Novel Challenges and Opportunities for Anesthesia and Perioperative Care in Microvascular Flap Surgery: A Narrative Review
Abstract
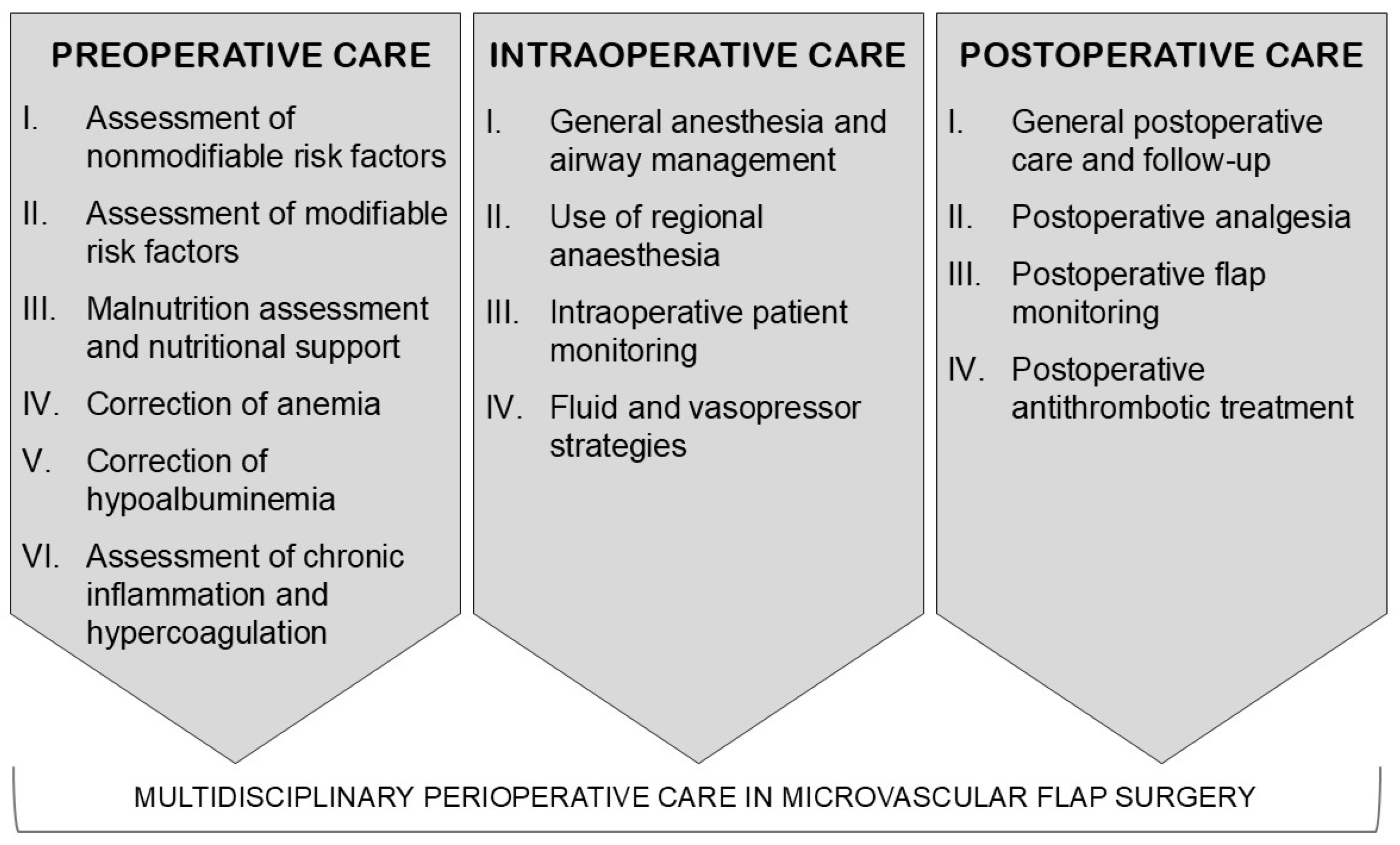
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Preoperative Assessment of Non-Modifiable Risk Factors
3.2. Preoperative Assessment of Comorbidities and Modifiable Risk Factors
3.3. Preoperative Malnutrition Risk Assessment and Nutritional Support
3.4. Correction of Preoperative Anemia
3.5. Correction of Hypoalbuminemia
3.6. Other Preoperative Biomarkers
3.7. General Anesthesia (GA) and Airway Management
3.8. Use of Regional Anesthesia
3.9. Intraoperative Monitoring and Surgical Aspects
3.10. Fluids, Vasopressors, and Red Blood Cell Transfusions
3.11. General Postoperative Care Principles and Postoperative Follow-Up
3.12. Postoperative Pain Control
3.13. Postoperative Antithrombotic Treatment
4. Areas of Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Min, K.; Hong, J.P.; Suh, H.P. Risk Factors for Partial Flap Loss in a Free Flap: A 12-Year Retrospective Study of Anterolateral Thigh Free Flaps in 303 Lower Extremity Cases. Plast. Reconstr. Surg. 2022, 150, 1071e–1081e. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.C.; Lin Tay, S.K.; Al Deek, N.F. Principles and techniques of microvascular surgery. Plast. Surg. 2024, 25, 414–415. [Google Scholar]
- Lese, I.; Biedermann, R.; Constantinescu, M.; Grobbelaar, A.O.; Olariu, R. Predicting risk factors that lead to free flap failure and vascular compromise: A single unit experience with 565 free tissue transfers. J. Plast. Reconstr. Aesthet. Surg. 2021, 4, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Rocans, R.P.; Zarins, J.; Bine, E.; Deksnis, R.; Citovica, M.; Donina, S.; Mamaja, B. The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery. J. Clin. Med. 2023, 12, 4794. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, J.P.; Suh, H.P.; Park, J.Y.; Kim, D.H.; Ha, S.; Lee, J.; Hwang, J.H.; Kim, Y.K. Prognostic Nutritional Index is a Predictor of Free Flap Failure in Extremity Reconstruction. Nutrients 2020, 12, 562. [Google Scholar] [CrossRef]
- Ishimaru, M.; Ono, S.; Suzuki, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. Risk Factors for Free Flap Failure in 2,846 Patients with Head and Neck Cancer: A National Database Study in Japan. J. Oral. Maxillofac. Surg. 2016, 74, 1265–1270. [Google Scholar] [CrossRef]
- Bishop, J.L.; Vasudev, M.; Garcia, N.; Heslop, G.; Pham, T.T.; Hicks, M.D.; Chowdhury, F.; Grayson, J.W.; Goddard, J.A.; Tjoa, T.; et al. Effect of Perioperative Antithrombotics on Head and Neck Microvascular Free Flap Survival After Anastomotic Revision. Otolaryngol. Head Neck Surg. 2023, 168, 1353–1361. [Google Scholar] [CrossRef]
- Stevens, M.N.; Freeman, M.H.; Shinn, J.R.; Kloosterman, N.; Carr, S.; Mannion, K.; Rohde, S.L. Preoperative Predictors of Free Flap Failure. Otolaryngol. Head Neck Surg. 2022, 168, 180–187. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chuang, H.C.; Lin, Y.T.; Lu, H.; Chen, W.C.; Fang, F.M.; Chien, C.Y. Clinical impact of albumin in advanced head and neck cancer patients with free flap reconstruction-a retrospective study. PeerJ 2018, 6, e4490. [Google Scholar] [CrossRef]
- Drizlionoka, K.; Zariņš, J.; Ozoliņa, A.; Ņikitina-Zaķe, L.; Mamaja, B. Polymorphism rs2066865 in the Fibrinogen Gamma Chain (FGG) Gene Increases Plasma Fibrinogen Concentration and Is Associated with an Increased Microvascular Thrombosis Rate. Med. 2019, 55, 563. [Google Scholar] [CrossRef]
