Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder
Abstract
1. Introduction
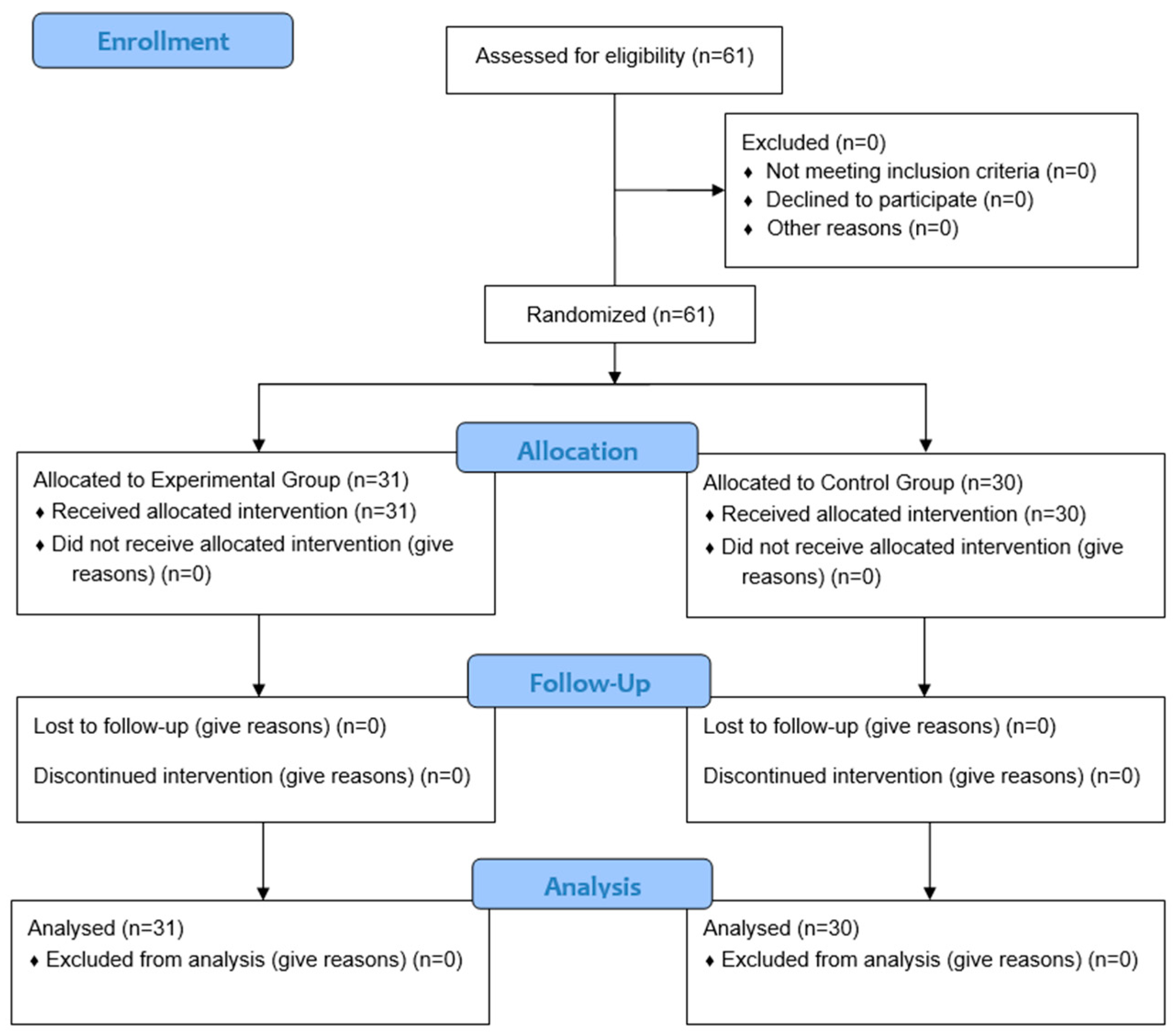
2. Materials and Methods
2.1. Study Design
2.2. The Study Population
2.3. Assessment
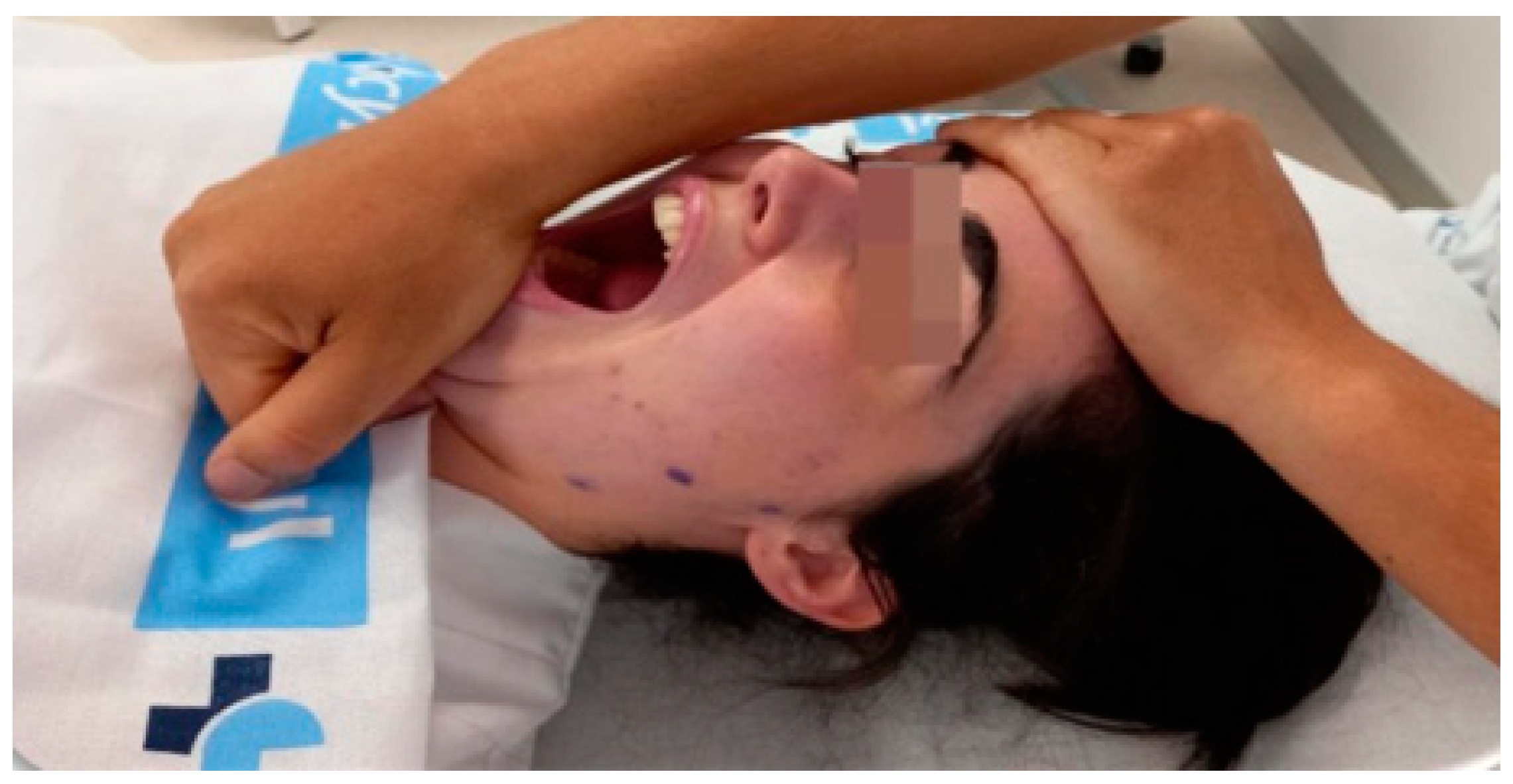
2.4. Interventions
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Results of the Outcome Variables
4. Discussion
4.1. Discussion of the Results
4.2. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; Dworkin, S.F. Diagnostic Criteria for Temporomandibular Disorders (DC/TMDS) for Clinical and Research Applications: Recommendations of the International RDC/TMDS Consortium Network and Orofacial Pain Special Interest Group. J. Oral. Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Bair, E.; Fillingim, R.B.; Gonzalez, Y.; Gordon, S.M.; Lim, P.F.; Slade, G.D. Clinical orofacial characteristics associated with risk of first-onset TMDS: The OPPERA prospective cohort study. J. Pain 2013, 14 (Suppl. S12), T33–T50. [Google Scholar] [CrossRef]
- Oral, K.; Bal Küçük, B.; Ebeoğlu, B.; Dinçer, S. Etiology of temporomandibular disorder pain. Agri 2009, 21, 89–94. [Google Scholar] [PubMed]
- Fricton, J.R.; Ouyang, W.; Nixdorf, D.R.; Schiffman, E.L.; Velly, A.M.; Look, J.O. Critical Appraisal of Methods Used in Randomized Controlled Trials of Treatments for Temporomandibular Disorders. J. Orofac. Pain 2010, 24, 139. [Google Scholar]
- Cooper, B.C.; Kleinberg, I. Examination of a Large Patient Population for the Presence of Symptoms and Signs of Temporomandibular Disorders. CRANIO 2007, 25, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.; Garg, K.; Gokhale, A.; Heaphy, N. Temporomandibular Disorder: A practical guide for dental practitioners in diagnosis and management. Aust. Dent. J. 2020, 65, 172–180. [Google Scholar] [CrossRef]
