filter | 8 |
| Age-Mean | 48.3 |
|---|---|
| Weight | 80.3 |
| Gender | |
| Male | 16 |
| Female | 13 |
| Comorbidities | |
| COVID-19 | 13 |
| Myasthenia Gravis | 3 |
| Polyradiculonevritis | 10 |
| Hypertriglyceridemia | 3 |
| Prismaflex with TPE 2000 plasma filter | 18 |
| HF 440 with Granopen 60 plasma filter | 11 |
| Variable | Average/Median Before TPE | Average/Median After TPE | p-Value for Median |
|---|---|---|---|
| IL-6, pg/mL | 799/129 | 480/79 | 0.0003 |
| Ferritin, µg/L | 2364/1529 | 1660/1120 | <0.0001 |
| D-dimers, µg/mL | 5.18/1.9 | 3.50/1.57 | 0.0024 |
| CRP, mg/L | 122/88 | 87/60 | 0.0001 |
| LDH, U/L | 579/512 | 461/419 | <0.0001 |
| PCT, ng/mL | 2.28/0.33 | 2.26/0.42 | 0.34 |
| Fibrinogen, g/L | 4.90/4.23 | 3.42/3.26 | <0.0001 |
| ESR, mm/h | 46/35 | 22/15 | <0.0001 |
| Leucocytes, ×103/µL | 14/13 | 16/15.5 | 0.19 |
| % Lymphocytes | 7.66/5.2 | 8.14/5.5 | 0.53 |
| Lymph abs, ×103/µL | 0.96/0.69 | 1.11/0.80 | 0.003 |
| TAM, mmHg | 80.9/77 | 81.9/80 | 0.9 |
| Temperature, °C | 36.5/36.4 | 36.6/36.4 | 0.26 |
| BUN, mg/dL | 72.7/58.5 | 74.2/62 | 0.29 |
| Creatinine, mg/dL | 1.02/0.8 | 1.08/0.81 | 0.98 |
| Variable | Average/Median Before TPE | Average/Median After TPE | p-Value for Median |
|---|---|---|---|
| TRIGLICERIDE | 3680 mg/dL | 969 mg/dL | p < 0.005 |
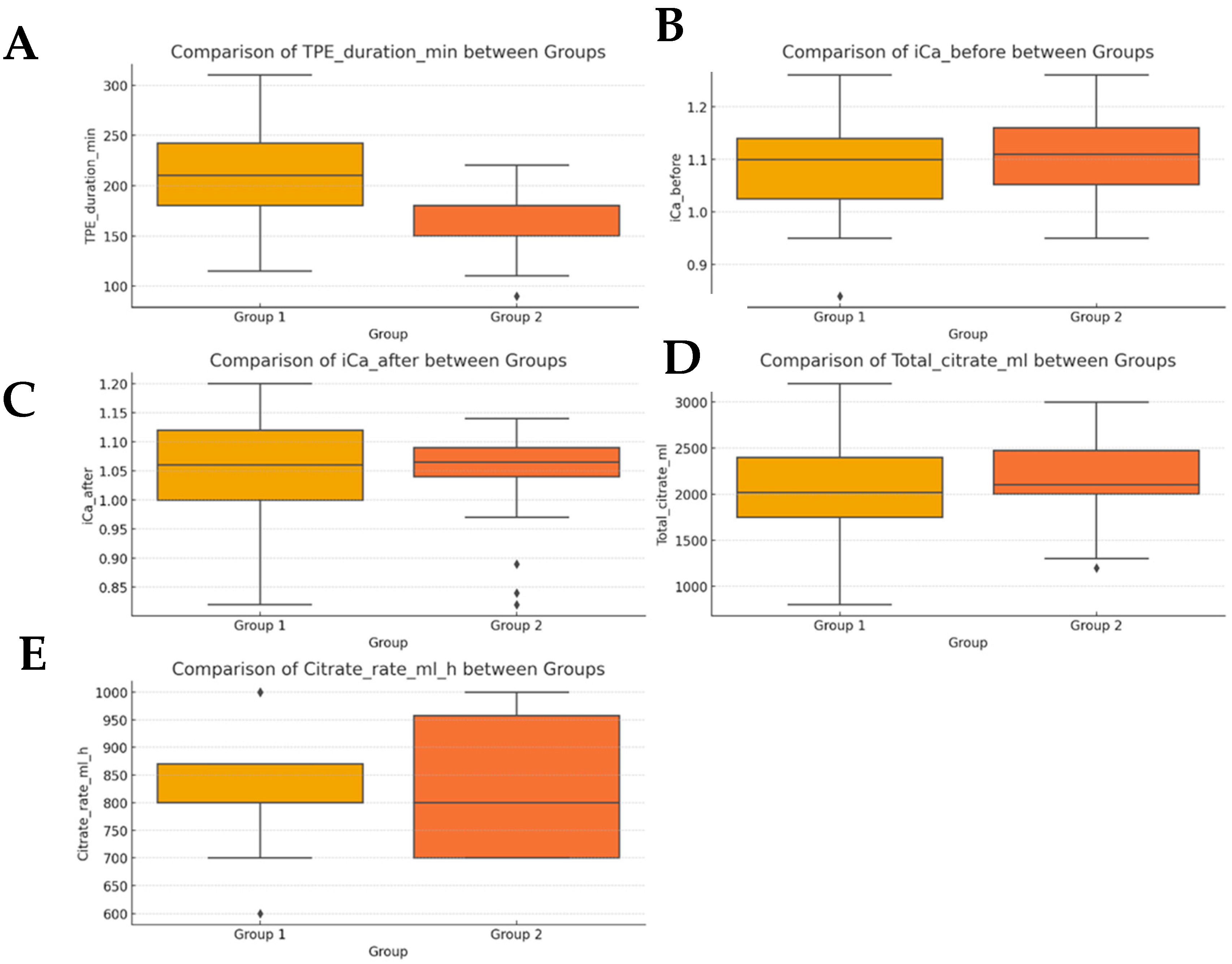
| Parameter | Group 1 (Mean ± SD) | Group 2 (Mean ± SD) |
|---|---|---|
| Age (years) | 49.4 ± 12.6 | 48.3 ± 12.1 |
| Weight (kg) | 86.8 ± 16.9 | 80.3 ± 12.1 |
| TPE duration (min) | 208.0 ± 5 | 164.0 ± 7.3 |
| Ionized calcium (before) | 1.09 ± 0.10 mmol/L | 1.11 ± 0.08 mmol/L |
| Ionized calcium (after) | 1.05 ± 0.10 mmol/L | 1.04 ± 0.08 mmol/L |
| Total citrate (mL) | 2030 ± 21.3 | 2206 ± 18.6 |