- Motakef, S.; Mountziaris, P.M.; Ismail, I.K.; Agag, R.L.; Patel, A. Emerging paradigms in perioperative management for microsurgical free tissue transfer: Review of the literature and evidence-based guidelines. Plast. Reconstr. Surg. 2015, 135, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Dort, J.C.; Farwell, D.G.; Findlay, M.; Huber, G.F.; Kerr, P.; Shea-Budgell, M.A.; Simon, C.; Uppington, J.; Zygun, D.; Ljungqvist, O.; et al. Optimal Perioperative Care in Major Head and Neck Cancer Surgery with Free Flap Reconstruction: A Consensus Review and Recommendations From the Enhanced Recovery After Surgery Society. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Hirsch, B.P.; Shah, A.A.; Reid, M.A.; Thomson, J.G. Mild intraoperative hypothermia reduces free tissue transfer thrombosis. J. Reconstr. Microsurg. 2011, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sun, Z.; Huang, N.; Hu, Z.; Cao, A.; Shen, Z.; Shao, Z.; Yu, P.; Miao, C.; Wu, J. Epidural Combined with General Anesthesia versus General Anesthesia Alone in Patients Undergoing Free Flap Breast Reconstruction. Plast. Reconstr. Surg. 2016, 137, 502e–509e. [Google Scholar] [CrossRef]
- Sayal, N.R.; Militsakh, O.; Aurit, S.; Hufnagle, J.; Hubble, L.; Lydiatt, W.; Lydiatt, D.; Lindau, R.; Coughlin, A.; Osmolak, A.; et al. Association of multimodal analgesia with perioperative safety and opioid use following head and neck microvascular reconstruction. Head Neck 2020, 42, 2887–2895. [Google Scholar] [CrossRef]
- Dawoud, B.E.S.; Kent, S.; Tabbenor, O.; Markose, G.; Java, K.; Kyzas, P. Does anticoagulation improve outcomes of microvascular free flap reconstruction following head and neck surgery: A systematic review and meta-analysis. Br. J. Oral. Maxillofac. Surg. 2022, 60, 1292–1302. [Google Scholar] [CrossRef]
- Chorath, K.; Go, B.; Shinn, J.R.; Mady, L.J.; Poonia, S.; Newman, J.; Cannady, S.; Revenaugh, P.C.; Moreira, A.; Rajasekaran, K. Enhanced recovery after surgery for head and neck free flap reconstruction: A systematic review and meta-analysis. Oral. Oncol. 2021, 113, 105117. [Google Scholar] [CrossRef]
- Sanati-Mehrizy, P.; Massenburg, B.B.; Rozehnal, J.M.; Ingargiola, M.J.; Hernandez Rosa, J.; Taub, P.J. Risk Factors Leading to Free Flap Failure: Analysis from the National Surgical Quality Improvement Program Database. J. Craniofac. Surg. 2016, 20, 1956–1964. [Google Scholar] [CrossRef]
- Bui, D.T.; Cordeiro, P.G.; Hu, Q.Y.; Disa, J.J.; Pusic, A.; Mehrara, B.J. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast. Reconstr. Surg. 2007, 119, 2092–2100. [Google Scholar] [CrossRef]
- Ooms, M.; Puladi, B.; Houschyar, K.S.; Heitzer, M.; Rashad, A.; Bickenbach, J.; Hölzle, F.; Modabber, A. Smoking and microvascular free flap perfusion in head and neck reconstruction: Radial free forearm flaps and anterolateral thigh flaps. Sci. Rep. 2022, 12, 13902. [Google Scholar] [CrossRef]
- Wong, J.; An, D.; Urman, R.D.; Warner, D.O.; Tønnesen, H.; Raveendran, R.; Abdullah, H.R.; Pfeifer, K.; Maa, J.; Finegan, B.; et al. Society for Perioperative Assessment and Quality Improvement (SPAQI) Consensus Statement on Perioperative Smoking Cessation. Anesth. Analg. 2019, 131, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Stechmiller, J.K. Understanding the role of nutrition and wound healing. Nutr. Clin. Pract. 2010, 25, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.Z.; Wang, Y.M.; Jeng, S.F.; Lee, Y.C.; Chen, T.S.; Su, S.Y.; Huang, C.C.; Lam, C.F. Intraoperative Enteral Nutrition Feeding in Free-Flap Healing after Reconstruction Surgery for Head and Neck Cancers. Otolaryngol. Head Neck Surg. 2023, 169, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K.; Kondrup, J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Sigurdsson, G.H. Perioperative fluid management in microvascular surgery. J. Reconstr. Microsurg. 1995, 11, 57–65. [Google Scholar] [CrossRef]
- Hill, J.B.; Patel, A.; Del Corral, G.A.; Sexton, K.W.; Ehrenfeld, J.M.; Guillamondegui, O.D.; Shack, R.B. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann. Plast. Surg. 2012, 69, 364–367. [Google Scholar] [CrossRef]