- Engel, G.L. The Need for a New Medical Model: A Challenge for Biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, J.Y.; Huh, K.H.; Park, J.W. Long-term Changes of Temporomandibular Joint Osteoarthritis on Computed Tomography. Sci. Rep. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Wu, M.; Almeida, F.; Friesen, R. A Systematic Review on the Association Between Clinical Symptoms and CBCT Findings in Symptomatic TMJ Degenerative Joint Disease. J. Oral Facial Pain Headache 2021, 35, 332–345. [Google Scholar] [CrossRef]
- Slade, G.D.; Ohrbach, R.; Greenspan, J.D.; Fillingim, R.B.; Bair, E.; Sanders, A.E.; Maixner, W. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res. 2016, 95, 1084. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and Treatment of Temporomandibular Disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Fernández-De-Las-Peñas, C.; Galán-Del-Río, F.; Alonso-Blanco, C.; Jiménez-García, R.; Arendt-Nielsen, L.; Svensson, P. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disoders. J. Pain 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Simons, D.; Travell, J.; Simons, L.S. Myofascial Pain and Dysfunction, 2nd ed.; Williams and Wilkins: Baltimore, MD, USA, 1999; pp. 1–1207. [Google Scholar]
- Poveda-Roda, R.; Bagán, J.V.; Sanchis, J.M.; Carbonell, E. Temporomandibular disorders. A case-control study. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e794. [Google Scholar] [CrossRef][Green Version]
- Martinez-Merinero, P.; Nuñez-Nagy, S.; Achalandabaso-Ochoa, A.; Fernandez-Matias, R.; Pecos-Martin, D.; Gallego-Izquierdo, T. Relationship between Forward Head Posture and Tissue Mechanosensitivity: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 634. [Google Scholar] [CrossRef]
- Gil-Martínez, A.; Paris-Alemany, A.; López-de-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMDS): Challenges and solutions. J. Pain Res. 2018, 11, 571–587. [Google Scholar] [CrossRef]
- Ingawalé, S.; Goswami, T. Temporomandibular joint: Disorders, treatments, and biomechanics. Ann. Biomed. Eng. 2009, 37, 976–996. [Google Scholar] [CrossRef]
