Effect of a Physical Exercise Intervention on Physical Function Parameters and Blood Analytical Changes in Lung Cancer Survivors: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
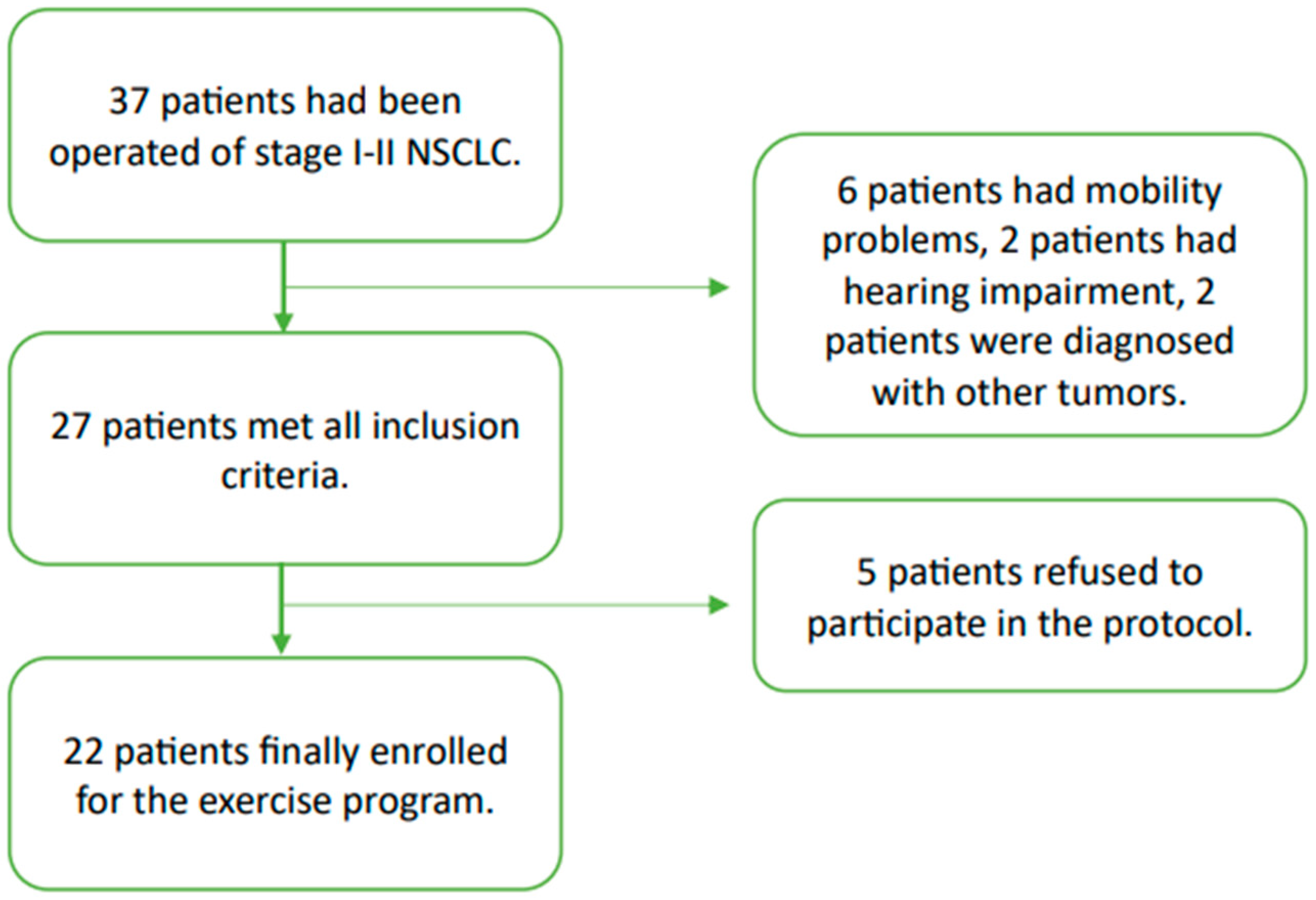
2.1.1. Participants
2.1.2. Intervention
- Warm-up and joint mobilisation (5–10 min).