- Sigaux, N.; Philouze, P.; Boucher, F.; Jacquemart, M.; Frobert, P.; Breton, P. Efficacy of the postoperative management after microsurgical free tissue transfer. J. Stomatol. Oral. Maxillofac. Surg. 2017, 118, 173–177. [Google Scholar] [CrossRef]
- Danan, D.; Smolkin, M.E.; Varhegyi, N.E.; Bakos, S.R.; Jameson, M.J.; Shonka, D.C., Jr. Impact of blood transfusions on patients with head and neck cancer undergoing free tissue transfer. Laryngoscope 2015, 125, 86–91. [Google Scholar] [CrossRef]
- Munting, K.E.; Klein, A.A. Optimisation of pre-operative anaemia in patients before elective major surgery—Why, who, when and how? Anaesthesia 2019, 74, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Barceló, L.; Artero, L.S.; García-Argüelles, J.S.; Gamir, P.C.; Gisbert, J.P.; Ferrer-Arranz, L.M.; Gallego, A.M.; Campos, L.P.; Malavés, J.M.H.; Sanchis, M.L.; et al. Randomised clinical trial: Intravenous vs. oral iron for the treatment of anaemia after acute gastrointestinal bleeding. Aliment. Pharmacol. Ther. 2019, 50, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enteral Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels during Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722. [Google Scholar] [CrossRef]
- da Silva, A.C.O.; Silva, G.B.; Cho, A.B.; Wei, T.H.; Mattar, R.; Iamaguchi, R.B. Hypoalbuminemia in microsurgical flaps of the musculoskeletal apparatus. Acta Ortop. Bras. 2020, 28, 168–171. [Google Scholar] [CrossRef]
- Shum, J.; Markiewicz, M.R.; Park, E.; Bui, T.; Lubek, J.; Bell, R.B.; Dierks, E.J. Low prealbumin level is a risk factor for microvascular free flap failure. J. Oral. Maxillofac. Surg. 2014, 72, 169–177. [Google Scholar] [CrossRef]
- Xu, H.; Han, Z.; Ma, W.; Zhu, X.; Shi, J.; Lin, D. Perioperative Albumin Supplementation is Associated with Decreased Risk of Complications Following Microvascular Head and Neck Reconstruction. J. Oral. Maxillofac. Surg. 2021, 79, 2155–2161. [Google Scholar] [CrossRef]
- Juang, L.J.; Hur, W.S.; Silva, L.M.; Strilchuk, A.W.; Francisco, B.; Leung, J.; Robertson, M.K.; Groeneveld, D.J.; La Prairie, B.; Chun, E.M.; et al. Suppression of fibrin(ogen)-driven pathologies in disease models through controlled knockdown by lipid nanoparticle delivery of siRNA. Blood 2022, 139, 1302–1311. [Google Scholar] [CrossRef]
- Handschel, J.; Burghardt, S.; Naujoks, C.; Kübler, N.R.; Giers, G. Parameters predicting complications in flap surgery. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 115, 589–594. [Google Scholar] [CrossRef]
- Kaplangoray, M.; Toprak, K.; Cicek, O.F.; Deveci, E. Relationship between the Fibrinogen/Albumin Ratio and Microvascular Perfusion in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Elevated Myocardial Infarction: A Prospective Study. Arq. Bras. Cardiol. 2023, 120, e20230002. [Google Scholar] [CrossRef]
- Tomita, K.; Ochiai, S.; Gunji, T.; Hikita, K.; Kobayashi, T.; Sano, T.; Chiba, N.; Kawachi, S. Prognostic Significance of Plasma Fibrinogen/Serum Albumin Ratio in the Postoperative Outcome of Pancreatic Ductal Adenocarcinoma. Anticancer. Res. 2020, 40, 7017–7023. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, S.; Feng, Y.; Zhang, J.; Peng, Y.; Wang, X.; Wang, H. The Fibrinogen/Albumin Ratio Index as an Independent Prognostic Biomarker for Patients with Combined Hepatocellular Cholangiocarcinoma After Surgery. Cancer Manag. Res. 2022, 14, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pi, J.; Ma, W.; Gu, W.; Zhang, H.; Xu, A.; Liu, Y.; Shi, T.; Yang, F.; Chen, L. Prognostic value of the fibrinogen-to-albumin ratio (FAR) in patients with chronic heart failure across the different ejection fraction spectrum. Libyan J. Med. 2024, 19, 2309757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.P.; Mao, X.F.; Wu, T.T.; Chen, Y.; Hou, X.-G.; Yang, Y.; Ma, X.; Zhang, J.-Y.; Ma, Y.-T.; Xie, X.; et al. The Fibrinogen-to-Albumin Ratio Is Associated with Outcomes in Patients With Coronary Artery Disease Who Underwent Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620933008. [Google Scholar] [CrossRef] [PubMed]