- Medlicott, M.S.; Harris, S.R. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys. Ther. 2006, 86, 955–973. [Google Scholar] [CrossRef]
- Vigotsky, A.D.; Bruhns, R.P. The Role of Descending Modulation in Manual Therapy and Its Analgesic Implications: A Narrative Review. Pain Res. Treat 2015, 2015, 292805. [Google Scholar] [CrossRef]
- Shoohanizad, E.; Garajei, A.; Enamzadeh, A.; Yari, A. Nonsurgical management of temporomandibular joint autoimmune disorders. AIMS Public Health 2019, 6, 554. [Google Scholar] [CrossRef]
- De Melo, L.A.; Bezerra de Medeiros, A.K.; Campos MF, T.P.; Bastos Machado de Resende, C.M.; Barbosa GA, S.; de Almeida, E.O. Manual Therapy in the Treatment of Myofascial Pain Related to Temporomandibular Disorders: A Systematic Review. J. Oral Facial Pain Headache 2020, 34, 141–148. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G.; CONSORT. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Touche RLa Pardo-Montero, J.; Cuenca-Martínez, F.; Visscher, C.M.; Paris-Alemany, A.; López-De-uralde-villanueva, I. Cross-Cultural Adaptation and Psychometric Properties of the Spanish Version of the Tampa Scale for Kinesiophobia for Temporomandibular Disorders. J. Clin. Med. 2020, 9, 2831. [Google Scholar] [CrossRef]
- Chaitow, L.; Crenshaw, K. Muscle Energy Techniques, 3rd ed.; Churchill Livingstone: Edinburgh, UK, 2006; p. 244. [Google Scholar]
- Heredia Rizo, A.M.; Pascual-Vaca, Á.O.; Cabello, M.A.; Blanco, C.R.; Pozo, F.P.; Carrasco, A.L. Immediate effects of the suboccipital muscle inhibition technique in craniocervical posture and greater occipital nerve mechanosensitivity in subjects with a history of orthodontia use: A randomized trial. J. Manip. Physiol Ther. 2012, 35, 446–453. [Google Scholar] [CrossRef]
- Bernal-Utrera, C.; Gonzalez-Gerez, J.J.; Anarte-Lazo, E.; Rodriguez-Blanco, C. Manual therapy versus therapeutic exercise in non-specific chronic neck pain: A randomized controlled trial. Trials 2020, 21, 1–6. [Google Scholar] [CrossRef]