- Main part of the session (45–50 min): 3 blocks of 3 sets combining an upper limb and a lower limb exercise followed by a break of 120–180 s, during which aerobic (balance and/or cardiovascular) exercises were introduced.
- Cool-down (3–5 min) with static stretching.
2.1.3. Data Collection
2.1.4. Evaluation of Physical Performance
2.1.5. Evaluation of Body-Mass Composition
2.1.6. Evaluation of Health-Related Quality of Life
2.1.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.S.; Trier, K.; Vibe-Petersen, J.; Missel, M.; Christensen, M.; Larsen, K.R.; Langer, S.W.; Hendriksen, C.; Clementsen, P.; Pedersen, J.H.; et al. Perioperative Rehabilitation in Operation for Lung Cancer (PROLUCA)—Rationale and Design. BMC Cancer 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Taylor-Stokes, G.; Roughley, A. Symptom Burden and Quality of Life in Advanced Non-Small Cell Lung Cancer Patients in France and Germany. Lung Cancer 2013, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Lehto, R.H. Symptom Burden in Lung Cancer: Management Updates. Lung Cancer Manag. 2016, 5, 61–78. [Google Scholar] [CrossRef]
- Yang, P.; Cheville, A.L.; Wampfler, J.A.; Garces, Y.I.; Jatoi, A.; Clark, M.M.; Cassivi, S.D.; Midthun, D.E.; Marks, R.S.; Aubry, M.C.; et al. Quality of Life and Symptom Burden among Long-Term Lung Cancer Survivors. J. Thorac. Oncol. 2012, 7, 64–70. [Google Scholar] [CrossRef]
- Ehsan, M.; Khan, R.; Wakefield, D.; Qureshi, A.; Murray, L.; ZuWallack, R.; Leidy, N.K. A Longitudinal Study Evaluating the Effect of Exacerbations on Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2013, 10, 559–564. [Google Scholar] [CrossRef]
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory Muscle Training in Adults with Chronic Obstructive Pulmonary Disease: An Update of a Systematic Review. Respir. Med. 2008, 102, 1715–1729. [Google Scholar] [CrossRef]
- Hung, R.; Krebs, P.; Coups, E.J.; Feinstein, M.B.; Park, B.J.; Burkhalter, J.; Ostroff, J.S. Fatigue and Functional Impairment in Early-Stage Non-Small Cell Lung Cancer Survivors. J. Pain Symptom Manag. 2011, 41, 426–435. [Google Scholar] [CrossRef]
- Maric, D.; Jovanovic, D.; Nagorni-Obradovic, L.; Stjepanovic, M.; Kisic-Tepavcevic, D.; Pekmezovic, T. Assessment of Health-Related Quality of Life in End-Stage Chronic Obstructive Pulmonary Disease and Non-Small-Cell Lung Cancer Patients in Serbia. Palliat. Support. Care 2016, 14, 60–68. [Google Scholar] [CrossRef]
- Phillips, I.; Stares, M.; Allan, L.; Sayers, J.; Skipworth, R.; Laird, B. Optimising Outcomes in Non Small Cell Lung Cancer: Targeting Cancer Cachexia. Front. Biosci. 2022, 27, 129. [Google Scholar] [CrossRef]
- Cavalheri, V.; Burtin, C.; Formico, V.R.; Nonoyama, M.L.; Jenkins, S.; Spruit, M.A.; Hill, K. Exercise Training Undertaken by People within 12 Months of Lung Resection for Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Cavalheri, V. Preoperative Exercise Training for People with Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2022, 9. [Google Scholar] [CrossRef]
- Pouwels, S.; Fiddelaers, J.; Teijink, J.A.W.; Woorst, J.F.T.; Siebenga, J.; Smeenk, F.W.J.M. Preoperative Exercise Therapy in Lung Surgery Patients: A Systematic Review. Respir. Med. 2015, 109, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Cennamo, A.; Cerqua, F.S.; Stefanelli, F.; Bianco, A.; Musella, S.; Rispoli, M.; Salvi, R.; Meoli, I. Effects of a High-Intensity Pulmonary Rehabilitation Program on the Minute Ventilation/Carbon Dioxide Output Slope during Exercise in a Cohort of Patients with COPD Undergoing Lung Resection for Non-Small Cell Lung Cancer. J. Bras. Pneumol. 2019, 45, e20180132. [Google Scholar] [CrossRef]