- Vanags, I.; Stepanovs, J.; Ozolina, A.; Mukans, M.; Bjertnaes, L.J.; Mamaja, B. Thromboelastometry for Assessing Risks of Free Flap Thrombosis in Patients Undergoing Microvascular Surgery. Front Med. 2020, 7, 289. [Google Scholar] [CrossRef]
- Leandro-Merhi, V.A.; Costa, C.L.; Saragiotto, L.; Aquino, J.L.B. Nutritional indicators of malnutrition in hospitalized patients. Arq. Gastroenterol. 2019, 56, 447–450. [Google Scholar] [CrossRef]
- Rocans, R.P.; Zarins, J.; Bine, E.; Mahauri, I.; Deksnis, R.; Citovica, M.; Donina, S.; Vanags, I.; Gravelsina, S.; Vilmane, A.; et al. Von Willebrand Factor Antigen, Biomarkers of Inflammation, and Microvascular Flap Thrombosis in Reconstructive Surgery. J. Clin. Med. 2024, 13, 5411. [Google Scholar] [CrossRef]
- Chargi, N.; Breik, O.; Forouzanfar, T.; Martin, T.; Praveen, P.; Idle, M.; Parmar, S.; de Bree, R. Association of low skeletal muscle mass and systemic inflammation with surgical complications and survival after microvascular flap reconstruction in patients with head and neck cancer. Head Neck 2022, 44, 2077–2094. [Google Scholar] [CrossRef]
- Pereira, C.M.; Figueiredo, M.E.; Carvalho, R.; Catre, D.; Assunção, J.P. Anesthesia and surgical microvascular flaps. Rev. Bras. Anestesiol. 2012, 62, 563–579. [Google Scholar] [CrossRef]
- Jayaram, K.; Rao, P.; Gurajala, I.; Ramachandran, G. Evaluation of the Effect of Regional Anaesthesia on Microvascular Free Flaps. Turk. J. Anaesthesiol. Reanim. 2018, 46, 441–446. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Kuriakose, R.; Koshy, R.C. Radiation induced changes in the airway—Anaesthetic implications. South. Afr. J. Anaesth. Analg. 2004, 10, 19–21. [Google Scholar] [CrossRef]
- Goswami, U.; Jain, A. Anaesthetic implications of free-flap microvascular surgery for head and neck malignancies—A relook. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Heindl, B.; Jacob, M.; Annecke, T.; Chen, C.; Rehm, M.; Conzen, P.; Becker, B.F. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011, 115, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, T.; Sencan, A.; Camgoz, N.; Ak, B.; Yavuzer, R. A challenging problem that concerns the aesthetic surgeon: Postoperative nausea and vomiting. Ann. Plast. Surg. 2008, 61, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Weibel, S.; Rücker, G.; Eberhart, L.H.; Pace, N.L.; Hartl, H.M.; Jordan, O.L.; Mayer, D.; Riemer, M.; Schaefer, M.S.; Raj, D.; et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 10, CD012859. [Google Scholar]
- Kaushal, J.; Gupta, M.C.; Kaushal, V.; Bhutani, G.; Dhankar, R.; Atri, R.; Verma, S. Clinical evaluation of two antiemetic combinations palonosetron dexamethasone versus ondansetron dexamethasone in chemotherapy of head and neck cancer. Singap. Med. J. 2010, 51, 871–875. [Google Scholar]
- Ciudad, P.; Escandón, J.M.; Manrique, O.J.; Escobar, H.; Pejerrey, M.B.; Arredondo, M.A. Efficacy of Combined Spinal-Epidural Anesthesia for Lower Extremity Microvascular Reconstruction. J. Surg. Res. 2023, 291, 700–710. [Google Scholar] [CrossRef]
- Owen, A.R.; Amundson, A.W.; Larson, D.R.; Duncan, C.M.; Smith, H.M.; Johnson, R.L.; Taunton, M.J.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Spinal Versus General Anesthesia in Contemporary Revision Total Knee Arthroplasties. J. Arthroplast. 2023, 38, S271–S274. [Google Scholar] [CrossRef]
- Galitzine, S.; Wilson, K.; Edington, M.; Burumdayal, A.; McNally, M. Patients’ reported experiences and outcomes following surgical excision of lower limb osteomyelitis and microvascular free tissue reconstruction under ‘awake’ epidural anaesthesia and sedation. Surgeon 2021, 19, 193–199. [Google Scholar] [CrossRef]