| Citrate rate (mL/h) | 826 ± 15.3 | 831 ± 17.4 |
| Variable | Mann–Whitney U p-Value | T-Test p-Value |
|---|---|---|
| TPE duration (min) | <0.001 | <0.001 |
| Ionized Ca (Before) | 0.220 | 0.188 |
| Ionized Ca (After) | 0.737 | 0.754 |
| Total citrate (mL) | 0.121 | 0.140 |
| Citrate rate (mL/h) | 0.942 | 0.870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gîndac, C.; Poroșnicu, T.M.; Kundnani, N.R.; Sgăvârdea, N.; Bârsac, C.R.; Meche, V.; Băloi, A.; Nussbaum, L.A.; Bedreag, O.H.; Săndesc, D.; et al. Safety and Efficacy of Citrate Anticoagulation in Therapeutic Plasma Exchange: A Clinical Study. Clin. Pract. 2025, 15, 172. https://doi.org/10.3390/clinpract15100172
Gîndac C, Poroșnicu TM, Kundnani NR, Sgăvârdea N, Bârsac CR, Meche V, Băloi A, Nussbaum LA, Bedreag OH, Săndesc D, et al. Safety and Efficacy of Citrate Anticoagulation in Therapeutic Plasma Exchange: A Clinical Study. Clinics and Practice. 2025; 15(10):172. https://doi.org/10.3390/clinpract15100172
Chicago/Turabian StyleGîndac, Ciprian, Tamara Mirela Poroșnicu, Nilima Rajpal Kundnani, Nicoleta Sgăvârdea, Claudiu Rafael Bârsac, Vlad Meche, Adelina Băloi, Laura Alexandra Nussbaum, Ovidiu Horea Bedreag, Dorel Săndesc, and et al. 2025. "Safety and Efficacy of Citrate Anticoagulation in Therapeutic Plasma Exchange: A Clinical Study" Clinics and Practice 15, no. 10: 172. https://doi.org/10.3390/clinpract15100172
APA StyleGîndac, C., Poroșnicu, T. M., Kundnani, N. R., Sgăvârdea, N., Bârsac, C. R., Meche, V., Băloi, A., Nussbaum, L. A., Bedreag, O. H., Săndesc, D., & Păpurică, M. (2025). Safety and Efficacy of Citrate Anticoagulation in Therapeutic Plasma Exchange: A Clinical Study. Clinics and Practice, 15(10), 172. https://doi.org/10.3390/clinpract15100172