- Rispoli, M.; Salvi, R.; Cennamo, A.; Di Natale, D.; Natale, G.; Meoli, I.; Gioia, M.R.; Esposito, M.; Nespoli, M.R.; De Finis, M.; et al. Effectiveness of Home-Based Preoperative Pulmonary Rehabilitation in COPD Patients Undergoing Lung Cancer Resection. Tumori J. 2020, 106, 203–211. [Google Scholar] [CrossRef]
- Granger, C.L.; McDonald, C.F.; Berney, S.; Chao, C.; Denehy, L. Exercise Intervention to Improve Exercise Capacity and Health Related Quality of Life for Patients with Non-Small Cell Lung Cancer: A Systematic Review. Lung Cancer 2011, 72, 139–153. [Google Scholar] [CrossRef]
- Pasqua, F.; Geraneo, K.; Nardi, I.; Lococo, F.; Cesario, A. Pulmonary Rehabilitation in Lung Cancer. Monaldi Arch. Chest Dis. 2013, 79, 73–80. [Google Scholar] [CrossRef]
- Avancini, A.; Sartori, G.; Gkountakos, A.; Casali, M.; Trestini, I.; Tregnago, D.; Bria, E.; Jones, L.W.; Milella, M.; Lanza, M.; et al. Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist 2020, 25, e555–e569. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Fan, J.; Huang, Y.; Ye, J.; Gu, F.; Li, Y. The Subjective Will and Psychological Experience of Home-Based Exercise in Lung Cancer Patients During Interval of Chemotherapy: A Qualitative Study. J. Multidiscip. Healthc. 2023, 16, 663–674. [Google Scholar] [CrossRef]
- Nguyen, T.; Tracy, K.; Ullah, A.; Karim, N.A. Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review. Clin. Pract. 2023, 13, 715–730. [Google Scholar] [CrossRef]
- Edbrooke, L.; Bowman, A.; Granger, C.L.; Burgess, N.; Abo, S.; Connolly, B.; Denehy, L. Exercise across the Lung Cancer Care Continuum: An Overview of Systematic Reviews. J. Clin. Med. 2023, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Phillips, M.; Sanders, L.H.; Hill, K. Patterns of Sedentary Behaviour and Physical Activity in People Following Curative Intent Treatment for Non-Small Cell Lung Cancer. Chronic Respir. Dis. 2016, 13, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Effect of Walking on Circadian Rhythms and Sleep Quality of Patients with Lung Cancer: A Randomised Controlled Trial. Br. J. Cancer 2016, 115, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Goldberg, S.; Vogel, P.D.; Sullivan, M.; Pirl, W.F.; Lynch, T.J.; Christiani, D.C.; Smith, M.R. A Structured Exercise Program for Patients with Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 595–601. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Brintnall, R.A. A Home-Based Exercise Intervention for Non-Small Cell Lung Cancer Patients Post-Thoracotomy. Semin. Oncol. Nurs. 2017, 33, 106–117. [Google Scholar] [CrossRef]
- Blakely, A.M.; Hu, H.; Wong, F.L.; Raz, D.J.; Erhunmwunsee, L.; Sun, V.; Kim, J.Y. Deterioration in Health-Related Quality of Life Diminishes Benefit of Lung Cancer Resection in Older Adults. Clin. Lung Cancer 2021, 22, e544–e551. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Milton, K.; Varela, A.R.; Strain, T.; Cavill, N.; Foster, C.; Mutrie, N. A Review of Global Surveillance on the Muscle Strengthening and Balance Elements of Physical Activity Recommendations. J. Frailty Sarcopenia Falls 2018, 3, 114–124. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, A.; Lord, S.R.; Sherrington, C. The Development and Validation of a Brief Performance-Based Fall Risk Assessment Tool for Use in Primary Care. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.L.; Holland, A.E.; Gordon, I.R.; Denehy, L. Minimal Important Difference of the 6-Minute Walk Distance in Lung Cancer. Chronic Respir. Dis. 2015, 12, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Wallace, D.; Chandler, J.M.; Duncan, P.W.; Rooney, E.; Fox, M.; Guralnik, J.M. Physical Performance Measures in the Clinical Setting. J. Am. Geriatr. Soc. 2003, 51, 314–322. [Google Scholar] [CrossRef]
- Barrachina-Igual, J.; Pablos, A.; Pérez-Ros, P.; Flor-Rufino, C.; Martínez-Arnau, F.M. Frailty Status Improvement after 5-Month Multicomponent Program PROMUFRA in Community-Dwelling Older People: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 4077. [Google Scholar] [CrossRef]