- Calixtre, L.B.; Oliveira, A.B.; Alburquerque-Sendín, F.; Armijo-Olivo, S. What is the minimal important difference of pain intensity, mandibular function, and headache impact in patients with temporomandibular disorders? Clinical significance analysis of a randomized controlled trial. Musculoskelet Sci. Pract. 2020, 46, 102108. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences. In Statistical Power Analysis for the Behavioral Sciences; Taylor & Francis: Abingdon, VA, USA, 2013. [Google Scholar]
- Trivedi, P.; Bhatt, P.; Dhanakotti, S.; Nambi, G. Comparison of muscle energy technique and myofascial release technique on pain and range of motion in patients with temporomandibular joint dysfunction: A randomized controlled study. Int. J. Physiother. Res. 2016, 4, 1788–1792. [Google Scholar] [CrossRef]
- Gouw, S.; de Wijer, A.; Kalaykova, S.I.; Creugers, N.H.J. Masticatory muscle stretching for the management of sleep bruxism: A randomised controlled trial. J. Oral Rehabil. 2018, 45, 770–776. [Google Scholar] [CrossRef]
- Kuć, J.; Szarejko, K.D.; Gołębiewska, M. Evaluation of Soft Tissue Mobilization in Patients with Temporomandibular Disorder-Myofascial Pain with Referral. Int. J. Environ. Res. Public Health 2020, 17, 9576. [Google Scholar] [CrossRef]
- American Academy of Orofacial Pain; McNeill, C. Epidemiology Temporomandibular Disorders: Guidelines for Classification, Assessment and Management, 2nd ed.; Quintessence: Chicago, IL, USA, 1993; pp. 19–22. [Google Scholar]
- Amorim, C.S.M.; Espirito Santo, A.S.; Sommer, M.; Marques, A.P. Effect of Physical Therapy in Bruxism Treatment: A Systematic Review. J. Manip. Physiol. Ther. 2018, 41, 389–404. [Google Scholar] [CrossRef]
- Martins, W.R.; Blasczyk, J.C.; de Oliveira MA, F.; Gonçalves KF, L.; Bonini-Rocha, A.C.; Dugailly, P.M.; de Oliveira, R.J. Efficacy of musculoskeletal manual approach in the treatment of temporomandibular joint disorder: A systematic review with meta-analysis. Man. Ther. 2016, 21, 10–17. [Google Scholar] [CrossRef]