- Erni, D.; Banic, A.; Signer, C.; Sigurdsson, G.H. Effects of epidural anaesthesia on microcirculatory blood flow in free flaps in patients under general anaesthesia. Eur. J. Anaesthesiol. 1999, 16, 692–698. [Google Scholar] [CrossRef]
- Koster, I.T.; Os, M.M.D.; Rutten, M.V.; Dungen, T.R.v.D.; de Jong, T.; Winters, H.A.; Driessen, C. The Effect of Regional Anaesthesia on Free Flap Survival in Lower Extremity Reconstructions. Strateg. Trauma. Limb Reconstr. 2024, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, K.Y.; Bai, S.J.; Hong, J.H.; Lee, J.; Park, J.M.; Kim, S.H. Comparison of the effects of remifentanil-based general anesthesia and popliteal nerve block on postoperative pain and hemodynamic stability in diabetic patients undergoing distal foot amputation: A retrospective observational study. Medicine 2016, 95, e4302. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J. Anaesthesia for reconstructive surgery. Anaesth. Intensive Care Med. 2006, 7, 31–35. [Google Scholar] [CrossRef]
- Wang, J.C.; Piple, A.S.; Mayfield, C.K.; Chung, B.C.; Oakes, D.A.; Gucev, G.; Lieberman, J.R.; Christ, A.B.; Heckmann, N.D. Peripheral Nerve Block Utilization is Associated with Decreased Postoperative Opioid Consumption and Shorter Length of Stay Following Total Knee Arthroplasty. Arthroplast. Today 2023, 20, 101101. [Google Scholar] [CrossRef] [PubMed]
- Salibian, A.A.; Frey, J.D.; Karp, N.S.; Choi, M. Abstract: Transversus Abdominis Plane Blocks in Microsurgical Breast Reconstruction: An Analysis of Pain, Narcotic Consumption, Length of Stay and Cost Implications. Plast. Reconstr. Surg. Glob. Open 2018, 6, 174–175. [Google Scholar] [CrossRef]
- Egan, R.J.; Hopkins, J.C.; Beamish, A.J.; Shah, R.; Edwards, A.G.; Morgan, J.D. Randomized clinical trial of intraoperative superficial cervical plexus block versus incisional local anaesthesia in thyroid and parathyroid surgery. Br. J. Surg. 2013, 100, 1732–1738. [Google Scholar] [CrossRef]
- Mak, Q.H.Y.; Chan, H.T.; Irwin, M.G. Anaesthesia for plastic and reconstructive surgery. Anaesth. Intensive Care Med. 2021, 24, 800–805. [Google Scholar] [CrossRef]
- Karamanos, E.; Walker, R.; Wang, H.T.; Shah, A.R. Perioperative Fluid Resuscitation in Free Flap Breast Reconstruction: When Is Enough Enough? Plast. Reconstr. Surg. Glob. Open. 2020, 8, e2662. [Google Scholar] [CrossRef]
- Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Gonzalez, J.; Losantos, I.; Gilsanz, F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers 2021, 13, 545. [Google Scholar] [CrossRef]
- Kouz, K.; Thiele, R.; Michard, F.; Saugel, B. Haemodynamic monitoring during noncardiac surgery: Past, present, and future. J. Clin. Monit. Comput. 2024, 38, 565–580. [Google Scholar] [CrossRef]
- Moellhoff, N.; Broer, P.N.; Heidekrueger, P.I.; Ninkovic, M.; Ehrl, D. Impact of Intraoperative Hypothermia on Microsurgical Free Flap Reconstructions. J. Reconstr. Microsurg. 2021, 37, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Young, V.L.; Watson, M.E. Prevention of perioperative hypothermia in plastic surgery. Aesthet. Surg. J. 2006, 26, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Laitman, B.M.; Ma, Y.; Hill, B.; Teng, M.; Genden, E.; DeMaria, S.; Miles, B.A. Mild hypothermia is associated with improved outcomes in patients undergoing microvascular head and neck reconstruction. Am. J. Otolaryngol. 2019, 40, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Schraven, S.P.; Kossack, B.; Strüder, D.; Jung, M.; Skopnik, L.; Gross, J.; Hilsmann, A.; Eisert, P.; Mlynski, R.; Wisotzky, E.L. Continuous intraoperative perfusion monitoring of free microvascular anastomosed fasciocutaneous flaps using remote photoplethysmography. Sci. Rep. 2023, 13, 1532. [Google Scholar] [CrossRef]