- Fayers, P.; Bottomley, A. Quality of Life Research within the EORTC—The EORTC QLQ-C30. Eur. J. Cancer 2002, 38, 125–133. [Google Scholar] [CrossRef]
- Remon, J.; Reguart, N.; García-Campelo, R.; Conde, E.; Lucena, C.M.; Persiva, O.; Navarro-Martin, A.; Rami-Porta, R. Lung Cancer in Spain. J. Thorac. Oncol. 2021, 16, 197–204. [Google Scholar] [CrossRef]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined Aerobic Exercise and High-Intensity Respiratory Muscle Training in Patients Surgically Treated for Non-Small Cell Lung Cancer: A Pilot Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef]
- Dhillon, H.M.; Bell, M.L.; van der Ploeg, H.P.; Turner, J.D.; Kabourakis, M.; Spencer, L.; Lewis, C.; Hui, R.; Blinman, P.; Clarke, S.J.; et al. Impact of Physical Activity on Fatigue and Quality of Life in People with Advanced Lung Cancer: A Randomized Controlled Trial. Ann. Oncol. 2017, 28, 1889–1897. [Google Scholar] [CrossRef]
- Mikkelsen, M.K.; Lund, C.M.; Vinther, A.; Tolver, A.; Johansen, J.S.; Chen, I.; Ragle, A.M.; Zerahn, B.; Engell-Noerregaard, L.; Larsen, F.O.; et al. Effects of a 12-Week Multimodal Exercise Intervention Among Older Patients with Advanced Cancer: Results from a Randomized Controlled Trial. Oncologist 2022, 27, 67–78. [Google Scholar] [CrossRef]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.C.; Rosario, D.J.; Thaha, M.A.; Bourke, L. Interventions for Promoting Habitual Exercise in People Living with and beyond Cancer. Cochrane Database Syst. Rev. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.J.; Crisafio, M.E.; Howell, M.J.; Nicklawsky, A.; Marker, R.J. A Group-Based, Videoconference-Delivered Physical Activity Program for Cancer Survivors. Transl. J. Am. Coll. Sports Med. 2023, 8, e000221. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 4, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.L.; Connolly, B.; Denehy, L.; Hart, N.; Antippa, P.; Lin, K.Y.; Parry, S.M. Understanding Factors Influencing Physical Activity and Exercise in Lung Cancer: A Systematic Review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 983–999. [Google Scholar] [CrossRef]
- Occhipinti, S.; Dunn, J.; O’Connell, D.L.; Garvey, G.; Valery, P.C.; Ball, D.; Fong, K.M.; Vinod, S.; Chambers, S. Lung Cancer Stigma across the Social Network: Patient and Caregiver Perspectives. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1443–1453. [Google Scholar] [CrossRef]
- Hawley-Hague, H.; Horne, M.; Skelton, D.A.; Todd, C. Review of How We Should Define (and Measure) Adherence in Studies Examining Older Adults’ Participation in Exercise Classes. BMJ Open 2016, 6, e011560. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Jiang, S.; Chen, C.; Zheng, J.; Liu, H.; Liang, X.; Chen, Z.; Wang, H.; Guo, Z.; et al. Spontaneous Ventilation Video-Assisted Thoracoscopic Surgery for Non-Small-Cell Lung Cancer Patients With Poor Lung Function: Short- and Long-Term Outcomes. Front. Surg. 2022, 9, 800082. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Hantel, A.; DuMontier, C.; Odejide, O.O.; Luskin, M.R.; Sperling, A.S.; Hshieh, T.; Chen, R.; Soiffer, R.; Driver, J.A.; Abel, G.A. Gait Speed, Survival, and Recommended Treatment Intensity in Older Adults with Blood Cancer Requiring Treatment. Cancer 2021, 127, 875–883. [Google Scholar] [CrossRef]
- Dociak-Salazar, E.; Barrueto-Deza, J.L.; Urrunaga-Pastor, D.; Runzer-Colmenares, F.M.; Parodi, J.F. Gait Speed as a Predictor of Mortality in Older Men with Cancer: A Longitudinal Study in Peru. Heliyon 2022, 8, e08862. [Google Scholar] [CrossRef] [PubMed]
- El Cáncer en Cifras|SEOM: Sociedad Española de Oncología Médica. Available online: https://seom.org/prensa/el-cancer-en-cifras (accessed on 11 September 2024).