- Busse, J.W.; Casassus, R.; Carrasco-Labra, A.; Durham, J.; Mock, D.; Zakrzewska, J.M.; Agoritsas, T. Management of chronic pain associated with temporomandibular disorders: A clinical practice guideline. BMJ 2023, 383, e076227. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Wolford, L.M.; Ellis, E.; Neff, A. The hierarchy of different treatments for arthrogenous temporomandibular disorders: A network meta-analysis of randomized clinical trials. J. Cranio-Maxillofac. Surg. 2020, 48, 9–23. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Conti, P.C.R.; Alyahya, A.; Alkebsi, K.; Elsharkawy, A.; Christidis, N. The hierarchy of different treatments for myogenous temporomandibular disorders: A systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac. Surg. 2022, 26, 519–533. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man. Ther. 2008, 14, 531. [Google Scholar] [CrossRef]
- Voogt, L.; de Vries, J.; Meeus, M.; Struyf, F.; Meuffels, D.; Nijs, J. Analgesic effects of manual therapy in patients with musculoskeletal pain: A systematic review. Man. Ther. 2015, 20, 250–256. [Google Scholar] [CrossRef]
- Ahmed, M.; Grillo, M.; Taebi, A.; Kaya, M.; Thibbotuwawa Gamage, P. A Comprehensive Analysis of Trapezius Muscle EMG Activity in Relation to Stress and Meditation. BioMedInformatics 2024, 4, 1047–1058. [Google Scholar] [CrossRef]
- Bartsch, T.; Goadsby, P.J. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 2003, 126 Pt 8, 1801–1813. [Google Scholar] [CrossRef]
- Zafar, H.; Nordh, E.; Eriksson, P.O. Temporal coordination between mandibular and head-neck movements during jaw opening-closing tasks in man. Arch. Oral Biol. 2000, 45, 675–682. [Google Scholar] [CrossRef]
| Outcome | EG (n = 31) | CG (n = 30) | p-Value |
|---|---|---|---|
| ± SD | ± SD | Sig. | |
| Age (Mean, SD) | 40.13 ± 10.28 | 38.47 ± 11.39 | 0.551 |
| Female, n (%) | 25 (80.64%) | 25 (83.33%) | 0.785 |
| Weight (kg) | 69.48 ± 15.43 | 64.93 ± 14.25 | 0.237 |