- Bombardelli, J.; Farhat, S.; De La Fuente Hagopian, A.; Hua, J.; Schusterman, M.A.; Echo, A. Evaluation of Intraoperative Anastomotic Patency with Angiography in Microsurgical Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open. 2023, 11, e5230. [Google Scholar] [CrossRef]
- Chen, K.-C.; Lin, C.-H.; Ma, H.; Wang, T.-H.; Shih, Y.-C.; Chen, M.-C.; Chiu, Y.-J.; Chen, C.-E. Outcome analysis of free flap reconstruction for head and neck cancer with intraoperative indocyanine green angiography. J. Plast. Reconstr. Aesthet. Surg. 2023, 85, 387–392. [Google Scholar] [CrossRef]
- Abdou, S.A.; Sharif-Askary, B.; Zolper, E.G.; Evans, K.K. Intraoperative Utility of the Implantable Doppler in Lower Extremity Reconstruction: A Matched Case-control Study. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3229. [Google Scholar] [CrossRef]
- Huang, T.C.T.; Ciudad, P.; Manrique, O.J.; Agko, M.; Chen, S.H.; Tang, P.Y.; Sabbagh, M.D.; Chen, H.C. Staged inset of free flaps for complex microsurgical head and neck reconstruction to ensure total flap survival. Microsurgery 2018, 38, 844–851. [Google Scholar] [CrossRef]
- Chang, E.I. My first 100 consecutive microvascular free flaps: Pearls and lessons learned in first year of practice. Plast. Reconstr. Surg. Glob. Open 2013, 1, e27. [Google Scholar] [CrossRef]
- Elia, J.; Diwan, M.; Deshpande, R.; Brainard, J.C.; Karamchandani, K. Perioperative Fluid Management and Volume Assessment. Anesthesiol. Clin. 2023, 41, 191–209. [Google Scholar] [CrossRef]
- Haughey, B.H.; Wilson, E.; Kluwe, L.; Piccirillo, J.; Fredrickson, J.; Sessions, D.; Spector, G. Free flap reconstruction of the head and neck: Analysis of 241 cases. Otolaryngol. Head Neck Surg. 2001, 125, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dooley, B.J.; Karassawa Zanoni, D.; Mcgill, M.R.; Awad, M.I.; Shah, J.P.; Wong, R.J.; Broad, C.; Mehrara, B.J.; Ganly, I.; Patel, S.G. Intraoperative and postanesthesia care unit fluid administration as risk factors for postoperative complications in patients with head and neck cancer undergoing free tissue transfer. Head Neck 2020, 42, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; McCluskey, S.A.; Hall, F.; Lipa, J.; Neligan, P.; Brown, D.; Irish, J.; Gullane, P.; Gilbert, R. Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck 2007, 29, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Chu, M.W.; Nelson, J.A.; Basta, M.; Gerety, P.; Kanchwala, S.K.; Wu, L.C. Complications and Cost Analysis of Intraoperative Arterial Complications in Head and Neck Free Flap Reconstruction. J. Reconstr. Microsurg. 2017, 33, 318–327. [Google Scholar]
- Chen, C.; Nguyen, M.D.; Bar-Meir, E.; Hess, P.A.; Lin, S.; Tobias, A.M.; Upton, J.I.; Lee, B.T. Effects of vasopressor administration on the outcomes of microsurgical breast reconstruction. Ann. Plast. Surg. 2010, 65, 28–31. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Kim, P.S.; Rabie, A.N.; Lee, B.T.; Lin, S.J. Vasopressors and reconstructive flap perfusion: A review of the literature comparing the effects of various pharmacologic agents. Ann. Plast. Surg. 2014, 73, 245–248. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Santamaria, E.; Hu, Q.Y.; Heerdt, P. Effects of vasoactive medications on the blood flow of island musculocutaneous flaps in swine. Ann. Plast. Surg. 1997, 39, 524–531. [Google Scholar] [CrossRef]
- Kelly, D.A.; Reynolds, M.; Crantford, C.; Pestana, I.A. Impact of intraoperative vasopressor use in free tissue transfer for head, neck, and extremity reconstruction. Ann. Plast. Surg. 2014, 72, S135–S138. [Google Scholar] [CrossRef]