- Collins, J.T.; Noble, S.; Chester, J.; Davies, H.E.; Evans, W.D.; Farewell, D.; Lester, J.F.; Parry, D.; Pettit, R.; Byrne, A. The Value of Physical Performance Measurements alongside Assessment of Sarcopenia in Predicting Receipt and Completion of Planned Treatment in Non-Small Cell Lung Cancer: An Observational Exploratory Study. Support. Care Cancer 2018, 26, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Eves, N.D.; Kraus, W.E.; Potti, A.; Crawford, J.; Blumenthal, J.A.; Peterson, B.L.; Douglas, P.S. The lung cancer exercise training study: A randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: Rationale and design. BMC Cancer 2010, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Coletta, G.; Phillips, S.M. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res. Rev. 2023, 86, 101883. [Google Scholar] [CrossRef]
- Machingura, A.; Taye, M.; Musoro, J.; Ringash, J.; Pe, M.; Coens, C.; Martinelli, F.; Tu, D.; Basch, E.; Brandberg, Y.; et al. Clustering of EORTC QLQ-C30 Health-Related Quality of Life Scales across Several Cancer Types: Validation Study. Eur. J. Cancer 2022, 170, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, J.; Gui, D.; Liu, F.; Li, J.; Tu, J. Functional and Postoperative Outcomes after High-Intensity Interval Training in Lung Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2023, 12, 1029738. [Google Scholar] [CrossRef]
- Edvardsen, E.; Skjønsberg, O.H.; Holme, I.; Nordsletten, L.; Borchsenius, F.; Anderssen, S.A. High-Intensity Training Following Lung Cancer Surgery: A Randomised Controlled Trial. Thorax 2015, 70, 244–250. [Google Scholar] [CrossRef]
- Bade, B.C.; Thomas, D.D.; Scott, J.A.B.; Silvestri, G.A. Increasing Physical Activity and Exercise in Lung Cancer: Reviewing Safety, Benefits, and Application. J. Thorac. Oncol. 2015, 10, 861–871. [Google Scholar] [CrossRef]
- Salhi, B.; Haenebalcke, C.; Perez-Bogerd, S.; Nguyen, M.D.; Ninane, V.; Malfait, T.L.A.; Vermaelen, K.Y.; Surmont, V.F.; Van Maele, G.; Colman, R.; et al. Rehabilitation in Patients with Radically Treated Respiratory Cancer: A Randomised Controlled Trial Comparing Two Training Modalities. Lung Cancer 2015, 89, 167–174. [Google Scholar] [CrossRef]
- Arbane, G.; Douiri, A.; Hart, N.; Hopkinson, N.S.; Singh, S.; Speed, C.; Valladares, B.; Garrod, R. Effect of Postoperative Physical Training on Activity after Curative Surgery for Non-Small Cell Lung Cancer: A Multicentre Randomised Controlled Trial. Physiotherapy 2014, 100, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hvid, T.; Winding, K.; Rinnov, A.; Dejgaard, T.; Thomsen, C.; Iversen, P.; Brasso, K.; Mikines, K.J.; Van Hall, G.; Lindegaard, B.; et al. Endurance Training Improves Insulin Sensitivity and Body Composition in Prostate Cancer Patients Treated with Androgen Deprivation Therapy. Endocr. Relat. Cancer 2013, 20, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, R.; Pedersen, M.; Solomon, T.P.J.; Knudsen, S.H.; Hansen, L.S.; Karstoft, K.; Lehrskov-Schmidt, L.; Pedersen, K.K.; Thomsen, C.; Holst, J.J.; et al. Normal Physical Activity Obliterates the Deleterious Effects of a High-Caloric Intake. J. Appl. Physiol. 