| Height (cm) | 164.14 ± 8.09 | 160.18 ± 8.90 | 0.074 |
| BMI (kg/m2) | 25.73 ± 5.27 | 25.25 ± 4.98 | 0.717 |
| VAS (mean, SD) | 5.69 ± 1.95 | 5.44 ± 2.67 | 0.681 |
| PTP, Trapezius R (kg/cm2) | 0.90 ± 0.44 | 1.03 ± 0.90 | 0.470 |
| PTP, Upper Trapezius L (kg/cm2) | 0.92 ± 0.43 | 1.00 ± 0.70 | 0.576 |
| PTP, Masseter R (kg/cm2) | 0.61 ± 0.30 | 0.63 ± 0.40 | 0.799 |
| PTP, Masseter L (kg/cm2) | 0.58 ± 0.28 | 0.65 ± 0.39 | 0.438 |
| PTP, External Pterygoid R (kg/cm2) | 0.66 ± 0.34 | 0.65 ± 0.40 | 0.900 |
| PTP, External Pterygoid L (kg/cm2) | 0.71 ± 0.35 | 0.72 ± 0.42 | 0.909 |
| PTP, Digastric Muscle R (kg/cm2) | 0.55 ± 0.27 | 0.54± 0.34 | 0.987 |
| PTP, Digastric Muscle L (kg/cm2) | 0.53 ± 0.26 | 0.58 ± 0.36 | 0.575 |
| ROM, Opening (mm) | 38.88 ± 9.08 | 32.47 ± 8.80 | 0.007 |
| ROM, Deviation R (mm) | 8.56 ± 2.3 | 7.82 ± 2.93 | 0.271 |
| ROM, Deviation L (mm) | 8.82 ± 2.4 | 7.46 ± 2.74 | 0.044 |
| Kinesophobia (mean. SD) | 30.32 ± 6.77 | 31.67 ± 7.19 | 0.455 |
| Experimental Group (n = 31) | Control Group (n = 30) | p. c | d | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | p. a | Pre | Post | p. b | |||
| ± SD | ± SD | Sig. | ± SD | ± SD | Sig. | Sig. | Sig. | |
| VAS (Mean, SD) | 5.69 ± 1.95 | 5.20 ± 1.81 | <0.001 | 5.44 ± 2.67 | 5.58 ± 2.59 | 0.013 | <0.001 | 0.427 |
| PTP, Trapezius R (kg/cm2) | 0.90 ± 0.44 | 1.33 ± 0.71 | 0.025 | 1.03 ± 0.90 | 1.04 ± 0.98 | 0.390 | <0.001 | 0.300 |
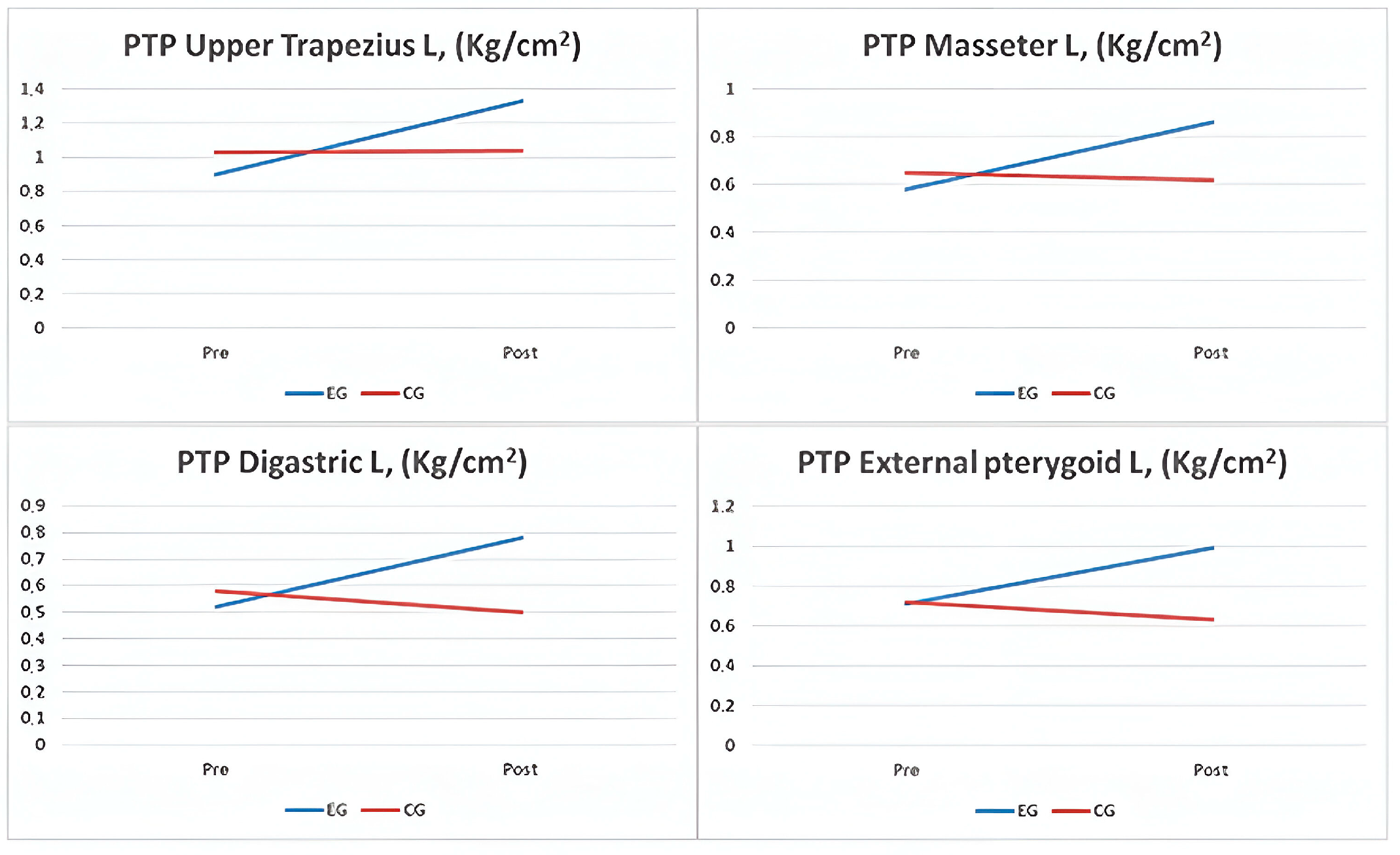
| PTP, Upper Trapezius L (kg/cm2) | 0.92 ± 0.43 | 1.30 ± 0.70 | 0.011 | 1.00 ± 0.70 | 0.94 ± 0.80 | 0.047 | <0.001 | 0.322 |
| PTP, Masseter R (kg/cm2) | 0.61 ± 0.30 | 0.89 ± 0.45 | 0.009 | 0.63 ± 0.40 | 0.60 ± 0.44 | 0.087 | <0.001 | 0.184 |
| PTP, Masseter L (kg/cm2) | 0.58 ± 0.28 | 0.86 ± 0.44 | 0.014 | 0.65 ± 0.39 | 0.62 ± 0.43 | 0.086 | <0.001 | 0.228 |
| PTP, External Pterygoid R (kg/cm2) | 0.66 ± 0.34 | 0.92 ± 0.52 | 0.012 | 0.65 ± 0.40 | 0.62 ± 0.45 | 0.075 | <0.001 | 0.234 |