- Suominen, S.; Svartling, N.; Silvasti, M.; Niemi, T.; Kuokkanen, H.; Asko-Seljavaara, S. The effect of intravenous dopamine and dobutamine on blood circulation during a microvascular TRAM flap operation. Ann. Plast. Surg. 2004, 53, 425–431. [Google Scholar] [CrossRef]
- Eley, K.A.; Young, J.D.; Watt-Smith, S.R. Power spectral analysis of the effects of epinephrine, norepinephrine, dobutamine and dopexamine on microcirculation following free tissue transfer. Microsurgery 2013, 33, 275–281. [Google Scholar] [CrossRef]
- Eley, K.A.; Young, J.D.; Watt-Smith, S.R. Epinephrine, norepinephrine, dobutamine, and dopexamine effects on free flap skin blood flow. Plast. Reconstr. Surg. 2012, 130, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Porro Gil, L.; Leon Vintro, X.; Lopez Fernandez, S.; Vega Garcia, C.; Pons Playa, G.; Fernandez Garrido, M.; Masia Ayala, J. The Effect of Perioperative Blood Transfusions on Microvascular Anastomoses. J. Clin. Med. 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, E.; Shah, A.R.; Kim, J.N.; Wang, H.T. Impact of Blood Transfusion in Free Flap Breast Reconstruction Using Propensity Score Matching. J. Reconstr. Microsurg. 2021, 37, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Remy, K.E.; Hall, M.W.; Cholette, J.; Juffermans, N.P.; Nicol, K.; Doctor, A.; Blumberg, N.; Spinella, P.C.; Norris, P.J.; Dahmer, M.K.; et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018, 58, 804–815. [Google Scholar] [CrossRef]
- Mantoani, C.C.; Margatho, A.S.; Dantas, R.A.S.; Galvão, C.M.; de Campos Pereira Silveira, R.C. Perioperative Blood Transfusion and Occurrence of Surgical Site Infection: An Integrative Review. AORN J. 2019, 110, 626–634. [Google Scholar] [CrossRef]
- Higgins, R.M.; Helm, M.C.; Kindel, T.L.; Gould, J.C. Perioperative blood transfusion increases risk of surgical site infection after bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 582–587. [Google Scholar] [CrossRef]
- Kim, M.J.; Woo, K.J.; Park, B.Y.; Kang, S.R. Effects of Transfusion on Free Flap Survival: Searching for an Optimal Hemoglobin Threshold for Transfusion. J. Reconstr. Microsurg. 2018, 34, 610–615. [Google Scholar] [CrossRef]
- Mashrah, M.A.; Aldhohrah, T.; Abdelrehem, A.; Sabri, B.; Ahmed, H.; Al-Rawi, N.H.; Yu, T.; Zhao, S.; Wang, L.; Ge, L. Postoperative care in ICU versus non-ICU after head and neck free-flap surgery: A systematic review and meta-analysis. BMJ Open 2022, 12, e053667. [Google Scholar] [CrossRef]
- Yalamanchi, P.; Thomas, W.W.; Workman, A.D.; Rajasekaran, K.; Chalian, A.A.; Shanti, R.M.; Newman, J.G.; Cannady, S.B. Value of Intensive Care Unit-Based Postoperative Management for Microvascular Free Flap Reconstruction in Head and Neck Surgery. Facial Plast. Surg. Aesthet. Med. 2021, 23, 49–53. [Google Scholar] [CrossRef]
- Stevens, M.N.; Prasad, K.; Sharma, R.K.; Gallant, J.N.; Habib, D.R.S.; Langerman, A.; Mannion, K.; Rosenthal, E.; Topf, M.C.; Rohde, S.L. Comparative Outcomes for Microvascular Free Flap Monitoring Outside the Intensive Care Unit. Otolaryngol. Head Neck Surg. 2024, 171, 381–386. [Google Scholar] [CrossRef]
- Puppo Moreno, A.M.; Abella Alvarez, A.; Morales Conde, S.; Pérez Flecha, M.; García Ureña, M.Á. The intensive care unit in the postoperative period of major abdominal surgery. Med. Intensiv. 2019, 43, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Wang, L.L.; Yi, D.I.; Prasanna, P.D.; Kandl, C. Opioid sparing multimodal analgesia treats pain after head and neck microvascular reconstruction. Laryngoscope 2020, 130, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Go, B.C.; Go, C.C.; Chorath, K.; Moreira, A.; Rajasekaran, K. Multimodal Analgesia in Head and Neck Free Flap Reconstruction: A Systematic Review. Otolaryngol. Head Neck Surg. 2022, 166, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wu, L.; Ding, W. The efficacy of preoperative administration of gabapentin/pregabalin in improving pain after total hip arthroplasty: A meta-analysis. BMC Musculoskelet. Disord. 2016, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Biermann, N.; Chak, J.C.; Wiesmeier, A.; Klein, S.M.; Ruewe, M.; Spoerl, S.; Kruppa, P.; Prantl, L.; Anker, A.M. Evidence-Based Approaches to Anticoagulation in Reconstructive Microsurgery-A Systematic Literature Review. Life 2024, 14, 82. [Google Scholar] [CrossRef]