2014, 116, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and Resistance Exercise Improves Physical Fitness, Bone Health, and Quality of Life in Overweight and Obese Breast Cancer Survivors: A Randomized Controlled Trial. Breast Cancer Res. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Rebelos, E.; Latva-Rasku, A.; Koskensalo, K.; Pekkarinen, L.; Saukko, E.; Ihalainen, J.; Honka, M.J.; Tuisku, J.; Bucci, M.; Laurila, S.; et al. Insulin-Stimulated Brain Glucose Uptake Correlates with Brain Metabolites in Severe Obesity: A Combined Neuroimaging Study. J. Cereb. Blood Flow Metab. 2024, 44, 407–418. [Google Scholar] [CrossRef]
- Kawai, H.; Ota, H. Low Perioperative Serum Prealbumin Predicts Early Recurrence after Curative Pulmonary Resection for Non-Small-Cell Lung Cancer. World J. Surg. 2012, 36, 2853–2857. [Google Scholar] [CrossRef]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A Tutorial on Pilot Studies: The What, Why and How. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef]
- van Teijlingen, E.; Hundley, V. The Importance of Pilot Studies. Nurs. Stand. 2002, 16, 33–36. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Sully, B.G.O.; Campbell, M.J. Pilot and Feasibility Studies: Is There a Difference from Each Other and from a Randomised Controlled Trial? Contemp. Clin. Trials 2014, 38, 130–133. [Google Scholar] [CrossRef]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of Nutrition and Physical Exercise Intervention in Palliative Cancer Patients: A Randomized Controlled Trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef]

| Median (IQR) or Frequency (%) | |
|---|---|
| Age (years) | 68 (63.5–76) |
| Sex (Female) | 7 (31.8) |
| Civil status: | |
| Married | 15 (78.9) |
| Divorced | 2 (10.5) |
| Widowed | 2 (10.5) |
| Coexistence: | |
| Living alone | 3 (15.8) |
| Living with partner | 16 (84.2) |
| Cancer stage: | |
| Stage I | 20 (90.9) |
| Stage II | 2 (9.1) |
| Time elapsed since surgery (months) | 5 (4–8) |
| Chemotherapy (%) | 3 (15) |
| Radiotherapy (%) | 3 (15) |
| Presence of COPD | 15 (68.2) |
| BMI (kg/m2) | 27.8 (26.3–31.5) |
| Lean mass (kg) | 50.3 (43–59) |
| Fat mass (%) | 31.9 (26.9–35.1) |
| Ability to perform the activities of daily life (Barthel index score) | 100 (100–100) |
| Nutritional status (MNA-SF score) | 12 (10.2–13) |
| Physical functional status (SPPB score) | 11 (8–12) |
| Comorbidities (CIRS-G score) | 7 (6–10) |
| Variables | Preintervention-M0 (Median [IQR]) | Postintervention-M1 (Median [IQR]) | p-Value Adjusted | Size Effect (r) If Significant p Value (p < 0.05) |
|---|---|---|---|---|
| Gait speed (m/s) | 1.09 (0.98–1.28) | 1.61 (1.46–1.80) | <0.001 | 0.75 |
| 30s-STS (repetitions) | 13.5 (10.8–14.0) | 16.0 (15.0–19.0) | <0.001 | 0.77 |
| Handgrip (kg) | 30.0 (19.5–34.3) | 25.0 (20.5–31.0) | N.S. | |
| 6MWT (m) | 436.0 (398.0–459.5) | 471.0 (421.5–522.5) | 0.012 | 0.58 |
| Physical functional status (SPPB score) | 11.0 (8.0–12.0) | 12.0 (12.0–12.0) | 0.007 | 0.66 |