| PTP, External Pterygoid L (kg/cm2) | 0.71 ± 0.35 | 0.99 ± 0.64 | 0.009 | 0.72 ± 0.42 | 0.63 ± 0.43 | <0.001 | <0.001 | 0.371 |
| PTP, Digastric Muscle R (kg/cm2) | 0.55 ± 0.27 | 0.78 ± 0.45 | 0.017 | 0.54± 0.34 | 0.56 ± 0.43 | 0.296 | <0.001 | 0.215 |
| PTP, Digastric Muscle L (kg/cm2) | 0.52 ± 0.26 | 0.78 ± 0.41 | 0.013 | 0.58 ± 0.36 | 0.50 ± 0.41 | 0.004 | <0.001 | 0.238 |
| ROM, Opening (mm) * | 38.77 (34.04–45.83) | 50.49 (46.27–51.87) | <0.001 | 33.39 (23.87–39.25) | 32.45 (24.65–37.61) | 0.149 | <0.001 | 0.742 |
| ROM, Deviation R (mm) | 8.56 ± 2.3 | 10.35 ± 1.96 | <0.001 | 7.82 ± 2.93 | 8.09 ± 2.57 | 0.275 | <0.001 | 0.453 |
| ROM, Deviation L (mm) * | 9.19 (6.98–10.22) | 11.09 (9.2–12.13) | <0.001 | 7.15 (5.62–8.86) | 7.7 (5.30–9.43) | 0.242 | <0.001 | 0.602 |
| Kinesophobia (Mean, SD) | 30.32 ± 6.77 | 23.58 ± 5.81 | <0.001 | 31.67 ± 7.19 | 30.83 ± 8.35 | 0.156 | <0.001 | 0.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Vera, A.; Polo-Ferrero, L.; Puente-González, A.S.; Méndez-Sánchez, R.; Blanco-Rueda, J.A. Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder. Clin. Pract. 2024, 14, 2568-2579. https://doi.org/10.3390/clinpract14060202
Márquez-Vera A, Polo-Ferrero L, Puente-González AS, Méndez-Sánchez R, Blanco-Rueda JA. Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder. Clinics and Practice. 2024; 14(6):2568-2579. https://doi.org/10.3390/clinpract14060202
Chicago/Turabian StyleMárquez-Vera, Antonio, Luis Polo-Ferrero, Ana Silvia Puente-González, Roberto Méndez-Sánchez, and José Antonio Blanco-Rueda. 2024. "Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder" Clinics and Practice 14, no. 6: 2568-2579. https://doi.org/10.3390/clinpract14060202
APA StyleMárquez-Vera, A., Polo-Ferrero, L., Puente-González, A. S., Méndez-Sánchez, R., & Blanco-Rueda, J. A. (2024). Immediate Effects of the Mandibular Muscle Energy Technique in Adults with Chronic Temporomandibular Disorder. Clinics and Practice, 14(6), 2568-2579. https://doi.org/10.3390/clinpract14060202