- Schug, S.A. Do NSAIDs Really Interfere with Healing after Surgery? J. Clin. Med. 2021, 10, 2359. [Google Scholar] [CrossRef]
- Ridha, H.; Jallali, N.; Butler, P.E. The use of dextran post free tissue transfer. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 951–954. [Google Scholar] [CrossRef]
- Kearns, M.C.; Baker, J.; Myers, S.; Ghanem, A. Towards standardization of training and practice of reconstructive microsurgery: An evidence-based recommendation for anastomosis thrombosis prophylaxis. Eur. J. Plast. Surg. 2018, 41, 379–386. [Google Scholar] [CrossRef]
- Disa, J.J.; Polvora, V.P.; Pusic, A.L.; Singh, B.; Cordeiro, P.G. Dextran-related complications in head and neck microsurgery: Do the benefits outweigh the risks? A prospective randomized analysis. Plast. Reconstr. Surg. 2003, 112, 1534–1539. [Google Scholar] [CrossRef]
- Bashir, M.M.; Yousaf, N.; Khan, F.A. The outcome of microvascular free flap surgery with or without the use of postoperative heparin. J. Coll. Physicians Surg. Pak. 2014, 24, 412–415. [Google Scholar]
- Lee, K.T.; Mun, G.H. The efficacy of postoperative antithrombotics in free flap surgery: A systematic review and meta-analysis. Plast Reconstr Surg. 2015, 135, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Lighthall, J.G.; Cain, R.; Ghanem, T.A.; Wax, M.K. Effect of postoperative aspirin on outcomes in microvascular free tissue transfer surgery. Otolaryngol. Head Neck Surg. 2013, 148, 40–466. [Google Scholar] [CrossRef] [PubMed]
- Rothweiler, R.; Gerlach, V.; Voss, P.; Poxleitner, P.; Ermer, M.; Gross, C.; Schwer, C.; Vach, K.; Kalbhenn, J.; Metzger, M. Aspirin, heparin and ischemia time in microvascular free flap surgery—Their influence and an optimal anticoagulation protocol. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, e556–e562. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Ardestani, S.H.; Jafari, M.; Hagh, A.B. Testing a New Anticoagulation Method for Free Flap Reconstruction of Head and Neck Cancers. Clin. Exp. Otorhinolaryngol. 2016, 9, 370–373. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojuva, A.M.; Rocans, R.P.; Zarins, J.; Bine, E.; Mahauri, I.; Donina, S.; Mamaja, B.; Vanags, I. Novel Challenges and Opportunities for Anesthesia and Perioperative Care in Microvascular Flap Surgery: A Narrative Review. Clin. Pract. 2024, 14, 2187-2201. https://doi.org/10.3390/clinpract14050172
Ojuva AM, Rocans RP, Zarins J, Bine E, Mahauri I, Donina S, Mamaja B, Vanags I. Novel Challenges and Opportunities for Anesthesia and Perioperative Care in Microvascular Flap Surgery: A Narrative Review. Clinics and Practice. 2024; 14(5):2187-2201. https://doi.org/10.3390/clinpract14050172
Chicago/Turabian StyleOjuva, Aleksi Matias, Rihards Peteris Rocans, Janis Zarins, Evita Bine, Insana Mahauri, Simona Donina, Biruta Mamaja, and Indulis Vanags. 2024. "Novel Challenges and Opportunities for Anesthesia and Perioperative Care in Microvascular Flap Surgery: A Narrative Review" Clinics and Practice 14, no. 5: 2187-2201. https://doi.org/10.3390/clinpract14050172
APA StyleOjuva, A. M., Rocans, R. P., Zarins, J., Bine, E., Mahauri, I., Donina, S., Mamaja, B., & Vanags, I. (2024). Novel Challenges and Opportunities for Anesthesia and Perioperative Care in Microvascular Flap Surgery: A Narrative Review. Clinics and Practice, 14(5), 2187-2201. https://doi.org/10.3390/clinpract14050172