| BMI (kg/m2) | 27.8 (26.3–31.5) | 28.3 (25.6–31.3) | N.S. | |
| Lean mass (kg) | 50.4 (43.0–59.0) | 48.8 (43.4–58.9) | N.S. | |
| Fat mass (%) | 31.9 (26.9–35.1) | 31.2 (27.6–35.4) | N.S. | |
| EORTC-QLQ-C30 health-status self-perception (points) | 52.0 (49.5–61.25) | 51.0 (46.0–59.0) | N.S. | |
| EORTC-QLQ-C30 function (points) | 23.5 (20.5–28.3) | 22.0 (19.5–29.0) | 0.016 | 0.55 |
| EORTC-QLQ-C30 symptoms (points) | 19.0 (16.0–24.5) | 17.0 (14.0–21.0) | 0.013 | 0.57 |
| Glucose (mg/dL) | 102.0 (95.0–114.5) | 101.0 (94.0–113.5) | N.S. | |
| Total cholesterol (mg/dL) | 185.0 (154.8–217.5) | 170.0 (128.3–200.0) | 0.013 | 0.58 |
| Triglycerides (mg/dL) | 94.0 (75.5–132.0) | 70.0 (61.0–102.0) | 0.033 | 0.46 |
| Total proteins (g/dL) | 7.3 (7.0–7.4) | 7.1 (6.8–7.3) | N.S. | |
| Albumin (g/dL) | 4.5 (4.3–4.6) | 4.5 (4.4–4.8) | N.S. | |
| Pre-albumin (mg/dL) | 25.0 (23.5–29.2) | 26.7 (24.2–29.9) | 0.037 | 0.45 |
| Creatinine (mg/dL) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | N.S. | |
| CRP (mg/L) | 4.0 (2.0–7.0) | 3.0 (1.3–4.0) | N.S. | |
| IGF-1 (ng/mL) | 151.5 (93.3–180.0) | 105.0 (75.0–136.0) | 0.029 | 0.48 |
| Hb (g/dL) | 13.8 (12.7–14.9) | 14.3 (13.2–15.2) | N.S. | |
| Haematocrit (%) | 41.5 (38.9–44.3) | 42.2 (39.8–44.3) | N.S. | |
| Haematites (×1012/L) | 4.8 (4.4–5.0) | 4.8 (4.5–5.0) | N.S. | |
| Leucocytes (×109/L) | 6.8 (5.6–8.1) | 6.7 (5.4–7.3) | N.S. | |
| Platelets (×109/L) | 231.5 (198.5–260.3) | 202.5 (181.8–229.5) | 0.029 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria-Comes, T.; Climent-Gregori, M.; Maestu-Maiques, I.; Inchaurraga-Álvarez, I.; Cuenca-Martínez, F.; Cauli, O.; Martínez-Arnau, F.M. Effect of a Physical Exercise Intervention on Physical Function Parameters and Blood Analytical Changes in Lung Cancer Survivors: A Feasibility Study. Clin. Pract. 2024, 14, 2202-2216. https://doi.org/10.3390/clinpract14050173
Soria-Comes T, Climent-Gregori M, Maestu-Maiques I, Inchaurraga-Álvarez I, Cuenca-Martínez F, Cauli O, Martínez-Arnau FM. Effect of a Physical Exercise Intervention on Physical Function Parameters and Blood Analytical Changes in Lung Cancer Survivors: A Feasibility Study. Clinics and Practice. 2024; 14(5):2202-2216. https://doi.org/10.3390/clinpract14050173
Chicago/Turabian StyleSoria-Comes, Teresa, María Climent-Gregori, Inmaculada Maestu-Maiques, Ignacio Inchaurraga-Álvarez, Ferrán Cuenca-Martínez, Omar Cauli, and Francisco M. Martínez-Arnau. 2024. "Effect of a Physical Exercise Intervention on Physical Function Parameters and Blood Analytical Changes in Lung Cancer Survivors: A Feasibility Study" Clinics and Practice 14, no. 5: 2202-2216. https://doi.org/10.3390/clinpract14050173
APA StyleSoria-Comes, T., Climent-Gregori, M., Maestu-Maiques, I., Inchaurraga-Álvarez, I., Cuenca-Martínez, F., Cauli, O., & Martínez-Arnau, F. M. (2024). Effect of a Physical Exercise Intervention on Physical Function Parameters and Blood Analytical Changes in Lung Cancer Survivors: A Feasibility Study. Clinics and Practice, 14(5), 2202-2216. https://doi.org/10.3390/clinpract14050173








